Healing the Chronic Wound in Alzheimer's Disease Brain: Early Targeting of Microglia as a Promising Strategy
Received: 17-Mar-2022 / Manuscript No. jcmhe-22-57622 / Editor assigned: 21-Mar-2022 / PreQC No. jcmhe-22-57622 (PQ) / Reviewed: 05-Apr-2022 / QC No. jcmhe-22-57622 / Revised: 17-May-2022 / Manuscript No. jcmhe-22-57622 (R) / Published Date: 25-May-2022
Abstract
Objectives: To outline the underlying pathophysiology and biochemistry in Alzheimer’s Disease (AD) development and progression, and then focus on microglial effect on AD microenvironment with exploitation of microglia as a promising approach to the development of effective therapeutic for AD.
Design: In this review, a search of the literature up to December 2020 in Scopus, Web of Science, Medline, and PubMed were included, using search terms that include Aβ peptide deposition, Aβ42 protofibril, senile plaque, hyperphosphorylated tau, neurofibrillary tangles, degenerated neurons, neurodegeneration, Microgliaphenotype, microenvironments, physiological functions, phagocytosis, neuroinflammation, atrophy, frontal lobe, temporal lobes, proteolytic degradation, Biochemical imbalances, proinflammatory mediators, intracellular molecules, redox signaling molecules, Angiotensin 1 converting enzyme 1, single nucleotide polymorphisms, blood brain barrier, perivascular drainage, Low density lipoprotein receptor related protein, interstitial fluid, P-glycoprotein, cerebral amyloid angiopathy, Ubiquitin-proteasome system.
Result: Success in developing an effective therapeutic approach of AD is limited due to incompleteness of our knowledge on the biochemical and physiological effects of the initial insult inflicted by Aβ peptide deposition and senile plaques in the extracellular space. Moreover, the complexity of AD is increased by the secondary insult caused by the spatiotemporal progression of intraneuronal fibrillary tangles of hyperphosphorylated tau. Taken together, these insults contribute greatly to neurodegeneration and cognitive malfunction.
Conclusion: There is no effective treatment and no known stimulus for effective repair of degenerated neurons, neuroregeneration or prevention of neuronal death. Microglia is the main innate immune cells of the brain and detects changes in the local microenvironments to maintain normal physiological functions. Changes in the microenvironment (e.g., infection, ischaemic injury, Aβ species, Aβ-plaque, tau proteins, proinflammatory mediators), soluble factors released from neurons and astrocytes, intracellular molecules, redox signaling molecules, and metabolic shift-mediated proteins negatively impact microglial clearing processes, such as change in phenotype, morphology, and proliferative responses. An imbalance in microglia phenotypes occurs in AD with AD progression and these results in increased microglia-derived neuroinflammatory activities.
Keywords: Alzheimer’s disease; Protofibril, Ischaemic injury, Proteolytic degradation
Introduction
Alzheimer’s Dementia (AD)
Dementia is a progressive cognitive malfunction, from a previously attained cognitive level, characterised by deterioration in the ability to perform daily activities with psychiatry symptoms [1]. Dementia is preceded by Mild Cognitive Impairment (MCI), where patients can still perform complex activities such as paying bills [2]. MCI does not always lead to dementia. People living with dementia are increasing in number especially in low and middle-income countries [3]. AD, the most common cause of dementia, represents sixty percent 60-80% cases of dementia-AD pathology alone represents half of these cases and the remaining ones are AD combined with vascular dementia/dementia with Lewy bodies [4]. AD consists of 3 stages: preclinical AD, Mild Cognitive Impairment (MCI due to AD), and dementia due to AD. In preclinical AD, there are no symptoms such as memory decline but there are changes in the brain, cerebrospinal fluid and blood biomarkers this is a proposed stage that needs to be validated [5]. MCI due to AD is characterised by changes in Alzheimer’s biomarkers and cognitive decline (deficits in short-term memory and episodic memory followed by progressive impairment in declarative and non-declarative memory) which does not significantly affect daily activities [6]. Dementia due to AD is also called Alzheimer’s dementia or early and late onset AD. Memory, thinking, and behaviours are impaired and this alters intellectual function/daily activities. There are significant changes in AD biomarkers and neuropathology [7]. Alzheimer’s dementia is not a normal part of ageing and older age alone could not cause Alzheimer’s dementia [8]. According to World Health Organisation (WHO), 47 million people live with AD in the whole world [9] and this number will rise one hundred million in the year 2050 [10]. In USA, number of Alzheimer’s dementia patients is five million seven hundred thousand (5.7 million) in the year 2018 [11].
