Handgrip Strength is a Reliable Outcome Measure and is Associated with Inspiratory Muscle Strength and Exercise Capacity in Hemodialysis Patients
Received: 20-Sep-2019 / Accepted Date: 07-Oct-2019 / Published Date: 14-Oct-2019 DOI: 10.4172/2165-7025.1000417
Abstract
Objective: To determine the reliability of the handgrip strength (HGS) and to evaluate the association between HGS, inspiratory muscle strength and exercise capacity (EC) in hemodialysis patients.
Methods: Hemodialysis patients performed HGS, maximal inspiratory pressure, and Incremental Shuttle Walking Test (ISWT) for EC evaluation. The data obtained from the ISWT were divided into tertiles, and the HGS results were compared among tertiles using analysis of variance. Reliability analyses were assessed by intra-class correlation coefficient (ICC) and minimal detectable change at the 90% CI (MDC90). Associations were determined by linear regression analysis.
Results: Thirty-six hemodialysis patients aged 49.1 years (95% CI 44.0–54.1) were evaluated. HGS presented excellent reliability (ICC=0.98) and MDC90 was 3.1 kg. HGS and age were the independent predictors to maximal inspiratory pressure and EC (R2 adjusted=0.52 and 0.42, respectively). Patients with inspiratory muscle weakness had lower HGS values [difference of 8.6 kg (95% CI 0.5–16.7]. Tertile 1 of the ISWT was different from tertile 2 for HGS [difference of 13.4 kg (95% CI 4.7–22.1)], as well as from tertile 3 [difference of 13.9 kg (95% CI 5.2–22.6)].
Conclusion: HGS is a reliable outcome measure and is directly related to the inspiratory muscle strength and EC in hemodialysis patients.
Keywords: Handgrip; Exercise capacity; Inspiratory strength; Hemodialysis
Introduction
End-Stage Renal Disease (ESRD) is a worldwide public health problem. The incidence of the disease is approximately 8% per year in the Brazilian population. The costs of treatment of the disease are 1.4 billion per year on average with hemodialysis [1], the most performed renal substitution therapy in worldwide [2].
Both the progression of renal disease as well the dialysis treatment itself is involved with the manifestation of uremic myopathy, which is marked by changes in muscular structure and function, with consequent reduction of strength and resistance [3]. Sedentary lifestyle and nutritional deficiency are factors that worsen myopathy in the ESRD and can progress with fatigue and reduced exercise capacity (EC) [4], which may predispose to restrictions on mobility, pain, psychological disturbances and worsening of quality of life [5,6]. In addition, the EC impairment is an independent predictor of mortality in this population [7].
Similarly to the muscles of the locomotor system, inspiratory muscles can also be affected by uremic myopathy. Some studies have demonstrated the reduction of inspiratory muscle strength in this population, represented by the reduction of Maximum Inspiratory Pressure (MIP) [8-10]. Although it’s clinical repercussions are not known in the long term, inspiratory muscle weakness is indicated as an independent predictor of EC impairment in hemodialysis patients [11].
Handgrip strength (HGS) is a non-invasive, fast-performing, objective and low-cost method for assessing muscle function [12], and the most used test to evaluate the muscular strength of hemodialysis patients [13]. It is a validated method to evaluate the nutritional status of this population, directly associated with lean body mass [14], and provides important prognostic information. Studies have demonstrated the influence of HGS on the risk of clinical long-term complications in ESRD patients [15-17].
Once the reduction of muscular function affects the EC and the ventilatory capacity, it is expected that the HGS may be a method able to identify individuals with EC impairment and inspiratory muscle weakness (IMW). In other health conditions, this association has already been demonstrated [18,19]. However, in ESRD this association not has been explained. In addition, the reliability of the HGS needs to be studied. The characterization of relative and absolute reliability is clinically important because refers to test-retest reliability and allows us to differentiate a true change from an individual variation in test performance. Therefore, the present study aimed to evaluate the association between HGS, inspiratory muscle strength and EC, and to assess the reliability of the HGS in hemodialysis patients.
Methods
Study design
This cross-sectional study was conducted between May 2013 and January 2015 in the hemodialysis unit of the Santa Casa de Caridade de Diamantina Hospital and the Cardiovascular Rehabilitation Laboratory (LABCAR) of the Universidade Federal dos Vales Jequitinhonha e Mucuri (Diamantina-Minas Gerais state, Brazil). The research was carried out in accordance with the declaration of Helsinki (2013) and was approved by ethics committee of the Universidade Federal dos Vales do Jequitinhonha e Mucuri (protocol 088/12). All the patients gave their written informed consent before participating in the study.
