Health Related Quality of Life in Patients with HCV Geno-Type 4 and Cirrhosis Receiving Direct Acting Anti-viral Drugs
Received: 24-Jul-2019 / Accepted Date: 21-Feb-2020 / Published Date: 28-Feb-2020 DOI: 10.4172/2161-0711.1000680
Abstract
Background: Chronic Hepatitis C virus (HCV) causes significant decline in the Health Related Quality of Life (HRQL). Recently, Direct Acting Anti-viral drugs (DAA) have been endorsed as standard of care for treatment for HCV.
Aim: To study the HRQL in patients with HCV and cirrhosis before and after DAA.
Subject and methods: We included 500 patients with HCV and cirrhosis fulfilled all eligibility criteria for treatment and completed the survey of HRQL using (SF-36 and CLDQ questioners) before and after treatment. Patients were treated by combination of one of the following regimens: a) single daily oral dose of 400 mg of Sofosbuvir (SOF) plus 60 mg of Daclatasvir (DAC) ± ribavirin (RBV) in 2 divided doses or b) 400 mg of SOF plus 150 mg of Simeprevir (SIM) ± RBV or c) 400 mg of SOF plus RBV.
Results: On treatment, 35.2% of the patients had a poor physical function and half of them had poor roleemotional, while 45.7% patients’ had excellent role-emotional, 49% noticed change in their mode, The evaluation of HRQL before and after treatment through SF-36 and CLDQ showed an improvement in different domains (p<0.05). The overall sustained virological response (SVR) was (89.6%).
Conclusions: Results from the current study suggest that HRQL decreased on treatment and significantly increased after treatment. We recommend addressing the patients’ quality of life into consideration as a part of the evaluation protocol before the initiation of DAA drugs and after cure to improve this particular aspect of patients’ life.
Keywords: HCV; DAA drugs; Liver cirrhosis; Quality of life
Introduction
Given HCV, is recognized as a major national and international health burden and represents around 14.7% prevalence rate among the general population in Egypt [1] with estimated worldwide prevalence of 150-170 million [2]. On one hand, HCV infection can lead to a diversity of hepatic related complications that ranged from mild form of chronic hepatitis to liver cirrhosis and hepatocellular carcinoma, with estimated mortality up to 350,000 people/year [3]. On the other hand, HCV may promote a panel of extra-hepatic manifestations, twothirds of infected patients experienced extra-hepatic manifestations at some time point of course of disease [3]. Some of these manifestation are well documented and more common, while others are infrequent [3-5]. Soon after HCV discovery, HCV-related autoimmune or lymphoproliferative disorders, from benign mixed cryoglobulinemia to frank lymphomas, have been reported [5-7]. Patients with HCV infection and associated extra hepatic manifestations have shown higher morbidity and mortality rates than patients with HCV alone [8-11]. Moreover patients with HCV infection have reported significant decline of the key dimensions of their quality of life [12-19].
Many patients with chronic HCV infection report unexplained symptoms including dyspepsia, fatigue, irritability, anorexia, nausea, headache muscle ache. These symptoms have been shown to negatively impact patients’ psychological well-being and quality of life [20,21].
Given the association of aforementioned extra hepatic comorbidities in absence of severity of liver disease, non-viral factors such as psychiatric and stigmatization elements associated with acquisition of the virus, may explain part of decline of quality of life associated with HCV [22,23]. HCV infection may be associated with the release of inflammatory cytokines and altered serotonergic and dopaminergic neurotransmission [24].
Health-related quality of life has also been reported to be influenced by the patient’s awareness of a serious illness [25].
Many clinical findings have shown that, viral eradication significantly reduced the rate of extra hepatic deaths [26-29]. Recent therapeutic advances in the treatment of HCV, with the possibility to eradicate HCV following newly introduced DAA [30] appears of major importance, for hepatic and extra hepatic manifestations. The HRQL in patients with HCV and cirrhosis before and after DAA wasn’t studied.
Subjects and Methods
Subjects
From March 2016 to March 2018; 19443 patients with chronic HCV infection have been evaluated for participation in National Egyptian program for treatment for HCV at National Liver Institute’ hospital- Menoufia University-Egypt. Among this number, 2127 patients have liver cirrhosis, from this number of patients, 780 met our inclusion criteria and agreed to sign the consent form to participate in the current study, 620 patients started DAAs treatment, but only 582 started the study. Finally, 500 patients fulfilled all eligibility criteria and completed the survey before and after the end of treatment.
All patients tested positive for antibodies to HCV in addition to having circulating HCV-RNA as measured by RT-PCR, all patients were geno-type 4 and lacked the clinical or laboratory indices of advanced liver disease according to the current national Egyptian program for treatment of HCV. All patients have Metavir score of F3 and F4 based on fibroscan results prior to initiation of treatment.
Exclusion criteria included: age under 18 or above 70 years, associated another liver diseases as co-infections with HBV, patients with diseases known to cause decline the quality of life as cancers, psychiatric illness, autoimmune disease, ischemic heart disease, addicts. Patients with depression were also excluded based on the validated Arabic version of Hospital Anxiety and Depression Scale [31].
Methods
Assessment of HRQL: Patients, who initially agreed, were interviewed by a trained research assistant and they were asked to sign an Arabic consent form in the presence of one of authors. The consent form stated that the answers would be anonymous and the obtained results would be used only for scientific research. For comprehensive assessment of HRQL, the Short Form-36 (SF-36) and The Chronic Liver Disease Quality score (CLDQ) were applied before and after treatment.