Literature review
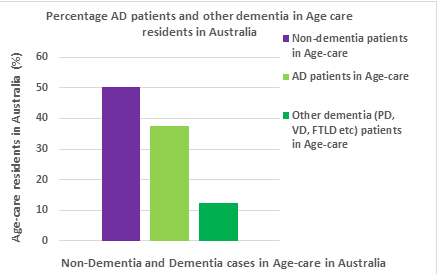
Australia has a growing incidence of AD and this causes a significant burden on families, healthcare, and economy. For instance, in year 2015, dementia patients in aged care represented 49.9% of all aged care recipients. AD patients are up to 75% (AD cases is 37.425 and all other dementia cases is 12.475) of the reported dementia cases (Figure 1) [12] and AD doubles in its prevalence every five years in ages beyond sixty five (65) years [3].
Impacts of AD on patients (personal wellbeing, experiences, and challenges)
AD begins with brain changes that commence years before the appearance of symptoms, continues for years with morbid symptoms, and ends severely when brain changes are so broad that patients struggle to communicate, cannot walk again, and eventually stop eating and breathing [13]. Nerve cells involved in cognitive function, walking, movement, and swallowing are affected. Damage to nerve cells controlling swallowing creates eating and drinking difficulties, which make food particles roll into the trachea instead of the esophagus. Similarly, food may also be deposited in the lungs and thereby causes aspiration pneumonia, a lung infection that causes death in many patients with AD. At the final stages of AD, AD patients are bed-bound and require around the clock care. As a result, they are susceptible to blood clots, skin infections, respiratory system diseases, and sepsis which induce inflammation that results in organ failure. AD progression is slow and insidious and AD patients pass through years of morbidity as the disease progresses.
AD patients experience many symptoms, which depend on the degree of damage inflicted on neuron in different parts of the brain. The rate at which symptoms progresses from mild to severe differs from person to person. At mild stage (MCI), most AD patients perform daily activities independently, but they may require help to perform some activities in order to enhance their independence and become safe. For instance, AD patients may still drive, work, and participates in favourite activities [6]. If MCI co-exists with higher A (i.e. positive: ˃0.64 ng/mL in plasma), AD patients may be in early stage of AD (MCI due to AD). In moderate stage, AD patients may find daily activities difficult, be confused of where they are and thus start wandering, and begin exhibiting change in personality and behaviour such as agitation and suspicion. In severe stage, they need help to perform activities of daily living such as using bathroom, bathing, dressing, and eventually have reduced ability to communicate verbally. AD is the greatest cause of disability and morbidity in USA. In Australia, AD is the overall cause of disability burden and greatest cause of disability in older Australians (65 years and above) [14].
Impact of AD on caregivers
AD symptoms affect the personality and behaviour of AD patients and this disrupts their family relationships, with the greatest challenges experienced by the family caregivers [15]. Family caregivers endure neuropsychiatry symptoms such as anxiety/apathy and grievous behavioural abnormalities of their patients. The more the symptoms worsen, the more the emotional stress, depression, income reduction (e.g. problems in employment, health bills and services for themselves and their care patients), and/or exacerbated health problems (such as physical problems and sleep) of the family caregivers [4]. About thirty to forty percent (30%-40%) family caregivers develop depression and anxiety compared to non-caregiver of similar ages [16]. Due to kinship, the risk of depression increases in family caregivers as cognitive impairment worsens in the care recipients [17]. For instance, family caregivers of AD patients have two and half higher times risk of having depression than informal caregivers of people of AD patients and are prone to chronic stress, which negatively impacts their physiological processes in cardiovascular and renal systems [18]. Caregivers (family and informal) undergo greater subjective cognitive decline compared to non-caregivers of the same age [19]. Caregivers that experience higher stress owing to care responsibilities are at higher risk of death compared to caregivers with little or no stress [20]. However, seventy two percent (72%) of family caregivers are relieved when AD patient dies and caring for people with AD or other dementia is more rewarding.
Genetic variance and its impact in AD pathogenicity
AD can be defined as early onset (EO-AD; 1-5% AD; <65 years) and late onset (LO-AD; 95%AD; >65 years) forms. EO-AD consists of a familiar AD (FAD). To date, FAD has been linked with mutation in three genes, specifically, those that encode Presenilin 1 and 2 (PS1 and PS2) and APP (Val717lle). PS1 and PS2 are the catalytic subunits of γ-secretase which is located in Endoplasmic Reticulum (ER), ER-Golgi compartments, Golgi, Trans-Golgi-Network (TGN), endosomes and cell membranes (including neuronal with astrocytic membranes). Contextually, γ-secretase is involved in proteolytic APP processing. FAD-linked presenilin and APP mutations have been demonstrated to increase lysosomal pathology in both post-mortem human brain tissue and transgenic mouse models and in A formation.