Subjects
ESRD patients older than 18 years who were receiving hemodialysis treatment three times a week for at least six months and had an arteriovenous fistula for hemodialysis access were included in the study. Exclusion criteria were contraindications or inability to perform the exercise tests. The sample size was calculated a priori, considering a correlation coefficient of 0.76 between MIP and HGS [18], statistical power of 99% and alpha error of 1%. The sample size was estimated in 19 volunteers.
Procedures
The selected patients underwent clinical evaluation by nephrologists following anthropometrics measurements [weight, height, body mass index (BMI) and waist circumference] and evaluation of HGS, inspiratory muscle strength and EC. All evaluations were performed during a week on dialysis days, always on the same day shift, in the following sequence: immediately before first weekly hemodialysis session – anamnesis and anthropometric measurements; immediately after second weekly hemodialysis session – inspiratory muscle strength; immediately before third weekly hemodialysis session – HGS and EC. Prior to all evaluations the volunteers remained seated for 10 minutes. The investigators were blinded to test results, and all volunteers had previously been trained to perform the functional tests. After 6-to 8-week (trial 2) patients performed the second HGS [20]. The interval forms part of a control period in a clinical trial. Pre- and post-data were used to reliability analysis.
Handgrip Strength (HGS)
The HGS was obtained using Jamar® mechanical dynamometer with a precision of 0.5 kg (Sammons Preston, Masan, Korea), in the arm without arteriovenous fistula [14,16,21]. The volunteers remained seated with the arm and forearm in neutral position and 90° at elbow flexion. Three measurements were performed with intervals of about 60 s between each run and the highest score was recorded in kilograms. HGS values less than the 10th percentile of a Brazilian-based reference study were considered low HGS [22].
Inspiratory muscle strength
Respiratory muscle strength was determined using a previously calibrated aneroid vacuum manometer (MV-150/300, Ger-Ar, São Paulo, Brazil) equipped with a 2 mm diameter hole in the nozzle to compensate for the pressure change induced by the oropharynx muscles, following recommendations of American Thoracic Society/ Europen Respiratory Society [23]. MIP was evaluated based on residual volume while the volunteers were seated, and the highest value of three valid measurements was retained [23]. The measurements were considered acceptable if the variance between them was less than 10%. Respiratory measurements are shown as absolute and relative values based on the percentage achieved compared to the maximum predicted by age and sex [24]. IMW was defined as MIP less than 70% of the predicted value [23].
Exercise Capacity (EC)
EC was evaluated by the Incremental Shuttle Walk Test (ISWT) [25]. Volunteers were instructed to walk or run [26] in a 10 m corridor and the minimum speed was determined by an audio signal. The ISWT has 12 progressive intensity levels, and the test is completed when the volunteer either completes the 12 levels of intensity or fails to reach the minimum speed required on a given level two consecutive times [11,27,28]. The distance walked was recorded, and the predicted values were estimated [29]. Prior to data collection, the test-retest reliability of the ISWT was evaluated in twenty-two hemodialysis patients [age, 55.0 years (95% CI 49.4–60.7)] and showed an intra-class correlation coefficient of 0.90 (95% CI 0.77-0.95).
Reliability testing procedures
After 6-to 8-week anthropometric measurements were reevaluated and patients performed the second HGS (trial 2). Patients were instructed to maintain their habitual lifestyle during interval and all were weekly monitored. The same researcher applied all the tests and the HGS followed exactly the same protocol in both trials. Test– retest was used to determine the relative reliability. Standard error of measurement (SEM) and minimal detectable change (MDC) scores were calculated to determine the absolute reliability of the HGS.