A: SF-36 Health Survey summary score. It is a simple selfadministered multidimensional questionnaire. It comprises 36 items from 8 dimensions, (Physical Functioning (PF), Mental Health (MH), General Health (GH), Bodily Pain (P), Role Physical (RP), Social Functioning (SF), Vitality (V), Role Emotional (RE), Results are expressed as a score ranging from 0 to 100 for each of the 8 dimensions, the higher scores is indicator of better quality of life [32].
B: CLDQ is a disease-targeted measure focuses on aspects of HRQL relevant to liver disease. It is composed of 29 questions distributed in 6 domains (abdominal symptoms, fatigue, (systemic symptoms, activity, emotional function, and worry. according to severity. Scores range from 1 (worst) to 7 (best), in which higher scores indicate better quality of life. The overall score is calculated by sum of average of the six dimensions [33,34].
DAA drugs: Patients were treated by combination of one of the following regimens: a) single daily oral dose of 400 mg of Sofosbuvir (SOF) plus single daily oral dose of 60 mg of Daclatasvir (DAC) ± RBV (12-15 mg/kg/day in 2 divided doses or b) single daily oral dose of 400 mg SOF plus single daily oral dose of 150 mg Simeprevir (SIM) ± weight based ribavirin (RBV as mentioned before) or c) single daily oral dose of 400 mg SOF plus weight based ribavirin (RBV as mentioned before).
Patients were treated for 12 weeks or 24 weeks based on the committee of treatment protocol. All patients were followed up during treatment every 4 weeks and at the end of treatment, clinically and by laboratory investigations.
Follow up: Patients were followed up by the research team at 12 weeks and 24 weeks after end of treatment. Adverse events and treatment discontinuation were reported. All collected data were documented in clinical records, specifically designed for the current study.
Ethical approval
The study protocol was approved by the ethics committee (for medical research) of Würzburg University in accordance with the Declaration of Helsinki and the Institutional Review Board of the National Liver Institute, Menoufia University-Egypt, (IRB number IRB00003413).
Data collection
A baseline and disease history data of the participants, Baseline data included age, time of diagnosis, other comorbidities, marital status, sex, and education. The history related to the disease comprised previous treatments and respective responses, assessment of hepatic impairment, stage of liver fibrosis and/or presence of cirrhosis, presence of extra-hepatic manifestations, and quantitative viral load (HCV-RNA).
Statistical analysis
Data recorded in Microsoft Office Excel were processed and then transferred to the SPSS software version 26, in which all statistical tests were performed. Qualitative variables were expressed as absolute and relative frequencies, and quantitative variables were presented as means and Standard Deviations (SD).
The Kolmogorov-Smirnov test was used to verify the normality distribution of the studied parameters. Non-parametric tests were applied in the comparative analysis as data wasn ’ t normally distributed. Wilcoxon-related samples test was used for statistical analysis of the HRQoL instruments which allowed the comparison of HRQoL within a single group of patients, in which domains were evaluated in pairs, with values referring to before and after treatment, for each instrument. Mann-Whitney U test was performed to compare quality of life in patients receiving different treatments. For all analyses, the significance level of p <0.05 was adopted, with a confidence level of 95%.
Results
Baseline characteristics
All baseline characteristics, socio-demographic data, degree of fibrosis and treatment response were presented in Table 1. Most of the studied patients were males (420 out of 500 patients), most of them were treated with SOF/DAC ± RBV in 300 patients, 190 patients were treated with SOF/SIM ± RBV and 10 patients received SOF/RBV, 395 patients were treated for 12 weeks, Metavir score was F3 in most of cases (278 patients), 310 patients (62%) of cases were not responders to interferon based therapy.
| Parameter | Total patients (n-=500) |
|---|---|
| Gender, n (%) | |
| Male | 420 (84%) |
| Female | 80(16%) |
| Age, n (%) | |
| 18-34 | 25 (5%) |
| 35-54 | 250 (50%) |
| 55-74 | 225 (45%) |
| Mean age (SD) years | 42.8 ± 8.2 |
| Treatment received, n (%) | |
| SOF + DAC ± RBV | 300 (68%) |
| SOF + SMV ± RBV | 190 (30%) |
| SOF + RBV | 10 (2%) |
| Time since diagnosis, years (SD) | 12.87 ± 10.07 |
| Treatment duration, n (%) | |
| 12 w | 395 (79%) |
| 24 w | 105 (21%) |
| Metavir, n (%) | |
| F3 | 278 (55.6%) |
| F4 | 222 (44.4%) |
| Patients previously treated, n (%) | 310 (62%) |
| Viral load = 800.000 IU mL-1, n (%) | 240 (48%) |
| Marital status, n (%) | |
| Married | 470 (94%) |
| Not Married (Single, Separated, Widowed) | 30 (6%) |
| Educational level, n (%) | |
| Illiterate | 60 (12%) |
| Elementary school | 90 (18%) |
| Secondary school | 185 (37%) |
| High school | 165 (33%) |
| Patients with addictions, n (%) | 0 (0%) |
| 50% of the patients were between 35-54 years old. Abbreviations: RBV: ribavirin; SOF: sofosbuvir; SMV: simeprevir |
|
Table 1: Socio-demographic and clinical variables of the study population.