Intracellular Aβ accumulation has been proven as an early event in the pathogenesis of AD. Both EO-AD and LO-AD have been linked with genetic mutations of apolipoprotein E (apoE) which is produced in the liver, macrophages, and astrocytes. ApoE regulates: the transport of cholesterol and other lipid between neuronal cells and glial cells; Ab aggregates; Ab clearance, and neuroinflammation. ApoE is made up of 299 amino acids and its polymorphic alleles, apoE2 (ε2, 8.4%), apoE3 (ε3, 77.9%), and apoE4 (ε4, 13.%) have amino acids 112 and 115, which can be cysteine or arginine, respectively (ε2 (Cys112, Cys115) ε3 (Cys 112, Arg158) and ε4 (Arg112, Arg158)). The differences in these amino acids affects the structure of ApoE and its binding to Aβ. The ε2 allele reduces risk to develop AD and ε4 is the strongest risk factor of LO-AD. The ε3 allele has been associated with mitigation of the loss of synaptic function induced by Aβ. The ε4 allele is also associated with age-related (middle aged, (40-59 years) elderly (60-85 years)) memory decline and this is related with severity of neuronal pathology. Therefore, apoE4 is a key target for research into both the diagnosis and therapeutic intervention of AD.
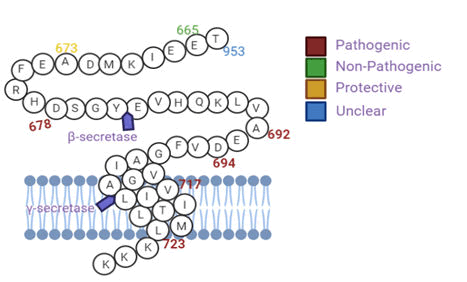
Known variants of the genes (e.g. PS1/2, APP, and ApoE) can be pathogenic while others elicit protective or risk modification. For example, APPA673T provides less APP for β-secretase cleavage and this reduces the formation of amyloidogenic Aβ peptides (40%) minimising Aβ deposition (Figure 2).
Results
This results in a protective effect against Aβ pathology, but has been observed within people of Caucasian dissent. The mutation of APPL723P elevates secretion of amyloidogenic Aβ peptides which is pathogenic in those of African descent.
Figure 2: APP variants with their respective form of pathogenicity within populations mutations (a) within and around Aβ are APP variants such as those at the N-terminus of Aβ (KM670/671NL (Swedish)), which provide more substrates (APP) for β-secretase, resulting in increased total Aβ (Ab1-40 and Ab1-42) production (b) beyond the C-terminus of Aβ are APP variants such as L723P (Australian), which also provides more APP for β-secretase and thereby results in increase production of Ab1-42, the main neurotoxic Ab species, and (c) in the mid-region are APP variants such as Dutch, which alters Aβ structure and thus enhances Aβ aggregation. Intracellular Aβ mutations can result in mixed amyloid pathologies (cerebral angiopathy and amyloid plaque).
The chances of developing AD is increased by risk factors such as age, family history, apolipoprotein E (apoε4) and triggering receptor expressed on myeloid cells 2 (TREM2) stress, poor dietary intake, and transient emotional state. Out of these risk factors, age, family history, apoε4 and TREM2 are thought to pose the highest risk likelihood for AD development.
