Editorial Open Access
hCG, Hyperglycosylated hCG, Pituitary hCG, Cancer hCG and Fetal hCG
| Laurence Cole* | |
| USA hCG Reference Service, 34 Broadmoor Way, Angel Fire, USA | |
| Corresponding Author : | Laurence Cole USA hCG Reference Service 34 Broadmoor Way Angel Fire, USA Tel: 575-377-1330 E-mail: Larry@hcglab.com |
| Received: January 19, 2016; Accepted: February 13, 2016; Published: February 20, 2016 | |
| Citation: Cole L (2016) hCG, Hyperglycosylated hCG, Pituitary hCG, Cancer hCG and Fetal hCG. J Preg Child Health 3:222. doi:10.4172/2376-127X.222 | |
| Copyright: © 2016 Cole L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Human chorionic gonadotropin (hCG) is a one-of-a-kind biochemical. The amino acid sequence of the α- and ß-subunits codes for six semi-independent molecules, binding two separate receptors, the lutropin (LH)/hCG hormone and the transforming growth factor-ß (TGFß) autocrine receptor. The six semi-independent molecules each vary by carbohydrate structure, merism and by the presence of mutations, and are produced by six different cells, or have six separate functions. This makes hCG the only molecule of its kind. Here we for the first time examine the structures, production and occurrence of the hCG six semi-independent molecules. The hormone hCG (hCG-1) structurally has 4 biantennary N-linked and 4 type one O-linked sugar side chains. It is made by fused placental syncytiotrophoblast cells and acts on an LH/hCG receptor, promoting hemochorial placentation during pregnancy, suppressing macrophage phagocytosis of fetal and placental tissues, and suppressing contractions during pregnancy. The molecule hyperglycosylated hCG (hCG-2) structurally has 4 biantennary N-linked and four type two O-linked sugar side chains. It is made by root cytotrophoblast cells but acts as an antagonist on an autocrine TGFß receptor on placental cells. It controls implantation and placental growth during pregnancy. The molecule previously called pituitary hCG (hCG-3) structurally is a variant of hCG-1 with sulfated N- and O-linked oligosaccharides. This is produced by pituitary gonadotrope cells and act on an LH/hCG receptor parallel to LH, promoting steroidogenesis, ovulation, oocyte growth and luteogenesis. hCG-4 is a variant of hCG with triantennary N-linked oligosaccharides and type two O-linked oligosaccharides. hCG-4 dimer is produced by trophoblastic cancers and hCG-4 free ß-subunit is produced by all other cancers. It is an autocrine that antagonizes a TGFß receptor promoting cancer cell growth and invasion. hCG-5 free ß-subunit is a variant of hCG-1 that is highly mutated. It is produced by the fetal liver and kidney during pregnancy, and promotes growth and development of fetal organs.
| Keywords |
| hCG; ß-Subunit Amino acid; Pregnancy; Blastocyst |
| hCG The Multi-Molecule |
| Human chorionic gonadotropin (hCG) is quite unlike any other human molecule, having a biochemistry unique to just hCG, it exists as six semi-independent structurally distinct forms, each differing primarily in carbohydrate side structure and merism, and each sharing a common α- and ß-subunit amino acid structure (Table 1). They each vary in secretion cells and receptors for action. |
| There is the hormone hCG with biantennary N-linked sugar side chains and type 1 O-linked sugar structures (Table 1). hCG, called here hCG-1, is produced by fused syncytiotrophoblast cells of the placenta during pregnancy. It is a hormone acting on a common lutropin (LH)/hCG receptor found on cytotrophoblast cells, uterine epithelial, stromal, myometrial and vascular cells, and on circulating macrophage cells. The primary function of hCG-1 is to promote hemochorial placentation, an independent fetal circulation during pregnancy [1-4], to block uterine muscular contractions during pregnancy [5,6], and to prevent macrophage rejection of foreign fetal and placental tissues during pregnancy [7,8]. |