On treatment, about 35.2% of the patients had a poor physical function and about half of them had poor role-emotional, while 45.7% patients’ had excellent role-emotional, 49% noticed change in their mode, and about 5% felt depressed to the extent of suicide, about (27%) had fair bodily-pain, (32.0%) had fair general health and (27%) had poor general health, 47.0% had poor vitality, 35.0% of patients had excellent social functioning, whereas (about 65.0%) ranging from poor to fair social functioning, about (36.0%) had poor mental health (Table 2).
| SF 36 scales | score | No. | Percentage (%) |
|---|---|---|---|
| Physical Functioning | (85 – 100 %) | 120 | 24 |
| (75 - < 85%) | 70 | 14 | |
| (65 - < 75%) | 55 | 11 | |
| (50 - < 65%) | 80 | 16 | |
| (< 50%) | 175 | 35 | |
| Role-Physical | (85 – 100 %) | 160 | 32 |
| (75 - < 85%) | 20 | 4 | |
| (65 - < 75%) | 0 | 0 | |
| (50 - < 65%) | 55 | 11 | |
| (< 50%) | 265 | 53 | |
| Role-Emotional | (85 – 100 %) | 230 | 46 |
| (75 - < 85%) | 0 | 0 | |
| (65 - < 75%) | 20 | 4 | |
| (50 - < 65%) | 0 | 0 | |
| (< 50%) | 250 | 50 | |
| Bodily-Pain | (85 – 100 %) | 120 | 24 |
| (75 - < 85%) | 25 | 5 | |
| (65 - < 75%) | 90 | 18 | |
| (50 - < 65%) | 135 | 27 | |
| (< 50%) | 130 | 26 | |
| General Health | (85 – 100 %) | 0 | 0 |
| (75 - < 85%) | 75 | 15 | |
| (65 - < 75%) | 130 | 26 | |
| (50 - < 65%) | 160 | 32 | |
| (< 50%) | 135 | 27 | |
| Vitality | (85 – 100 %) | 60 | 12 |
| (75 - < 85%) | 50 | 10 | |
| (65 - < 75%) | 45 | 9 | |
| (50 - < 65%) | 110 | 22 | |
| (< 50%) | 235 | 47 | |
| Social Functioning | (85 – 100 %) | 175 | 35 |
| (75 - < 85%) | 40 | 8 | |
| (65 - < 75%) | 0 | 0 | |
| (50 - < 65%) | 140 | 28 | |
| (< 50%) | 145 | 29 | |
| Mental Health | (85 – 100 %) | 75 | 15 |
| (75 - < 85%) | 60 | 12 | |
| (65 - < 75%) | 75 | 15 | |
| (50 - < 65%) | 110 | 22 | |
| (< 50%) | 180 | 36 |
Table 2: Distribution of patients attending National Liver Institute Hospital according to SF 36 scales (N=500).
The evaluation of HRQL before and after treatment through SF-36 showed an improvement in different domains. We noticed that the HRQL was improved after treatment, patients with SVR gained significantly better scores in most of dimensions of SF-36 and CLDQ instruments (Table 3).
| Domains of SF-36 and CLDQ | Before treatment | After treatment | p-value | Treatment responders | Treatment Non-responders | p-value | |
|---|---|---|---|---|---|---|---|
| SF-36 | PF | 52.1 ( ± 64.5) | 70.3 ( ± 26.3) | 0.04* | 78.9 ( ± 23.7) | 55.3 ( ± 24.3) | 0.03* |
| PH | 43.1 ( ± 42.1) | 62.5 ( ± 42.6) | 0.01* | 68.3 ( ± 43.2) | 44.7 ( ± 39.6) | 0.01* | |
| P | 53.6 ( ± 26.1) | 63.4 ( ± 25.7) | 0.005* | 76.4 ( ± 22.5) | 60.2 ( ± 22.3) | 0.02* | |
| GH | 55.3 ( ± 25.2) | 61.6 ( ± 24.2) | 0.001* | 81.2 ( ± 21.2) | 60.7 ( ± 26.2) | 0.001* | |
| V | 51.4 ( ± 27.1) | 62.3 ( ± 25.5) | 0.006* | 66.3 ( ± 22.5) | 52.3 ( ± 24.9) | 0.03* | |
| SF | 65.6 ( ± 32.4) | 74.2 ( ± 33.2) | 0.07 | 79.6 ( ± 30.4) | 69.3 ( ± 32.8) | 0.04* | |
| EW | 46.8 ( ± 46.2) | 53.6 ( ± 44.6) | 0.09 | 55.1 ( ± 41.3) | 47.1 ( ± 45.1) | 0.046* | |
| MH | 59.2 ( ± 23.9) | 68.1 ( ± 21.9) | 0.005* | 71.1 ( ± 22.2) | 63.2 ( ± 23.3) | 0.003* | |
| CLDQ | AS | 4.08 ( ± 1.82) | 4.11 ( ± 1.76) | 0.8 | 4.43 ( ± 1.75) | 4.11 ( ± 1.68) | 0.05 |
| FA | 3.44 ( ± 1.68) | 3.81 ( ± 1.74) | 0.08 | 3.92 ( ± 1.71) | 3.63 ( ± 1.65) | 0.06 | |
| AT | 4.36 ( ± 1.72) | 4.52 ( ± 1.84) | 0.5 | 4.73 ( ± 1.86) | 4.37 ( ± 1.74) | 0.07 | |
| EF | 3.75 ( ± 1.66) | 4.08 ( ± 1.84) | 0.006* | 4.24 ( ± 1.79) | 3.79 ( ± 1.81) | 0.003* | |
| WO | 3.62 ( ± 1.72) | 5.05 ( ± 1.61) | 0.001* | 5.51 ( ± 1.75) | 3.76 ( ± 1.65) | 0.001* | |
| SS | 4.09 ( ± 1.37) | 4.49 ( ± 1.24) | 0.03* | 4.55 ( ± 1.26) | 4.11 ( ± 1.27) | 0.04* | |
| Overall score | 3.88 ( ± 1.29) | 4.35 ( ± 1.23) | 0.002* | 4.55 ( ± 1.24) | 3.94 ( ± 1.26) | 0.003* | |
| Note: *p<0.05. Abbreviations: AS: Abdominal Symptoms; AT: Activity; EW: Emotional Well-being; EF: Emotional Function; FA: Fatigue; GH: General Health; MH: Mental Health; P: Pain; PF: Physical Functioning; PH: Role Limitations due to Physical Health; SF: Social Functioning; SS: Systemic Symptoms; V: Vitality; WO: Worry | |||||||
Table 3: Comparison of SF-36 and CLDQ scores between patients before and after treatment (N=500).