Abnormal APP and p-tau protein dysregulate cellular physiological processes in AD brain microenvironment
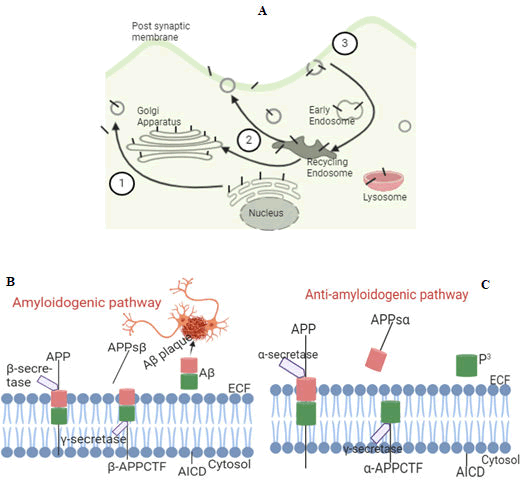
APP is an integral protein. APP is synthesised in ER, transported via the golgi to the Trans-Golgi Network (TGN) and from TGN via TGN derived secretory vesicles to the cell surface. During intracellular transport, APP undergoes post-translational modification by N and O-glycosylation, ectodomain/cytoplasmic phosphorylation, and tyrosine sulfation. When present at the cell surface, APP can be re-internalised by endocytosis with an only 0.1% recycled to the cell surface. This fraction (0.1%) facilitates APP trafficking via b-secretase and protein kinase C activities (Figure 3A). In endocytic compartments and recycling steps, b-secretase cleaves a soluble large portion of ectodomain of APP (sAPPβ) from cell and retains membrane bound-C-terminal fragments (βAPPCTF). γ-secretase cleaves βAPPCTF within the hydrophobic regions of the cell membrane and liberates Aβ peptides between 38-43 residues into the extracellular fluid (ECF: plasma, CSF, and ISF), a process called amyloidogenic (abnormal) pathway (Figure 3B). Liberated Aβ peptides slowly build up within ECF to form amyloid plaques. Aβ is normally degraded in the lysosomes or the ECF via vesicular trafficking. In non/anti-amyloidogenic (normal) pathway which usually commences at cell surface, b-secretase cleaves APP in the middle of Aβ (removing sAPPb (APPsb) from cell) and this generates truncated bAPPCTF which lacks amino terminal portion of Ab domain. γ-secretase cleaves bAPPCTF and this leads liberation of truncated Aβ (p3) which is pathologically irrelevant (Figure 3C).
Figure 3: Intracellular trafficking of APP (A) and Proteolytic processing of APP (B and C). In figure A, developing APP (black bars) becomes mature via the constitutive secretory pathway (step 1). When APP reaches the cell surface, it is internalised rapidly (step 2) and it then traffics via endocytic and recycling compartments back to the cell surface (step 3) or degraded in the lysosome. The N-terminus of APP lies within the extracellular fluid while the C terminus lies within the cytosol, a clear liquid portion of the cytoplasm (Figures 3B-C). APP proteolytic processing consists of 2 major pathways [amyloidogenic (abnormal), which generates Aβ and anti/non-amyloidogenic (normal), which prevents Aβ generation]. In amyloidogenic pathway, β-secretase cleaves a soluble large portion of ectodomain of APP (sAPPβ) from cells and retains membrane bound C-terminal fragments (βAPPCTF). γ-secretase cleaves βAPPCTF within the hydrophobic regions of the cell membrane and liberates Aβ peptides between 38-43 residues into the ECF (plasma and CSF) where it slowly builds up to form amyloid plaques (Figure 3B). In non-amyloidogenic pathway, b-secretase cleaves APP in the middle of Aβ (removing sAPPb from cells) and this generates truncatedbAPPCTF which lacks amino terminal portion of Aβ domain. γ-secretase cleaves bAPPCTF and this leads liberation of truncated Aβ (p3) which is pathologically irrelevant (Figure 3C).
APP plays important roles in cellular physiological processes during both development and adulthood. APP can bind to cell surface proteins and aids in the migration of neurons during early development and neurogenesis before and after birth. APP exerts neurotrophic properties that link with neurogenesis, synaptogenesis, and synaptic plasticity. The type and level of APP expression is influenced by cell steepness and the pregnancy related hormone human chorionic gonadotrophin, particularly during early stages of human embryogenesis. Non-amyloidogenic APP soluble fragment (sAPPb) and amyloidogenic APP soluble fragment (sAPPb) are strong drivers of neuronal differentiation; however, sAPPb is more potent at inducing hESCs neural differentiation. Neural stem cells are generated from the subependymal zone of the lateral ventricles and from the hippocampus. The hippocampus of the adult brain undergoes continuous neurogenesis and is the brain region most affected by AD. Alterations in AD APP processing negatively impacts the levels of sAPPb, sAPPb, Aβ and potentially results in aberrant neurogenesis. Increased APP processing in AD brains is a mechanism for inducing neural stem cell proliferation and repopulating neurons in and around the hippocampus. Aβ regulates synaptogenesis, axonal guidance and development, oxidative stress, synaptic plasticity, learning and memory, induces immune related transcriptional factors (NF-kB) produces regulator of inflammation, and exhibits a potent antimicrobial activity. Aβ1-42 has a significant role in hESCs proliferation but oligomeric Aβ decreases hESC proliferation.