| The molecule hyperglycosylated hCG, called here hCG-2, is also made during pregnancy by root cytotrophoblast cells (Table 1). This has biantennary N-linked oligosaccharides and type 2 O-linked sugar structures [9,10]. As demonstrated by Butler (unpublished observation), this molecules α- and ß-subunit fold differently to hCG-1 making an independent molecule [11]. hCG-2 is an autocrine, not a hormone, functioning by antagonizing a transforming growth factor (TGFß) receptor [12,13]. The function of hCG-2 is to control the invasion of12 uterus by the blastocyst or implantation of pregnancy [14-16]. hCG- 2 also acts during pregnancy to control placental tissue growth, by promoting expansion of root cytotrophoblast cells [14-16]. |
| In evolution, hCG-1 and hCG-2 evolved from a deletion mutation in the LH ß-subunit in the lower simian primate. hCG-1 and hCG-2 shared a common α-subunit with LH. hCG evolved as a pituitary hormone to supplement LH, even though it has applications beyond the pituitary. The human pituitary produces a form of hCG aside LH, at approximately one hunredth the concentration of LH [17]. The hCG is approximately 100X more potent than LH, so that the concentration and potency balance each other out and biological activity of this hCG form approximately equals that of LH, binding a common LH/hCG receptor. It is correct to say the hCG and LH share equally actions in women, promoting steroidogenesis, promoting follicle growth, promoting ovulation and luteogenesis, and in men, promotion sperm production and testosterone synthesis [17]. The pituitary gonadotrope cell hCG is structurally different to hCG-1. Pituitary hCG, or the molecule called here hCG-3 (Table 1), is uniquely sulfated on the oligosaccharides. It has biantennary N-linked oligosaccharides and type-1 O-linked oligosaccharides terminating in sulfate groups rather than sialic acid groups. |
| hCG forms also have a major role in carcinogenesis, driving cancer invasion and growth or malignancy [18-20]. It appears that cancers steal the invasion and growth functions of hCG-2 in pregnancy. Cancers use a superglycosylated form of hCG-2 to drive malignancies, this we call here hCG-4 (Table 1). Trophoblastic cancers (choriocarcinoma, testicular germ cell malignancies and ovarian germ cell malignancies) use hCG-4 α-/ß-subunit dimer with triantennary N-linked sugars and type-2 O-linked oligosaccharides to drive malignancies. This antagonizes a cancer cell TGFß receptor to promote cancer functions [12]. Non-trophoblastic malignancies (all other malignancies) do not make hCG dimer. α- and ß-subunit do not combine outside of placental and pituitary cells, so they produce and hCG-4 free ß-subunit (Table 1). This contains the common TGFß sequences and similarly antagonizes the cancer cell TGFß receptor to promote cancer cell functions [12]. |
| The least is known about form of hCG is fetal hCG, the final form of hCG we call hCG-5 free ß-subunit (Table 1), produced by fetal kidney and fetal liver cells. A genetic disorder “Familial hCG Syndrome” is known of in which background fetal hCG is seeming produced through adult life [21,22]. We do know is that what we see in Familial hCG Syndrome cases is a free ß-subunit made by these non-endocrine conditions. This molecule is highly mutated yielding contradictory hCG immunoassay results in differing cases. A recent case gave the hCG concentration as 32 mIU/ml using the Siemens Immulite hCG assay, 17 mIU/ml using the Abbott AxSym hCG assay, 14 mIU/ml using the Siemens Dimension hCG assay, and 7.0 mIU/ml using the Siemens Centaur assay. The 4 fold difference being due to mutations in the hCG free ß-subunit structure. Old studies with the human fetus indicate that this fetal molecule promotes fetal organ growth and development [23-25]. With the limitations on fetal research we know little about the structure of hCG-5, whether it has type 1 or type 2 O-linked sugar structures, or whether it acts through an LH/hCG receptor, a TGFß receptor or other hCG receptor. |
| This is 6 independent hCG forms (hCG-1 – hCG-5), each have separate function and seemingly binding separate receptors (Table 1). All molecules made using the same hCG α- and ß-subunit peptide sequences. Research shows that hCG has one α-subunit gene and as many as six ß-subunit genes in humans [26]. Recent studies indicate that cancer cell may express ß-subunit genes 1 and 2, while other cells seeming express the other four ß-subunit genes [27,28]. While amino acid sequence studies have not been completed on cancer cell hCG, no evidence suggests differences in peptide structure [9]. |
| hCG-1 – hCG-5 Structure |