In SF-36 score, an increase in patients’ HRQL in six of the eight domains was noticed (p<0.05). In four of domains of CLDQ score: EF, WO, SS and overall score, patients presented significantly higher averages after treatment (p<0.05). Another analysis was performed to compare the quality of life in patients receiving different treatments SOF/DAC vs. SOF/SIM, with or without RBV), patients’ receiving SOF/DAC ± RBV had significantly lower scores before and after treatment compared to the ones receiving SOF/SIM (Table 4).
| Domains of SF-36 and CLDQ | Before treatment with SOF + DAC ± RBV |
Before treatment with SOF + SMV ± RBV |
p-value | After treatment with SOF + DAC ± RBV |
After treatment with SOF + SMV ± RBV |
p-value | |
|---|---|---|---|---|---|---|---|
| SF-36 | PF | 64.1 ( ± 31.0) | 70.0 ( ± 31.0) | 0.5 | 69.1 ( ± 28.7) | 75.3 ( ± 28.3) | 0.43 |
| PH | 40.3 ( ± 43.1) | 56.8 ( ± 44.1) | 0.22 | 52.1 ( ± 44.1) | 78.0 ( ± 39.5) | 0.04* | |
| P | 51.1( ± 25.0) | 61.0 ( ± 24.1) | 0.23 | 57.9 ( ± 24.7) | 71.8 ( ± 23.0) | 0.04* | |
| GH | 49.1 ( ± 25.9) | 62.4 ( ± 18.2) | 0.09 | 59.0 ( ± 26.0) | 69.9 ( ± 19.1) | 0.19 | |
| V | 45.9 ( ± 28.7) | 58.1 ( ± 20.8) | 0.13 | 54.1 ( ± 28.2) | 70.1 ( ± 25.0) | 0.04* | |
| SF | 56.0 ( ± 31.9) | 84.2 ( ± 16.1) | 0.003* | 65.2 ( ± 32.0) | 83.9 ( ± 29.0) | 0.01* | |
| EW | 38.1 ( ± 44.0) | 69.3 ( ± 43.0) | 0.03* | 42.0 ( ± 46.0) | 82.1 ( ± 33.6) | 0.007* | |
| MH | 53.2 ( ± 24.9) | 73.9 ( ± 15.1) | 0.0027* | 61.9 ( ± 22.4) | 77.0 ( ± 18.0) | 0.02* | |
| CLDQ | AS | 4.0 ( ± 1.9) | 4.3 ( ± 1.8) | 0.88 | 4.2 ( ± 1.6) | 4.3 ( ± 1.7) | 0.96 |
| FA | 3.2 ( ± 1.7) | 4.0 ( ± 1.3) | 0.07 | 3.6 ( ± 1.4) | 4.4 ( ± 1.4) | 0.14 | |
| AT | 4.1 ( ± 1.7) | 4.9 ( ± 1.8) | 0.04* | 4.4 ( ± 1.3) | 4.5 ( ± 1.8) | 0.62 | |
| EF | 3.0 ( ± 1.7) | 4.2 ( ± 1.1) | 0.02* | 3.8 ( ± 1.2) | 4.7 ( ± 1.3) | 0.06 | |
| WO | 3.4 ( ± 1.9) | 4.1 ( ± 1.4) | 0.2 | 4.5 ( ± 1.2) | 5.5 ( ± 1.1) | 0.037* | |
| SS | 4.0 ( ± 1.4) | 4.4 ( ± 1.4) | 0.63 | 4.3 ( ± 1.0) | 4.9 ( ± 1.2) | 0.04* | |
| Overall score | 3.7 ( ± 1.2) | 4.4 ( ± 1.0) | 0.09 | 4.1 ( ± 1.0) | 4.6 ( ± 1.0) | 0.25 | |
| Note: *p<0.05. Abbreviations: AS: Abdominal Symptoms; AT: Activity; EW: Emotional Well-being; EF: Emotional Function; FA: Fatigue; GH: General Health; MH: Mental Health; P: Pain; PF: Physical Functioning; PH: Role Limitations due to Physical Health; SF: Social Functioning; SS: Systemic Symptoms; V: Vitality; WO: Worry | |||||||
Table 4: Comparison of SF-36 and CLDQ scores between SOF / DAC versus SOF / SMV patients before and after treatment (N=500).