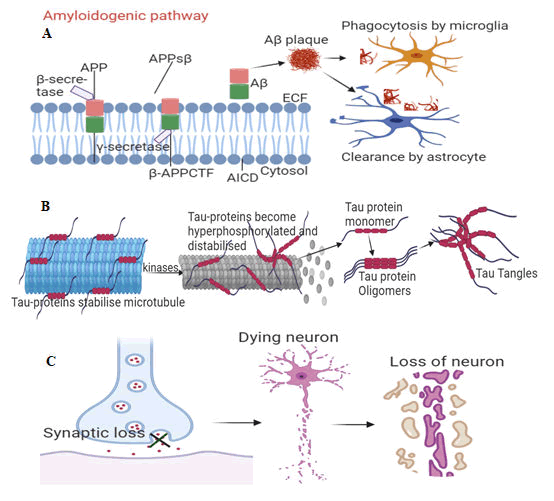
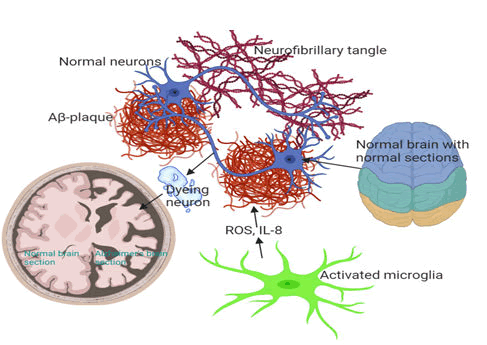
Abnormal cleavage of APP by b and γ-secretases (usually occurs at 40 years of age) causes formation of Ab which aggregates into Ab plaques, resulting in the activation of microglia and astrocytes, which clear the toxic proteins (Ab plaques) and debris of dead and dying cells Figure 4A. Ab plaques induces hyperphosphorylation of Tau protein (p-tau). This leads to formation of Tau tangles (t-tau) 10 years after initiation of Ab plaques (Figure 4B). Aβ plaques and tau tangle form a b-sheeted plate, which ultimately creates NTFs and a downstream effect on synaptic and neuronal loss 20 years after initiation of Ab plaques (Figure 4C).
Figure 4A-C: The pathogenic hallmarks of AD in human brain in relation with age related severity. (4A) Abnormal cleavage of APP by b and γ-secretases causes formation of Ab which aggregates into Ab plaques, resulting in the activation of microglia and astrocytes for Abclearance. (4B) Aβ plaques also induce hyperphosphorylation and distabilisation of Tau protein and leads to formation of Tau tangles 10 years after initiation of Aβ plaques. (4C) Aβ plaques and tau tangle form a β sheeted plate which ultimately creates neutritic fibrillatory tangles (NTFs) with a downstream effect on synaptic and neuronal loss 20 years after initiation of Aβ plaques.
Chronic inflammation sets in when microglia and astrocytes could not keep up with amounts of toxic proteins and debris of dead and dying cells to be cleared. This is followed by symptomatic cognitive decline. Accumulation of Aβ and p-tau can occur independent of each other. Tau is a microtubule associated protein controlled by phosphorylation. Tau stabilises microtubules with axons and supports cell functions. In AD brain, tau accumulates higher phosphates (3 times) than healthy brain (2-3 mol of phosphate). This lowers Tau’s affinity for microtubules, reduces its degradation by proteases, and leads to its fibrillisation and accumulation into neurofibrillary tangles, NFTs. NFTs clump together within the cytoplasm of neurons to form Senile Plaques (SP). The SP and the surrounding microenvironment of AD brain exhibit high expression of different Extracellular Matrix (ECM) molecules including the proteoglycans.
NFTs are insoluble and are pathological hallmark of AD. NFTs levels correlate with the severity of cognitive dysfunction in humans. However, NFT may not be the primary pathogenic form of Tau. Soluble forms of tau (such as tau monomer and tau oligomer) with aberrant PTMs mediate neurotoxicity. The most prevalent PTM on Tau is phosphorylation. Aberrant alteration of precise residues on tau plays a key role in Tau toxicity. Soluble Tau species decreases cognitive and memory function. It has been demonstrated that caspase-2 (Casp2) cleaves tau at aspartate 314. This leads to the formation of tau314 proteins, which induce synaptic dysfunction and loss of memory in a mouse model. This facilitates neurodegeneration and the precise cellular mechanisms are unknown. Mitochondria dysfunction is a feature of AD and other form of neurodegenerative dementia. Evidence has shown that pathological tau species may negatively impact mitochondria (via disproportions in mitochondria dynamics, impaired oxidative phosphorylation, and higher level of reactive oxygen species) and thereby compromise neuronal function.
In neuropathology, AD is characterised by accumulation of hyperphosphorylated tau (p-tau) protein and Ab plague, loss of synapse, oxidative stress, chronic inflammation, and altered cholesterol (Figure 5). This results in loss of brain weight and volume in the brain of AD patients.