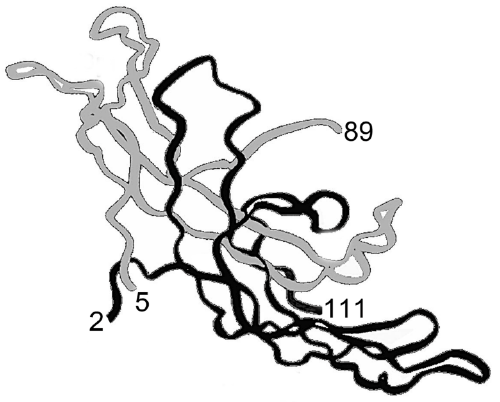
| It is just a tiny carbohydrate variation; type 2 verses type 1 O-linked sugar structures, that permits hCG-2, hCG-4 and hCG-4 free ß-subunit,and no other forms of hCG (hCG-1, hCG-3 and hCG-5) to bind a TGFß autocrine receptor. How does this change the three dimensional structure of hCG. Three separate research groups investigated the hCG three dimensional structure using X-ray crystallography methods. They each found that all 8 sugar structures had to be removed from hCG before a crystal could be made [29-31]. They also found the ß-subunit C-terminal peptide, residues ß112-145 had to be removed along with amino acids from the N-terminus of α- and ß-subunit. |
| The final crystals were made by the three groups of investigators from a dimeric molecule comprising α-subunit residues 5-89 and ß-subunit residues 2-111 and missing all 8 sugar chains. This was just 50% or half of a 92 and 145 amino acid residue molecule in which the 8 sugar side chains, 30% of the molecular weight, were also removed. The question is, was this root molecule hCG-1, hCG-2, hCG-3, hCG-4 or hCG-5? It was none of them specifically, but rather a root molecule representing them all. The structure found by the three groups is shown in Figure 1. We can tell nothing from this route structure about what differentiates hCG-1, hCG-2, hCG-3, hCG-4 or hCG-5. |
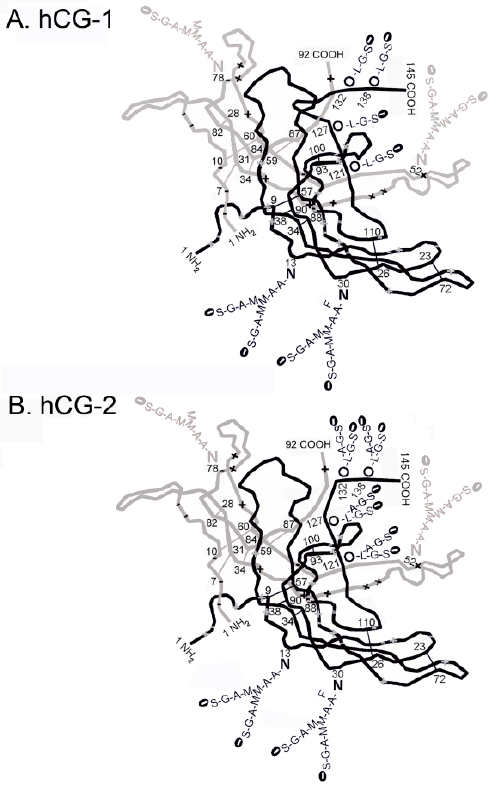
| Stephen Butler PhD in London needed to know what whole hCG-1 and hCG-2 looked like. They used specialized computer programs, Raptor X software, to thermodynamically model this X-ray crystallography core model, and to model the three dimensional structures of hCG-1 and hCG-2 with the missing carbohydrate structures and peptide sequences returned to the molecules (Butler SA, unpublished data). The final computer-modelled structures of hCG-1 and hCG-2 are shown in Figure 2. These computer modelled structures indicate clear difference between hCG-1 and hCG-2, possibly indicating how hCG-2, hCG-4 and hCG-4 free ß-subunit molecules bind a TGFß receptor. |
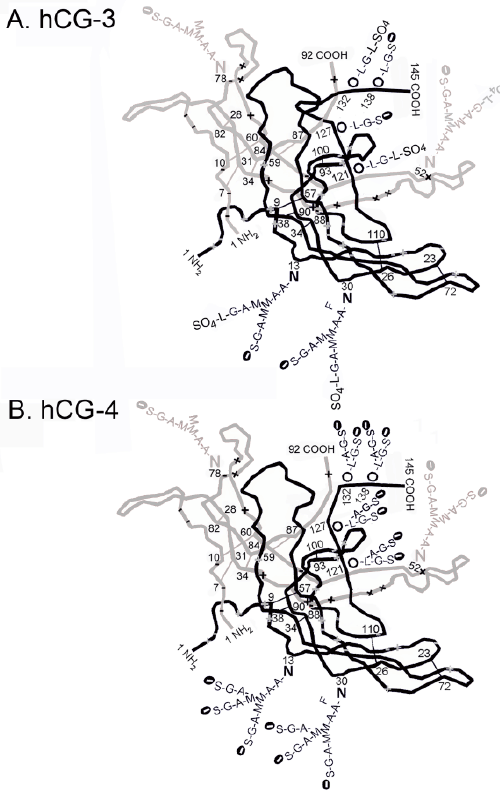
| Figure 3 shows the predicted structure of hCG-3 and hCG-4. hCG- 3 has been shown to contain 30% sulfated oligosaccharides, terminating in GalNAc-sulfate rather than acidic NeuAc [32]. The predicted model is a variation on hCG-1 based on this observation. Research studies show that hCG-2 has type 2 O-linked oligosaccharides and biantennary N-linked oligosaccharides [9,10]. Studies show that cancer hCG (hCG- 4) is analogous to hCG-2, both binding a TGFß receptor but has, as shown in Figure 3 triantennary N-linked oligosaccharides [10]. |
| Looking at the structure of hCG-1 (Figure 2), the ß-subunit C-terminal peptide, starting at amino acid 110, folds back on top of the hCG structure, it finally folds into the nicking loop (ß40-ß55) on hCG ß-subunit before terminating at residue 145. In contrast, on hCG-2, the C-terminal peptide does not fold into the nicking loop (ß40-ß55). This difference must be due to the larger type 2 oligosaccharides on hCG-2. |
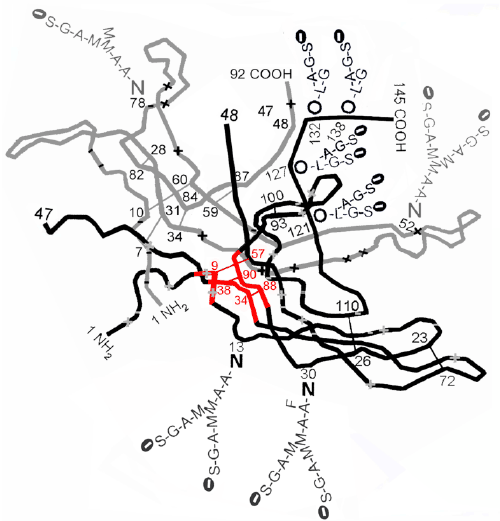
| Back in 1991 I showed that hCG-2 and hCG-4 are more rapidly nicked or cleaved in this ß40-ß55 loop than hCG-1 [33]. This is seemingly due to the blockage of nicking or cleavage in the loop by intercepting ß-subunit C-terminal peptide on hCG-1. Interestingly, nicking at ß47/ ß48 or cleavage in this loop, which occurs rapidly in hCG-2 and hCG-4, but not hCG-1 [33], exposes the common cystine knot structure in hCG ß-subunit, that the hCG group of molecules inherited in its evolution from TGFß (TGFß evolved to gonadotropin ancestral hormone, which evolved to LH and then to hCG). It is possible that nicking or cleavage is required for TGFß receptor binding, and that this nicking and nicking site blockage in hCG-1, explains the small structural differences and alternate receptor binding preferences of hCG1 (binds endocrine hCG/ LH receptor) and hCG2 plus hCG-4 (binds autocrine TGFß receptor). Figure 4 shows result of nicking hCG-2 and exposure of common TGFß knot. The ß40-ß55 loop is ampipathic, ß40-47 is charged and ß48-ß55 is hydrophobic, causing the wide opening of this loop that may lead to TGFß receptor binding, the charged arm stretching to the hydrophilic surface of this molecule, and the hydrophobic component stretching to hydrophobic center of the molecule. |