Outcomes regarding treatment effectiveness and safety
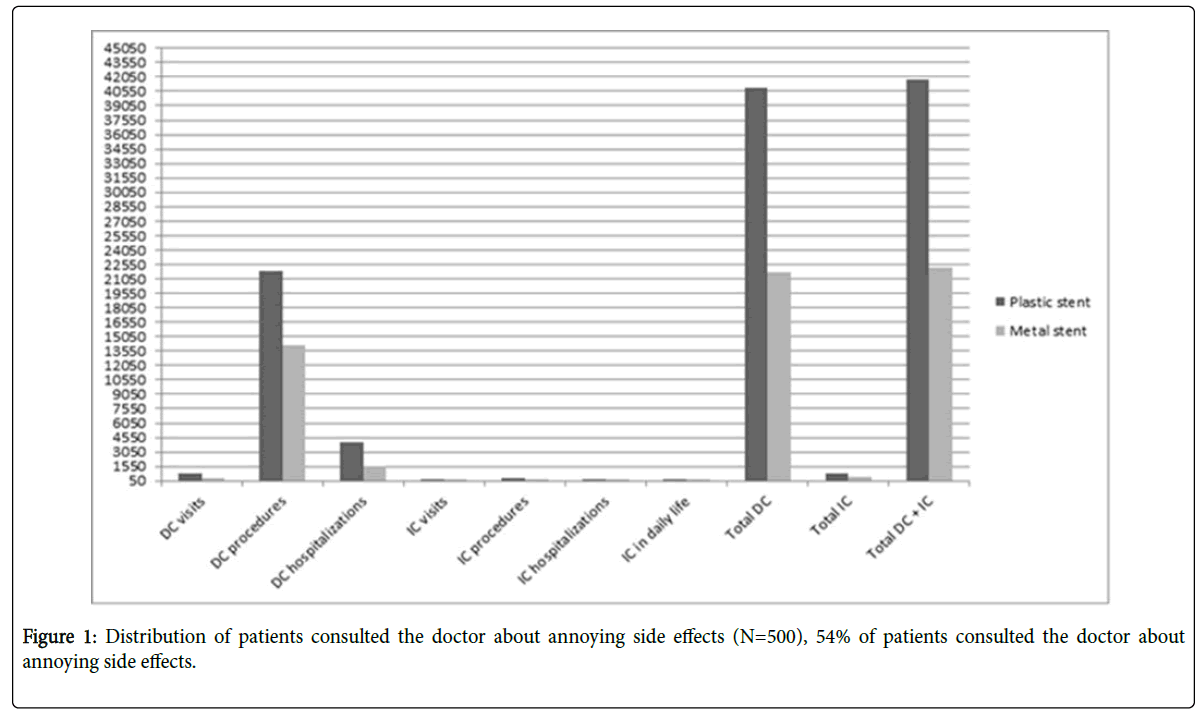
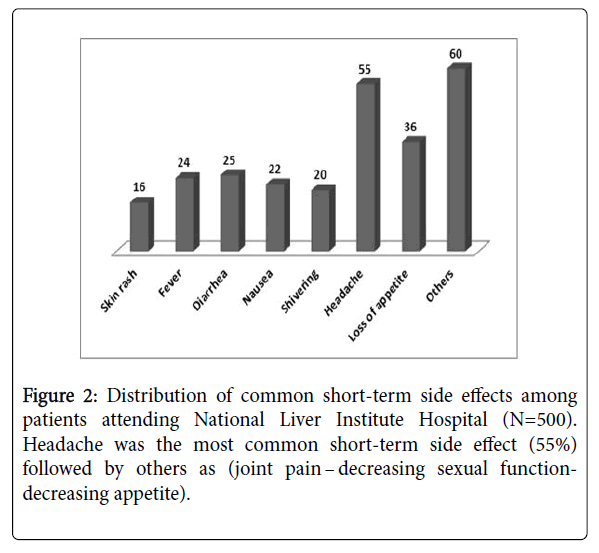
SVR was found in 448 out of 500 patients (89.6%). Regarding treatment regimens, 264 out of 300 patients who received SOF/DAC ± RBV, 174 out of 190 patients who received SOF/SMV ± RBV and 10 out of 10 patients who received SOF/RBV achieved SVR (88 % and 91.58% and 100% respectively). Only forty five of the 500 patients (9.0%) who were evaluated for safety outcomes did not present any adverse events. 270 patients (54%) sought medical advices for annoying side effects (Figure 1). The most commonly reported adverse events during treatment were headache (55%), loss of appetite (36%), shivering (20%), skin rash (16%), nausea (22%), Fever (24%), diarrhea (25%) and other problems (60%). No patients discontinued treatment due to adverse events (Figure 2).
Discussion
The concept of quality-of-life in health had been introduced in 1970 as an outcome measure in healthcare development in the context of medical progress [35]. Given the progress brings an increment in life expectancy and acute illness previously lethal could be curable, and chronic diseases could also be efficiently controlled. Consequently, HRQL has become of great significance to evaluate how patients live their ‘next times [36]. HRQL has intersections with functional and biological aspects, such as disability, health and functional statuses, social and psychological concepts such as well-being, happiness and satisfaction. Moreover, the American Food and Drug Administration have emphasized that, the HRQL should be considered when evaluating the outcomes of chronic disease and the impact of therapeutic modalities [37]. The HRQL can be classified as generic or disease specific. Generic measures are tailored to comparing patient groups across different diseases and for comparing diseased to healthy people, the disease-specific measures are suited to determination of the efficacy of treatment modalities for a specific disease [38].
In recent years, hundreds of thousands of Egyptians have been recruited for the current Egyptian national program for combating HCV infection, aimed at reducing the prevalence of HCV and eradication by 2030. The plenty of affordable generic DAAs in Egypt allowed us to recruit patients from the National Liver Institute, a nationally representative, well-equipped government university center. The DAA drugs have been emerged in recent years, however the treatment regimens have undergone numerous changes. The interferon-free schemes, have been approved in 2014 in terms of significantly better SVR with less adverse events and adherence to therapy when compared to previous regimens [39-41]. Moreover, in patients with cirrhosis or advanced fibrosis with SVR, the histologic analysis and or elastography showed that the fibrosis stage of HCVinfected patients was improved, consequently, improvement of HRQL is expected based on expected improvement of the synthetic power of liver in cured patients [42-44].