Figure 5: Typical loss of brain weight and volume in the brain of AD patients. In AD, accumulation of amyloid beta and tau leads to death of neurons or neurodegeneration. Brain of AD patients shows severe tissue shrinkage compared brains of non-AD patients of the same age. The most common problem of AD patients early in their disease state is cognitive malfunction (memory loss), mostly induced by activated microglia neuroinflammatory/neurodegeneration cascades. Clinically, this memory loss is a consequence of where the most severe neurodegeneration is taking place in the brain.
Aβ aggregates and NFT dysregulate LTP and synaptic plasticity: Pathophysiology of Alzheimer’s dementia
In AD, Aβ aggregates decrease Ca2+ in ER and thereby increases cytosolic Ca2+. This reduces quantity of CaMKII in cytosol, which negatively impacts CaMKII intracellular signal transduction such as binding of CaMKII with GluA1/GluR1; decreases distribution of CaMKII in the synapse and cytosol and; decreases distribution of alpha Amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptors, AMPARs (key regulator of synaptic function and cognition) at the synapse. Moreover, Aβ binds to postsynaptic proteins such as PSD-95 and this to leads to reduced concentration of PSD-95, AMPAR, and synaptophysin in the synapse this destroys NR2A function. Within the intracellular fluid, Aβ oligomers triggers overexpression of Arc gene (which is involved in memory function) and this brings about loss of NMDA receptors with cell morphology. In addition, cytoskeletal proteins such as tau, tubulin, and MAP2 are substrates for CaMKII. β CaMKII is hyperactive due to its autophosphorylation and high expression in CA1 neurons. Inside the cells, β CaMKII phosphorylates tau protein and causes hyperphosphorylated tau aggregates, which dimerises to paired helical filaments (PHF) that accumulate as NFT. However, the relationship between β CaMKII and Aβ production is unclear. D-amino acids are involved in AD cognitive decline but their relationship with NMDA receptors is not elucidated. Aβ could alter expression and function of integrin in AD brain microenvironment. Aβ peptides interaction with integrins leads to inhibition of LTP, mitochondria and synaptic dysfunction and gliosis. In AD animal model, it has been demonstrated that Aβ oligomers, integrin β1, and CaMKII determine spine changes in hippocampal neurons.
Clinically, loss of synapse (induced by Aβ aggregation, inflammation, p-tau, inhibition of LTP) is a hallmark of the pathophysiology of AD, following this are neurodegeneration and beginning of AD symptoms. AD pathophysiology is marked by chronic and progressive neurodegeneration such as synaptotoxicity, loss of neurophil, neurotransmitters dysregulations, extracellular amyloid/senile plaques, intracellular NFTs, gliosis, and blatant neuronal loss with degeneration. Principally, the hippocampus and the cortex are negatively affected at the earlier stage and this is connected with cognitive/memory decline as AD progresses, approximately (80%) of neurons in the hippocampus die and these results in cognitive decline and little or no ability to carry out daily activities. Extracellular amyloid/senile plaques around the neurons and glia are insoluble and can continuously fill all available space (quasi-crystalline). The main part of this plaque is Aβ which is known to drive AD development. However, Aβ oligomers (soluble) have been reported to be principally accountable for neurodegeneration, synaptic dysfunction, tauhyperphosphorylation, and imbalance in glutamate neurotransmission in AD.
Long-term potentiation and synaptic plasticity: molecular mechanisms of memory formation
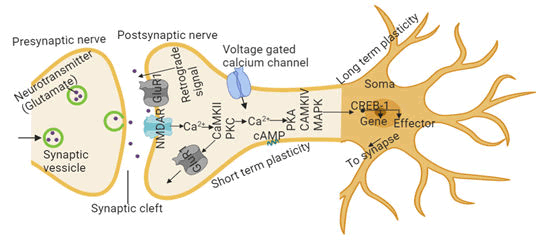
The neuronal synapse is the region most impacted by AD pathological proteins, Aβ and Tau. Synapses are key neural regions that permit the creation, storage, and access of memories. Memory is the ability to encode, store, and recall information to affect or influence behaviour. Learning and memory occurs if the synaptic strength is enhanced after consecutive and frequent excitation of the presynaptic and post synaptic neurons. Processes involved in synaptic strengthening (neurotransmitter-receptor activation) are called Long-Term Potentiation (LTP) which causes information storage and resulting in learning and memory. Synaptic plasticity is a short-term and long-term dynamic change in synaptic connections, which enhances LTP by increasing synaptic connections or results in a new pathway and memory forming via development of new synapses. During the induction of short-term plasticity, neurotransmitter (such as glutamate) binds on N-Methyl-D-Aspartate Receptors (NMDAR) which triggers Ca2+ influx, leading to the activation of protein kinases like calcium calmodulin-dependent kinase II (CaMKII), Protein Kinase C (PKC), and recruitment of new glutamate receptors to the synapse (Figure 6). Memory consists of three main classifications, including sensory, short-term, and long-term forms. In short-term (working) memory, neural network maintains task relevant information for a short-term (seconds to minutes). This occurs in pre-frontal cortex, posterior parietal cortex, cerebellum. In healthy cohorts, short term memory is impacted by ApoE4, bridging integrator 1 (BIN1, second most risk factor for AD and modulator of Tau pathology) and synaptosomal-associated protein, 25 (SNAP25).