| A structure in not shown for hCG-5 mutant hCG [21], dozens of different mutations are possible leading to structural changes. hCG-5 is an hCG free ß-subunit produced by liver and kidney cells [23-25]. Familial hCG Syndrome has been recognized in adults, a syndrome in which liver and kidney hCG is produced throughout life. This is a dominant inherited disorder. It is thought, that Familial hCG Syndrome may be a consequence of failure to turn off liver and kidney hCG production at birth. The hCG produced in Familial hCG Syndrome is a mutated free ß-subunit. |
| It is known that cells other that trophoblast or pituitary cells produce a variant of hCG subunits which cannot combine to form dimer. These cells lack the specific disulfide isomerase needed to complete the final two disulfide bridges on hCG ß-subunit, ß93-100 and ß26-110, this blocks subunit combination [34]. The same limitation explains why non-trophoblastic cancer produces hCG-4 free ß-subunit. |
| hCG-1 – hCG-5 Production and Occurrence |
| Multiple research studies are presented in this review from the USA hCG Reference Service. All these studies have been published previously, and are referenced accordingly. All subjects referred to the USA hCG Reference Service completed forms giving permission for their results to be used for research. |
| Table 2presents a large study (961 serum samples) showing changes in hCG-1 and hCG-2 concentration during the course of pregnancy [35]. As shown, both hCG-1 and hCG-2 peak at 10 weeks gestation, but remain elevated during the course of pregnancy. While hCG-2 concentrations are much greater than hCG-1 in early pregnancy, hCG- 1 concentrations start to exceed hCG-2 at 5 weeks gestation and exceed hCG-2 during the course of pregnancy. hCG-1 is critical for managing hemochorial placentation, macrophage suppression and contraction suppression during the course of pregnancy [1-8]. hCG-2 is critical to promote blastocyst implantation and trophoblast cell growth during the course of pregnancy [14-16]. While no deficiency of hCG-1 is known of during pregnancy. A deficiency of hCG-2 does quite commonly occur. It causes pregnancy failure at implantation, and preeclampsia and hypertension in the second and third trimesters of pregnancy [36-39]. |
| Table 3 shows urine samples and concentrations of hCG-1 and hCG- 2 in 93 women on the first possible day of hCG detection or implantation of pregnancy. All 93 women had term outcome pregnancies. As shown, concentrations of hCG-2 are generally higher than hCG-1. This is because hCG-2 functions in pregnancy to control implantation. The mean concentration of hCG-1 was 0.13 ng/ml while the mean concentration of hCG-2 was 0.56 ng/ml. In detecting early pregnancy it is critical to use a test that equally measures both hCG-1 and hCG-2, whether a serum hCG test or a point-of-care or home pregnancy test. There are 11 automated hCG tests used by laboratories today. Only one test, the Siemens Immulite test, as used here, equally detects hCG-1 and hCG-2. This is one of the least used automated hCG tests. The other 10 use a C-terminal peptide specific antibody which is O-linked oligosaccharide sensitive, leading to poor detection of hCG-2 [40,41]. |
| hCG-3 is produced by the pituitary at extremely low concentrations. Recently, we had the opportunity to examine urine concentrations during the menstrual cycle in 260 women [17]. We examined LH and hCG-3 on the day of the LH peak. hCG-3 parallels LH and also peaks at this time. As shown in Table 4, on this day, peak LH averages 142 mIU/ml, while peak hCG-3 averages 1.12 mIU/ml. hCG-3 is roughly 100 fold more biologically potent than LH, so that LH and hCG-3 have quite similar biological potency during the menstrual cycle. hCG-3 and LH work together to accomplish all luteotropic functions, promoting steroidogenesis in men and women, promoting follicle growth, sperm maturation, ovulation and corpus luteum formation. Pituitary hCG is barely detectable at other times, other that at the LH/hCG peaks during the menstrual cycle. |