From this context, we evaluated 500 Egyptian patients with chronic HCV infection and cirrhosis. We matched our patients in terms of factors that might influence the responses on the HRQL questionnaires, including age, educational level, socioeconomic class, Matavir score and previous treatments with interferon based therapy, all studied patients were treated with the three approved regimens at the time of study conduction namely, SOF/DAC with or without RBV, SOF/SIM with or without RBV and SOF/RBV in small number of patients.
In the present study, none of the studied patients discontinued treatment, and the aforementioned regimens have wide safety margins, however, we have found that significant proportions of patients developed significant adverse effects, and about 5% of them felt depressed to the extent of suicide during the period of treatment based on the results of SF-36. Our studied patients, experienced marked improvement after treatment than before treatment based on the SF-36 results, either in the total score or in most of its sub domains (p<0.05). Additionally, patients also presented significantly higher averages after treatment than before treatment (p<0.05) in the overall score and in some domains of CLDQ: namely EF, WO, SS.
In the sub analysis of our findings, the quality of life in patients receiving SOF/DAC with or without RBV had significantly lower scores before and after treatment compared to patients receiving SOF/ SIM. In the study population, a minority of patients (9.0%) did not present any adverse events, considerable number of patients, (54%) sought medical advice for some distressing adverse effects during treatment: headache (55%), loss of appetite (36%), shivering (20%), skin rash (16%), nausea (22%), fever (24%), diarrhea (25%) and other problems (60%). However all of the aforementioned adverse effects were self-limited.
The over-all SVR in our study population was (89.6. %). Regarding treatment regimens, the SVR was in SOF/DAC ± RBV, SOF/SIMV ± RBV and SOF/RBV got SVR of (88 %, 91.58% and 100%) respectively). Given all of the study’ populations are cirrhotic and were classified in the category of difficult to treat, moreover, significant number of them were non responders to interferon based therapy.
Our findings supported the results of Siqueira et al. [45] who discussed the quality of life during the treatment with DAA drugs and by the same regimens, however their study was limited by inclusion of small number of patients and the heterogeneity of study population by inclusion of patients with and without cirrhosis and associated comorbidities, the aforementioned factors may confound the results during and after treatment.
Our findings clearly supported the superiority of SOF/SIM as it related to patients’ HRQL when compared to other regimens. Given the SOF/DAC was considered as the treatment modality in patients with advanced cirrhosis, In fact, patients treated with second generation DAA drugs with (FN-free regimen) have a rapid improvement of their HRQL scores after treatment.
In our study patients with SVR gained better HRQL than nonresponders. The SVR, induced as an impact of HCV clearance, has been linked to the positive impacts of disease progression and survival outcomes, in addition SVR normalizes the elevated low-level of inflammatory cytokines and optimizes metabolites at the level of basal ganglia, that in turn improves the cerebral immune activation and cognitive function among patients who clear the virus [46,47].
In the era of DAA, similar studies have been conducted in different geographical areas with use of different or similar regimens [48-55]. Our findings are consistent with the data reported for patients with HCV worldwide who had been treated with the same regimens [51-55]. However, the direct comparison between the cohorts is difficult given the cohorts are not matched with different baseline clinical and socio-demographic characteristics in addition to use of different instruments in assessment of HRQL.
Many limitations are notable in this study, first, the use of English versions of SF 36 and CLDQ scores with Egyptian patients with native Arabic language tacked substantial effort for translation, second, the patients had different baseline characteristics and different treatment regimens, third the different age groups among the study population with different dimensions of HRQL. Moreover our study and others suffered from the lacking of standardized instruments for assessment of HRQL among patients with liver disease.
The many strengths of the current study include its status as the first to estimate an extensive assessment of HRQL in Egyptian patients with HCV related cirrhosis treated with interferon free regimens plus the exclusion of other comorbidities that confound the result. Moreover we compared between responders and non-responders in term of HRQL. The relatively large number of the study population, re-enforces the validity of our study.
Conclusion
DAA therapies have wide safety margins in the treatment of patients with HCV and cirrhosis. Results from the current study suggest that HRQL decreased during the therapy and significantly increased after treatment. Patients with SVR have significantly better HRQL than non-responders. We recommend addressing the patients’ quality of life into consideration as a part of the evaluation protocol before the initiation of DAA therapy and after cure to improve this particular aspect of patients’ life. Finally, it is important for hepatologists and health educators to encourage the patients to voice their concerns about quality of life, especially given the comings of potent and safer DDA drugs.
Key issues
• This study examined the HRQL in patients with HCV and cirrhosis before and after DAA wasn’t studied.
• We included 500 patients with HCV and cirrhosis fulfilled all eligibility criteria for treatment and completed the survey of HRQL using (SF-36 and CLDQ questioners) before and after treatment
• Results from the current study suggest that HRQL decreased on treatment and significantly increased after treatment.
• We recommend addressing the patients ’ quality of life into consideration as a part of the evaluation protocol before the initiation of DAA drugs and after cure to improve this particular aspect of patients’ life.
• There is much strength in the current study as its status as the first to estimate an extensive assessment of HRQL in Egyptian patients with HCV related cirrhosis treated with interferon free regimens plus the exclusion of other comorbidities that confound the result. The relatively large number of the study population, re-enforces the validity of our study.