During short-term processes in both declarative (facts and events) and procedural (skills and tasks) memories, proteins present at the synaptic connections undergo covalent modification, which modulates strength of that connection. Protein kinase A (PKA), Mitogen-Activated Protein Kinase (MAPK), cAMP responsive element binding proteins 1 and 2 (CREB-1 and CREB-2) convert short-term memory to long-term memory. PKA, CAMKII, and MAPK activate CREB-1, which causes downstream genes regulation, leading to the formation of long-term plasticity (Figure 6).
Ca2+ signaling to protein kinases and the recruitment of new glutamate receptors to the synapse. Long-term plasticity (lasting days) requires CREB-dependent gene activation in the nucleus by the action of multiple protein kinases. Long-term plasticity and memory also require synthesis of a constitutively PKC.
Episodic memory enables long-term storage and conscious recollection of past events. Episodic memory occurs in the hippocampus and depends on the medial temporal lobe and neocortex. Impairment in episodic memory is the initial clinical sign of AD and this is due to early hippocampal dysfunction. It is associated with ApoE4 and E2. Memory decline in AD are due to neurodegeneration, which results in abnormal physiological changes in synapse.
SWATH-MS as an emergent strategy in AD diagnostic discovery and development
AD begins slowly and pathologically from preclinical/early phases into a full clinical syndrome. A biomarker is an indicator of a specific biological phase such as a normal physiological process or pathologic process associated with a disease state. Biomarkers are used in clinical practice to predict, diagnose, and monitor diseases. A unique biomarker for AD is expected to accurately indicate the presence and/or progression of AD to provide a better prognosis for early diagnosis. Several studies have reported existing and potential AD biomarkers. In clinical diagnostics and drug development of AD, proteomics is an indispensable approach, especially in the identification of novel diagnostic and therapeutic biomarkers. Aspects that contribute to the fundamental problems in AD treatment are inaccurate interpretation of pathological results,lack of definitive early diagnostic AD biomarkers (identifying early/preclinical systematic changes in AD brain), and viable treatments (to prevent/slow/reverse these changes). To achieve these, data generated must be reproducible, complete, and accurate. Sequential Window Acquisition of All Theoretical Mass Spectra (SWATH) Mass Spectrometry (MS) is an emerging strategy in resolving AD data reproducibility, completeness, and accuracy. SWATH-MS comprises a high specificity Data Independent Acquisition (DIA) with a targeted data extraction strategy on a high resolution MS instrument in essence; this approach is a combination of discovery and targeted methods together at the same time. In DIA mode, all ionised compounds of a given sample that fall within a specified mass range are fragmented in systematic and unbiased fashion, using a number of consecutive, slightly overlapping precursor isolation windows to provide comprehensive coverage.
Discussion
Animal models are crucial in understanding the aetiology, pathological/pathophysiology mechanisms, designing, and validating diagnostic/therapeutic approaches of AD. Many failures and standstills in AD clinical trials are due to limited understanding of AD and the strength of the current animal models with their respective methods of generation. A good AD model should exhibit synaptic and neuronal degeneration with key phenotypes or histopathological features of AD (i.e. amyloid plaques and neurofilament tangles) at a cellular level these changes (i.e. biochemical and histopathological) should begin at preferred sites, affect a number of brain regions in a stereotypical manner as the disease progresses, and result in a progressive alteration of behavioural functions. Similarly, key molecular pathways that are inhibited or altered in human AD should be dysregulated in animal model. The model should exhibit morphological and structural abnormalities such as atrophy of human AD brain. However, many animal (rodent) models do not exhibit significant neuronal/synaptic loss and there is elusive degeneration these cause potential problem in therapeutic intervention. Moreover, different brain regions develop AD pathology at different times in AD processes and this adds a spatial element (to AD), which is not captured in AD cell culture models and frequently overlooked in AD human studies. Pathological results are at times inaccurately interpreted, for examples, discovery of hyperphosphorylated tau is not an evidence of neurofibrillary tangle (NFT) formation and immunoreactivity with anti-Aβ antibody is not an evidence of plaque pathology.