| In perimenopause and menopause ovarian estradiol production starts to close down. This prevents estradiol feedback control of hypothalamus gonadotropin releasing hormone (GnRH). Pulses of GnRH are sent to the pituitary more rapidly at this time than during the menstrual cycle, leading to excessive LH, follitropin (FSH) and hCG- 3 production. hCG-3 production in perimenopausal and menopausal women is more detectable. Table 5 shows hCG-3 production in 104 menopausal women referred to the USA hCG Reference Service. hCG-3 concentrations ranged from perimenopausal women (Mean 9.28 ± 6.05 mIU/ml, range 1.40-29 mIU/ml), to women having induced menopause from bilateral oophorectomy (Mean 9.81 ± 8.91 mIU/ml, range 2.0-38 mIU/ml) to maximal production observed in menopause (Mean 11.42 ± 8.67 mIU/ml, range 3.0-54 mIU/ml). It is quite simple to prove that hCG-3 is coming from menopause. Place a woman on a high estrogen contraceptive pill for 3 weeks, if hCG-3 is completely suppressed (>1 mIU/ml) then it must be coming from the hypothalamic-pituitary tract. |
| hCG-4 and hCG-4 free ß-subunit are produced by cancer cells. They are autocrines which feedback to the cancer cells antagonizing a TGFß receptor. This physically drives cancers, blocking apoptosis, promoting cancer cell growth and promoting cancer cell invasion of neighboring cells [18-20]. Trophoblastic malignancies, choriocarcinoma, testicular germ cell malignancies and ovarian germ cell malignancies (germ cells cancer take on trophoblastic morphology) produce hCG-4, while all other cancers cannot combine subunits [34], and produce hCG-4 free ß-subunit [9,10,18,19]. |
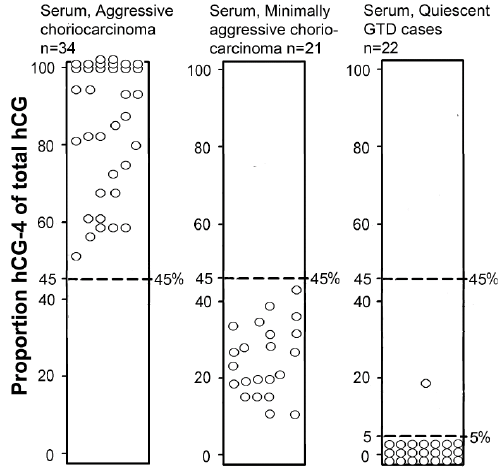
| Examining trophoblastic malignancies, Table 6 shows the range of hCG-4 production in choriocarcinoma patients referred to the USA hCG Reference Service, and the varying proportions of hCG-4 of total hCG in choriocarcinoma cases [34]. As shown in Figure 5, the USA hCG Reference Service uses proportion of hCG in diagnosis of choriocarcinoma [34]. hCG-4 drives choriocarcinoma, so that cases producing mostly hCG-4 are aggressive malignancies. Cases producing low hCG-4 are minimally aggressive or chemorefractory cases, and those producing no hCG-4 are quiescent or inactive disease [42]. The disease is dependent on hCG-4. As used by the USA hCG Reference Service, >45% hCG-4 is aggressive disease, <45% is minimally aggressive or chemorefractory disease, and <5% hCG-4 is quiescent or inactive disease [42] (Table 6). |
| Table 7 describes non-trophoblastic malignancies. Shown is data on 27 cases of ovarian cancer, 3 stage I, 5 stage II, 14 stage III, and 5 stage IV [43]. As shown, 27 of 27 cases produced hCG-4 free ß-subunit of variable concentration, 0.20–58.6 pmol/ml [19,43]. This was detected in urines using antibody B202, an antibody detecting free ß-subunit plus ß-core fragment. Unlike the tumor marker CA125, concentrations were not dependent on stage of cancer. A mean of 1.6 pmol/ml were detected in stage I malignancies, 20.2 pmol/ml in stage II, 8.4 pmol/ ml in stage III, and 7.8 pmol/ml in stage IV malignancies. It is thought, since hCG-4 free ß-subunit physically drives malignancies, that concentration of hCG free ß-subunit may be more related to the speed of growth or aggressiveness of the malignancies, rather than the stage or advancement the cancer reached. |
| Fetal hCG, hCG-5 free ß-subunit is produce by the normal fetus in all pregnancies [23-25]. Research indicates that hCG-5 free ß-subunit drives organ growth and development in the fetus [23-25]. While no fetal blood is available to show in this review. There is a disorder of hCG-5 free ß-subunit, from which hCG-5 free ß-subunit may be analyzed. Familial hCG syndrome is an inherited disorder in which male and female individuals may produce variable levels of hCG-5 free ß-subunit through their lives [21,22]. While it is inferred that this is hCG-5, the link is not proven. |
| Familial hCG syndrome seemingly is the result of failure of the body to turn off hCG-5 free ß-subunit production at birth [21,22]. In the 21 cases seen by the USA hCG Reference Service cases had no other symptoms, and no other signs other than production of hCG-5 free ß-subunit [21,22]. In every case, familial hCG syndrome was confirmed by showing that one parent had the disorder, indicating inherited dominance [21,22]. |