Acknowledgments
We wish to thank all the patients and medical staff who participated in this study. We also wish to thank Prof. Dr Imam Waked for his input during the development of this manuscript.
Authors’ Contributions
EE and WM contributed to study concept, design, and data collection. EE, NS, and WO were clinical investigators in the study. All authors contributed to the analysis and interpretation of the data, and all authors read, critically revised, and approved the final manuscript.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the International Conference on Harmonisation guideline for good clinical practice and the ethical principles of the Declaration of Helsinki. All patients gave written informed consent, which was reviewed and approved by an independent ethics committee or institutional review board of National Liver Institute (IRB00003413). This study was approved (approval numbers 00125/2017).
References
- Mohamoud YA, Mumtaz GR, Riome S, Miller D, Raddad LA (2013) The epidemiology of hepatitis C virus in Egypt: A systematic review and data synthesis. BMC Infect Dis 13: 288.
- Chack E, Talal AH, Sherman KE, Schiff ER, Saab S (2011) Hepatitis C virus infection in USA: An estimate of true prevalence. Liver Int 31: 1090-1101.
- Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, et al. (1999) Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment virus C. Arthritis Rheum 42: 2204-2212.
- Cacoub P, Renou C, Rosenthal E, Cohen P, Loury I, et al. (2000) Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. Medicine 79: 47-56.
- Zignego A, Ferri C, Pileri S, Caini P, Bianchi F, et al. (2007) Extrahepatic manifestations of hepatitis C virus infection: A general overview and guidelines for a clinical approach. Dig Liver Dis 39: 217.
- Terrier B, Cacoub P (2013) Renal involvement in HCV-related vasculitis. Clin Res Hepatol Gastroenterol 37: 334-339.
- Zignego A, Bre´chot C (1999) Extrahepatic manifestations of HCV infection: Facts and controversies. J Hepatol 31: 369-376.
- Maasoumy B, Wedemeyer H (2012) Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol 26: 401-412.
- Uto H, Stuver S, Hayashi K, Kumagai K, Sasaki F, et al. (2009) Increased rate of death related to presence of viremia among hepatitis C virus antibody-positive subjects in a community-based cohort study. Hepatology 50: 393-399.
- Bonkovsky HL, Woolley JM (1999) Reduction in health-related quality of life in chronic hepatitis C and improvement with interferon therapy. The Consensus Interferon Study Group. Hepatology 29: 264-270.
- Svirlith N, Pavic S, Terzic D, Delic D, Simonovic J, et al. (2008) Reduced quality of life in patients with chronic viral liver disease as assessed by the SF-12 questionnaire. J Gastrointest Liver Dis 17: 405-409.
- DiBonaventura MD, Wagner JS, Yuan Y, L'Italien G, Langley P, et al. (2010) Humanistic and economic impacts of hepatitis C infection in the United States. J Med Econ 13: 709-718.
- Hsu PC, Federico CA, Krajden M, Yoshida EM, Bremner KE, et al. (2012) Health utilities and psychometric quality of life in patients with early- and late-stage hepatitis C virus infection. J Gastroenterol Hepatol 27: 149-157.
- Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, et al. (2003) Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 98: 630-638.
- Bezemer G, van Gool AR, Verjeij-Hart E, Hansen BE, Lurie Y, et al. (2012) Long-term effects of treatment and response in patients with chronic hepatitis C on quality of life: an international, multicenter, randomized, controlled study. BMC Gastroenterol 12: 11.
- Ware JE, Bayliss MS, Mannocchia M, Davis GL (1999) Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. The Interventional Therapy Group. Hepatology 30: 550-555.
- Carithers RL, Sugano D, Bayliss M (1996) Health assessment for chronic HCV: Results of HQL. Dig Dis Sci 41: 75S-80S.
- Forton DM (2012) Hepatitis C treatment-Clearing the mind. J Hepatol 56: 513-514.
- Senzolo M, Schiff S, D’Aloiso CM, Crivellin C, Cholongitas E, et al. (2011) Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol 17: 3369-3374.
- Bouffard P, Hayashi P, Acevedo R, Levy N, Zeldis JB (1992) Hepatitis C virus is detected in monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis 166: 1276-1282.
- Foster GR, Goldin RD, Thomas HC (1998) Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 27: 209-212.
- Younossi Z, Kallman J, Kincaid J (2007) The effects of HCV infection and management on health-related quality of life. Hepatology 45: 806-816.
- Adinolfi L, Restivo L, Zampino R, Guerrera B, Lonardo A, et al. (2012) Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis 221: 496-502.
- Backus L, Boothroyd D, Phillips B, Belperio P, Halloran J, et al. (2011) A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 9: 509-516.
- Hsu Y, Lin J, Ho H, Kao Y, Huang Y, et al. (2014) Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 59: 1293-1302.
- Kallman J, O’Neil M, Larive B, Boparai N, Calabrese L, et al. (2007) Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci 52: 2531-2539.
- Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N (1999) The impact of diagnosis of hepatitis C virus on quality of life. Hepatology 30: 1299-1301.
- Rasenack J, Zeuzem S, Feinman SV, Heathcote EJ, Manns M, et al. (2003) Peginterferon alpha-2a (40kD) [Pegasys] improves HR-QOL outcomes compared with unmodified interferon alpha-2a [Roferon-A]: In patients with chronic hepatitis C. Pharmacoeconomic 21: 341-319.
- Hassanein T, Cooksley G, Sulkowski M, Smith C, Marinos G, et al. (2004) The impact of peginterferon alfa- 2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol 40: 675-681.