Conclusion
Some of these memory-related AD biomarkers are reported in post-mortems and aged animals’ studies. Besides, knowledge surrounding the severity and nature of these dysfunctions is limited. The distributions of unphosphorylated CaMKII is unknown. How CaMKII (at CaMKII sites T654/S665) phosphorylates APP and the overall effects on APP is unclear. Similarly, the relationship between glycine/D-amino acids (D-serine, D-glutamate, D-alanine) and function of NMDA receptors in AD progression is highly essential. Moreover, most of the time, the concentration of Aβ used in some studies do not produce negative effect on neuronal function.
References
- Lin YT, Wu PH, Kuo MC, ChenCS, Chiu YW, et al. (2015) Comparison of dementia risk between end stage renal disease patients with hemodialysis and peritoneal dialysis-a population based study. Sci Rep 5: 8224.
- Davy V, Brendon S, Elvira L, Mathieu V (2019) Correlates of sedentary behaviour in middle-aged and old age people with mild cognitive impairment: a multinational study. Intl Psychogeriatr 31:579-589.
- Prince MJ, Guerchet M, Prina M (2013)The Global Impact of Dementia 2013-2050: Policy Brief for Heads of Government: Doctoral dissertation, London. Alzheimer's Disease International.
- Alzheimer's Association (2018) 2018 Alzheimer's disease facts and figures. Alzheimer's Dementia 14:367-429.
- VillemagneVL, Burnham S, BourgeatP, Brown B, EllisK, et al. (2013) Amyloid β deposition, neurodegeneration, cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 12:357-367.
- RobertsR, KnopmanDS (2013) Classification and epidemiology of MCI. Clinics in geriatric medicine, 29:753-772
- Robinson RA, Amin B, Guest PC (2017) Multiplexing biomarker methods proteomics and considerations for Alzheimer’s disease. Adv Exp Med Biol 974: 21-48:Springer.
- DolginE (2016) How to defeat dementia. Nature News 539:156-158.
- Wilcox KC, Lacor PN, Pitt J, Klein WL(2011). Aβ oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol Neurobiol 31:939-948.
[Crossref] [Google Scholar] [Indexed]
- HebertLE, WeuveJ, ScherrPA, EvansDA (2013) Alzheimer disease in the United States (2010–2050)estimated using the 2010 census. Neurology 80:1778-1783.
- Söderqvist S, McNab F, Peyrard Janvid M, Matsson H, Humphreys K, et al. (2010)The SNAP25 gene is linked to working memory capacity and maturation of the posterior cingulate cortex during childhood. J Biological psychiatry 68:1120-1125.
- AlbertMS, DeKoskyST, DicksonD, DuboisB, FeldmanHH(2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia 7:270-279.
- Baborie A, Griffiths TD, Jaros E, McKeith IG, Burn D(2011)Australian Institute of Health(2012)Dementia in Australia: AIHW. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta neuropathologica, 121:365-371
- Vaingankar JA, Chong SA, Abdin E, PiccoL, ShafieS et al. (2016) Psychiatric morbidity and its correlates among informal caregivers of older adults. Comprehensive psychiatry 68:178-185.
- Mausbach BT, Chattillion EA, Roepke SK, PattersonTL, Grant I(2013) A comparison of psychosocial outcomes in elderly Alzheimer caregivers noncaregivers. Am J Geriatric Psychiatr 21:5-13.
- SallimAB, Sayampanathan AA, CuttilanA, Ho RCM(2015)Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Directors Association,16:1034-1041.
- Gouin JP, GlaserR, MalarkeyWB, BeversdorfD, Kiecolt-Glaser J(2012) Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology 31:264.
- Dassel KB, Carr DC, Vitaliano P(2017) Does caring for a spouse with dementia accelerate cognitive decline? Findings from the Health and Retirement Study. The Gerontologist 57:319-328.
- Perkins M, Howard VJ, Wadley VG, Crowe M., Safford M et al.(2012) Caregiving strain and all-cause mortality: evidence from the REGARDS study. Journals of Gerontology Series B: Psychol Sci Soc Sci 68:504-512.
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K, Neurology E (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960-969.
[Crossref] [Google Scholar] [Indexed]
Citation: Akeem GO, Francesca F, Lyn RG, Daniel AB (2022) Healing the Chronic Wound in Alzheimer’s Disease Brain: Early Targeting of Microglia as a Promising Strategy. J Comm Med Health Educ 10:753.
Copyright: © 2022 Owoola AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2266
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1829
- PDF downloads: 437