| Studies conducted by Stephen Butler PhD in England, and Ulf Stenman PhD in Finland show that the hCG-5 free ß-subunit produced is highly mutated. Generating a molecule that is invariably detected by different hCG immunoassays [21,22]. Table 8 shows the 21 cases diagnosed by the USA hCG Reference Service. As shown, the syndrome effects both males and females, and in every case a parent was demonstrated to be carrying the syndrome, making it a dominant disease [21,22]. |
| As shown hCG-5 free ß-subunit concentration may widely vary from 1.0 mIU/ml to 216 mIU/ml. Individual concentrations may change every day from non-detection up to >200 mIU/ml [21,22]. While the USA hCG Reference Service used a free ß-subunit assay to diagnose familial hCG syndrome based on the detection of free ß-subunit, and the demonstration of the syndrome in a parent. Today, they are now detecting this syndrome based upon widely varying results measuring hCG using the Siemens Dimension hCG assay as a proportion of measuring hCG using the Siemens Immulite hCG assay. The Siemens Immulite assay uses two antibodies to the ß-subunit three dimensional core structures. Part of the molecule that should not be affected by mutations. While the Siemens Dimension assay uses an antibody to the hCG ß-subunit C-terminal peptide, part of the molecule where amino acid sequence may be critical. This assay may be maximally affected by mutations. The most recent cases, case 17-21 were detected by this method. |
| Here data is presented on the production and occurrence of hCG- 1, hCG-2, hCG-3, hCG-4, hCG-4 free ß-subunit and hCG-5 free ß-subunit. All are semi-independent molecule with different functions. As indicated, hCG is produce by the placenta in pregnancy, by the normal pituitary, by trophoblastic and non-trophoblastic cancers and by the fetus during pregnancy. This story does not end here. Recent research demonstrates that brain cells have an hCG/LH receptor [44,45]. Nothing is known yet about what action hCG has on the brain [44,45]. |
| hCG is an exception to the general rules of biochemistry, one amino acid sequence leading to 6 semi-independent molecules, varying in carbohydrate structure, merism and because of mutations. Each is produced by different cells, and acts as a hormone or autocrine on multiple receptors, LH/hCG hormone receptor and TGFß autocrine receptor. |
| hCG-1 - hCG-5 and hCG Tests |
| Here I address the basic question, do all total hCG test detect hCG-1, hCG-2, hCG-3, hCG-4, gCG-4 free ß-subunit and hCG-5 free ß-subunit? To give a simple answer I would say invariable yes. In the United States there are 11 automated total hCG assays sold today, the Abbott Architect, Abbott AxSym, the Beckman Access, Beckman DXI800, Ortho Vitros ECiQ, Roche Elecsys hCG + ß, Siemens Centaur, Siemens Dimension, Siemens Immulite, Siemens Stratus and Tosoh A1A. These are used at all commercial labs across the country. The Siemens Immulite assay uses two antibodies to the core ß-subunit three dimensional epitope, all the others use antibody the ß-subunit C-terminal peptide. |
| hCG-2, hCG 4 and hCG-4 free ß-subunit have type 2 O-linked oligosaccharides. These are invariable detected by all assay except the Siemens Immulite. hCG-2 hCG-4 and hCG-4 free ß-subunit are as described above rapidly nicked molecules. Examining Table 9, nicked hyperglycosylated hCG best represents the detection of these molecules. The Abbott Architect detect these molecules with 40% efficiency, Abbott AxSym with 46%, the Beckman Access with 46%, Beckman DXI800 with 51%, Ortho Vitros ECiQ with 80%, Roche Elecsys hCG+ß with 100%, Siemens Centaur with 40%, Siemens Dimension with 80%, Siemens Immulite with 103%, Siemens Stratus with 88% and Tosoh A1A with 70% efficiency. |
| hCG-4 free ß-subunit and hCG-5 free ß-subunit are free ß-subunit forms of hCG. As published [33] 100% of free ß-subunit molecules in serum are nicked. We examine Table 9 and the category nicked hCG free ß-subunit. The Abbott Architect detected the molecules with 33% efficiency, Abbott AxSym with 51%, the Beckman Access with 56%, Beckman DXI800 with 63%, Ortho Vitros ECiQ with 19%, Roche Elecsys hCG+ß with 53%, Siemens Centaur with 19%, Siemens Dimension with 41%, Siemens Immulite with 107%, Siemens Stratus with 70% and Tosoh A1A with 60% efficiency. |