- Feeney ER, Chung RT (2014) Antiviral treatment of hepatitis C. BMJ 348: g3308.
- Terkawi AS, Tsang S, AlKahtani GJ, Al-Mousa SH, Al Musaed S, et al. (2017) Development and validation of Arabic version of the hospital anxiety and depression scale. Saudi J Anaesth 11: S11-S18.
- Lins L, Carvalho FM (2016) SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med 4: 205.
- Campolina AG, Bortoluzzo AB, Ferraz MB, Ciconelli RM (2011) Validação da versão brasileira do questionário genérico de qualidade de vida short-form 6 dimensions (SF-6D Brasil). Cienc Saude Coletiva 16: 3103-3110.
- Häuser W, Holtmann G, Grandt D (2004) Determinants of health-related quality of life in patients with chronic liver diseases. Clin Gastroenterol Hepatol 2: 157-163.
- Panzini RG, Mosqueiro BP, Zimpel RR, Bandeira DR, Rocha NS, et al. (2017) Quality-of-life and spirituality. Int Rev Psychiatry 29: 263-282.
- Barker A (1990) The quality of life: The missing measurement in health care. J R Soc Med 83: 539-540.
- Wilson IB, Cleary PD (1995) Linking clinical variables with health-related quality of life. JAMA 273: 59-65.
- Drossman DA (1996) Gastrointestinal illness and the biopsychosocial model. J Clin Gastroenterol 22: 252–254.
- World Health Organization (2016) Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection: Updated version, April 2016, guidelines. Geneva.
- Osoba D (1999) Guidelines for measuring health-related quality of life in clinical trials. New York: Oxford University Press 19-36.
- Gralnek IM, Hays RD, Kilbourne A, Rosen HR, Keeffe EB, et al. (2000) Development and evaluation of the Liver disease quality of life instrument in persons with advanced, chronic liver disease–the LDQOL 1.0. Am J Gastroenterol 95: 3554-3565.
- Mascia C, Vita S, Zuccala P, Marocco R, Tieghi T, et al. (2017) Changes in inflammatory biomarkers in HCV-infected patients undergoing direct acting antiviral-containing regimens with or without interferon. PLoS One 12: e0179400.
- Dolmazashvili E, Abutidze A, Chkhartishvili N, Karchava M, Sharvadze L, et al. (2017) Regression of liver fibrosis over a 24-week period after completing direct-acting antiviral therapy in patients with chronic hepatitis C receiving care within the national hepatitis C elimination program in Georgia: results of hepatology clinic HEPA experience. Eur J Gastroenterol Hepatol 29: 1223-1230.
- Tada T, Kumada T, Toyoda H, Mizuno K, Sone Y, et al. (2017) Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J Gastroenterol Hepatol 32: 1982-1988.
- Siqueira FM, Ferreira VL, Borba HHL, Pontarolo R (2018) Quality of life of Brazilian chronic hepatitis C patients treated with interferon-free therapies. Rev Inst Med Trop Sao Paulo 60: e72.
- Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, et al. (2005) Impact of hepatitis C on health related quality of life: A systematic review and quantitative assessment. Hepatology 41: 790-800.
- Ferreira VL, Leonart LP, Tonin FS, Borba HH, Pontarolo R (2018) Sustained virological response in special populations with chronic hepatitis C using interferon-free treatments: a systematic review and meta-analysis of observational cohort studies. Clin Drug Investig 38: 389-400.
- Ferreira VL, Tonin FS, Assis Jarek NA, Ramires Y, Pontarolo R (2017) Effcacy of interferon-free therapies for chronic hepatitis C: A systematic review of all randomized clinical trials. Clin Drug Investig 37: 635-646.
- Ferreira VL, Borba HH, Wiens A, Pedroso ML, Radunz VF, et al. (2018) Effectiveness and tolerability of directacting antivirals for chronic hepatitis C patients in a Southern state of Brazil. Braz J Infect Dis 22: 186-192.
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, et al. (2014) ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370: 1973-1982.
- Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, et al. (2014) Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 12: 1349-1359.
- Gerber L, Estep M, Stepanova M, Escheik C, Weinstein A, et al. (2016) Effects of viral eradication with ledipasvir and sofosbuvir, with or without ribavirin, on measures of fatigue in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 14: 156-164.
- Younossi ZM, Stepanova M, Pol S, Bronowicki JP, Carrieri MP, et al. (2016) The impact of ledipasvir/sofosbuvir on patient-reported outcomes in cirrhotic patients with chronic hepatitis C: The SIRIUS study. Liver Int 36: 42-48.
- Younossi ZM, Stepanova M, Sulkowski M, Foster GR, Reau N, et al. (2016) Ribavirin-free regimen with sofosbuvir and velpatasvir is associated with high efficacy and improvement of patient-reported outcomes in patients with genotypes 2 and 3 chronic hepatitis C: Results from astral-2 and -3 clinical trials. Clin Infect Dis 63: 1042-1048.
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, et al. (2014) ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370: 1973-1982.
Citation: Morad WS, Elshimi E, Shebl N, Othman W (2020) Health Related Quality of Life in Patients with HCV Geno-Type 4 and Cirrhosis Receiving Direct Acting Anti-viral Drugs. J Community Med Health Educ 10:680. DOI: 10.4172/2161-0711.1000680
Copyright: © 2020 Morad WS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1567
- [From(publication date): 0-2020 - Dec 03, 2024]
- Breakdown by view type
- HTML page views: 1004
- PDF downloads: 563