| As discussed above familial hCG syndrome generates a mutant free ß-subunit (hCG-5 free ß-subunit). The Siemens Immulite use antibodies focused on hCG ß-subunit three dimensional core, while the other 10 assays use antibodies focused on the ß-subunit C-terminal peptide, a non-folded amino acid sequence. Assay focused on the C-terminal peptide will be more sensitive to mutations, and poorly detect hCG-5 free ß-subunit. |
| In conclusion author recommends the Siemens Immulite assay (Siemens Immulite-1000 and -2000 assays) for measuring hCG-1, hCG- 2, hCG-3, hCG-4, hCG-4 free ß-subunit and hCG-5 free ß-subunit. |
| Are their specific antibodies for measuring hCG-1, hCG-2, hCG-3, hCG-4, hCG-4 free ß-subunit and hCG-5 free ß-subunit? No, there are not. There is a specific antibody for measuring molecules with type 2 O-linked sugar side chains, hCG-2, hCG-4 and hCG-4 free ß-subunit, but that is it. The existence of the six semi-independent molecules was demonstrated by structure studies [9,10,46]. |
References
- Cole LA (2013) hCG and HyperglycosylatedhCG, Promoters of Villous Placenta and Hemochorial Placentation. Placenta: Functions, Development and Disease. Nova Publishers 2: 155-166.
- Cole LA, Khanlian SA, Kohorn EI (2008) Evolution of the Human Brain, Chorionic Gonadotropin and Hemochorial Implantation of the Placenta: Origins of Pregnancy Failures, Preeclampsia and Choriocarcinoma. J Reprod Med 53: 449-557.
- Toth P, Li X, Rao CV, Lincoln SR, Sanfillipino JS, et al. (1994) Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J ClinEndocrinolMetab 79: 307-315.
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, et al. (2002) Characterization of human chorionic gonadotropin as a novel angiogenic factor. J ClinEndocrinolMetab 87: 5290-5296.
- Eta E, Ambrus G, Rao V (1994) Direct regulation of human myometrial contractions by human chorionic gonadotropin. J ClinEndocrinolMetab 79: 1582-1586.
- Doheny HC, Houlihan DD, Ravikumar N, Smith TJ, Morrison JJ (2003) Human chorionic gonadotrophin relaxation of human pregnant myometrium and activation of the BKCa channel. J ClinEndocrinolMetab 88: 4310-4315.
- Akoum A, Metz CN, Morin M (2005) Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J ClinEndocrinolMetab 90: 2904-2910.
- Wan H, Marjan A, Cheung VW, Leenen PJM, Khan NA, et al. (2007) Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J Leukocyte Biol 82: 926-933.
- Elliott MM,Kardana A, Lustbader JW, Cole LA (1997) Carbohydrate and peptide structure of the alpha- and beta-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine 7: 15-32.
- Cole LA (2015) hCG and HyperglycosylatedhCG Carbohydrate Structures Corrected. J Glycobiol In press.
- Cole LA (2013) hCG physiology. Placenta 34: 1257.
- Butler SA, Ikram MS, Mathieu S, Iles RK (2000) The increase in bladder carcinoma cell population induced by the free beta subunit of hCG is a result of an anti-apoptosis effect and not cell proliferation. Brit J Cancer 82: 1553-1556.
- BerndtS, BlacherS, MunautC, DetilleuxJ, d'HauteriveP S, et al. (2013) Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-ß receptor activation. J FASEB 27: 1309-1321.
- Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI (2006) Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylatedhCG. GynecolOncol 102: 145-150.
- Handschuh K, Guibourdenche J, Tsatsari V, Guesnon M, Laurendeau I, et al. (2007) Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-a. Endocrinol 148: 5011-5019.
- Guibourdenche J,Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, et al. (2010) HyperglycosylatedhCG is a marker of early human trophoblast invasion. J ClinEndocrinolMetab 95: E240-244.
- Cole LA, Gutierrez JM (2009) Production of human chorionic gonadotropin during the normal menstrual cycle. J Reprod Med 54: 245-250.
- Cole LA, Butler SA (2015) B152 anti-hyperglycosylated human chorionic gonadotropin free β-Subunit. A new, possible treatment for cancer. J Reprod Med 60: 13-20.
- Cole LA, Butler S (2012) HyperglycosylatedhCG, hCGβ and Hyperglycosylated hCGβ: interchangeable cancer promoters. Mol Cell Endocrinol 349: 232-238.
- Iles RK (2007) Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol 260-262: 264-70.
- Cole LA, Butler S (2014) Familial hCG syndrome: production of variable, degraded or mutant forms of hCG. J Reprod Med 59: 435-442.
- Cole LA (2012) Familial HCG syndrome. J ReprodImmunol 93: 52-57.
- McGregor WG, Raymoure WJ, Kuhn RW, Jaffe RB (1981) Fetal tissues can synthesize a placental hormone Evidence for chorionic gonadotropin ?-subunit synthesis by human fetal kidney. J Clin Invest 68: 306-309.
- Goldsmith PC, McGregor WG, Raymoure WJ, Kuhn RW, Jaffe RB (1983) Cellular localization of chorionic gonadotropin in human fetal kidney and liver. J ClinEndocrinolMetab 57: 654-661.
- Abdallah MA, Lei ZM, Li X, Greenwold N, Nakajima ST et al. (2004) Human Fetal nongonadal tissues contain human chorionic gonadotropin/ luteinizing hormone receptors. J ClinEndocrinolMetab 89: 952-956.
- Cole LA (2014) The Molecular Genetics of hCG. In: Human chorionic gonadotropin (hCG)J ClinEndocrinolMetab 2: 141-149.
- Kubiczak M, Grzegorz P, Walkowiak EN, Jankowska A (2013) Human Chorionic Gonadotropin Beta Subunit Genes CGB1 and CGB2 are Transcriptionally Active in Ovarian Cancer. Int J MolSci 14: 12650-12660.
- BurczynskaBB, KobroulyL, ButlerSA, NaaseM, IlesRK (2014) Novel insights into the expression of CGB1 & 2 genes by epithelial cancer cell lines secreting ectopic free hCG?. Anticancer Res 34: 2239-2248.
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, et al. (1994) Crystal structure of human chorionic gonadotropin. Nature 369: 455-461.
- Lustbader JW,Lobel L, Wu H, Elliott MM (1998) Structural and molecular studies of human chorionic gonadotropin and its receptor. Recent ProgHorm Res 53: 395-424.
- Wu H, Lustbader JW, Liu Y, Canfield RE, Hendricksonl WA (1994) Structure of human chorionic gonadotropin at 2.6A resolution from MAD analysis of the selenomethionyl. Protein Structure 2:545-558.
- Birken S,Maydelman Y, Gawinowicz MA, Pound A, Liu Y, et al. (1996) Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology 137: 1402-1411.
- Cole LA,Kardana A, Andrade-Gordon P, Gawinowicz MA, Morris JC, et al. (1991) The heterogeneity of human chorionic gonadotropin (hCG). III. The occurrence and biological and immunological activities of nicked hCG. Endocrinology 129: 1559-1567.
- Beebe JS,Huth JR, Ruddon RW (1990) Combination of the chorionic gonadotropin free beta-subunit with alpha. Endocrinology 126: 384-391.
- Cole LA (2012) hCG, five independent molecules. ClinChimActa 413: 48-65.
- Sasaki Y, Ladner DG, Cole LA (2008) Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. FertilSteril 89: 1781-1786.
- Cole LA (2012) HyperglycosylatedhCG and pregnancy failures. J ReprodImmunol 93: 119-122.
- Bahado-Singh RO, Oz AU, Kingston JM, Shahabi S, Hsu CD, et al. (2002) The role of hyperglycosylatedhCG in trophoblast invasion and the prediction of subsequent pre-eclampsia. PrenatDiagn 22: 478-481.
- Brennan MC, Wolfe MD, Murray-Krezan CM, Cole LA, Rayburn WF (2013) First Trimester Hyperglycosylated Human Chorionic Gonadotropin and Development of Hypertension. PrenatDiagn 33: 1075-1079.
- Cole LA, Sutton JM, Higgins TN, Cembrowski GS (2004) Between-method variation in human chorionic gonadotropin test results. ClinChem 50: 874-882.
- Cole LA,DuToit S, Higgins TN (2011) Total hCG tests. ClinChimActa 412: 2216-2222.
- Cole LA, Muller Y (2010) hCG in the Management of Quiescent and Chemorefractory Gestational Trophoblastic Diseases. GynecolOncol 116: 3-9.
- Cole LA, Nam JH (1989) Urinary gonadotropin fragment (UGF) measurements in the diagnosis and management of ovarian cancer. Yale J Biol Med 62: 367-378.
- Lei ZM,Rao CV, Kornyei JL, Licht P, Hiatt ES (1993) Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 132: 2262-2270.
- Lei ZM, Rao CV (2001) Neural actions of luteinizing hormone and human chorionic gonadotropin. SeminReprod Med 19: 103-109.
- Valmu L, Alfthan H, Hotakainen K, Birken S, Stenman UH (2006) Site-specific glycan analysis of human chorionic gonadotropin beta-subunit from malignancies and pregnancy by liquid chromatography - electrospray mass spectrometry. Glycobiol 16: 1207-1218.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
| Table 6 | Table 7 | Table 8 | Table 9 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11105
- [From(publication date):
February-2016 - Dec 18, 2024] - Breakdown by view type
- HTML page views : 10248
- PDF downloads : 857