Statistical analysis
Date analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The normal distribution and homoscedasticity was assessed by Shapiro Wilk test and Levene test, respectively. Categorical variables are presented as absolute and relative frequencies, and continuous variables are presented as the mean (95% CI). Correlation analysis was carried out using the Pearson or Spearman tests (continuous variables), as appropriate. We considered a moderate to good correlation when “r”=0.50 to 0.75 and values above 0.75 were considered to represent a strong or excellent correlation [30]. The associations between HGS and MIP and EC were assessed by univariate regression analysis, followed by stepwise multivariate linear regression analysis, with adjustment for age, sex and BMI. The comparisons of the HGS between groups stratified by MIP (with or without IMW) were performed by unpaired two-tailed t-tests. Data from the ISWT (distance walked) was divided into tertiles. The tertiles were defined by stratifying the sample into three different EC levels to verify the effectiveness of the HGS in identifying different functional status (low, moderate and high) [31,32]. The comparison of the HGS results among groups divided by EC levels was performed by the one-way analysis of variance with a post hoc analysis by Bonferroni test. To assess the accuracy of using HGS to discriminate between those who had low inspiratory muscle weakness and low EC, a receiver operating characteristic (ROC) curve was constructed. The area under the curve was calculated to represent the accuracy of the test at discriminating those with inspiratory muscle weakness and low EC. An area under the curve of 1.0 corresponds to perfect discrimination. The ROC curve constructed was also used to determine the sensitivity and specificity of different cut-off values of the HGS for the prediction of inspiratory muscle weakness and low EC. The optimal cut-off value was defined by the value with the best combination of sensitivity and specificity. The test-retest reliability of data for all repeated tests was assessed with the intra-class correlation coefficient (ICC), model alpha, 2-way random effects model. We considered an ICC ≥ 0.90 as “excellent” [33]. The absolute reliability was evaluated by standard error of measurement (SEM) for repeated measures and minimal detectable change (MDC) scores following formulas previously [34] described. SEM was calculated by following equations: SEM=SD * √ (1-r), where r=ICC for the participant group. The MDC at individual and group levels were calculated at 90% CI (MDC90). The MDC90 was calculated as: MDCindiv=SEM * 1.65 * √2, where the 1.65 represents the z-score at the 90%CI. The √2 represent the account for errors associated with repeated measures. Differences between trials 1 and 2 were evaluates by Wilcoxon test and agreement by Bland–Altman plot. The significance level set at 0.05 in all analyses.
Results
Forty-one ESRD patients were selected and 36 volunteers were enrolled in the study (one did not provide consent, one had angina and three were unable to perform all the steps of the evaluation protocol). The baseline characteristics are shown in Table 1. The volunteers were predominantly male (66.7%) and with overweight. Systemic arterial hypertension was the most prevalent etiology of ESRD (50.0%) and kt/v indexes of 1.6 (95% CI 1.5–1.7) demonstrated the efficiency of hemodialysis treatment. All volunteers were taking vitamin C and B complex, and 31 (86.1%) were using erythropoietin.
| Parameters | n (%) |
|---|---|
| Sex | |
| Male | 24 (66.7) |
| Female | 12 (33.3) |
| Age, years | 49.1 (44.0 – 54.1) |
| BMI, kg/m2 | 25.1 (23.1 – 27.0) |
| Duration of hemodialysis, years | 4.0 (2.7 – 5.4) |
| Biochemist | |
| Kt/V index | 1.6 (1.5 – 1.7) |
| Hemoglobin, mg/dL | 10.6 (10.1 – 11.0) |
| Hematocrit, % | 33.0 (31.6 – 34.3) |
| Handgrip strength | |
| Male, kg | 33.5 (29.1 – 37.8) |
| Female, kg | 22.5 (20.0 – 25.1) |
| Total, kg | 29.9 (26.4 – 33.3) |
| Low handgrip strength, n(%) | 9 (25.0%) |
| Inspiratory muscle strength | |
| MIP, cmH2O | 90.8 (81.7 – 99.9) |
| MIP predict, % | 85.2% (78.4 – 91.9) |
| Inspiratory muscle weakness, n(%) | 8 (22.2%) |
| ISWT | |
| Distance, m | 416.8 (343.9–489.7) |
| Predict distance, % | 69.2 (59.1– 79.3) |
| Data represented as mean (95% CI) or n(%). BMI: body mass index; ESRD: End-Stage Kidney Disease; Kt/V: dialysis eficiency; MIP: maximal inspiratory pressure; ISWT: Incremental Shuttle Walk Test | |
Table 1: Characteristic of the sample (n=36).
There was a significant and good correlation between HGS and MIP and EC (r=0.58 and 0.62, respectively) and the HGS was an independent predictor of MIP and EC (Table 2). HGS and age explained 52% of changes in MIP and 42% of changes in EC.
| Dependent variable | r | R2 adjusted | Predictors | b | ß | p |
|---|---|---|---|---|---|---|
| MIP | 0.58* | 0.52 | HGS | 1.2 ± 0.3 | 0.46 | 0.001 |
| Age | -0.9 ± 0.2 | -0.47 | <0.001 | |||
| ISWT | 0.62* | 0.42 | HGS | 8.0 ± 0.7 | 0.39 | 0.006 |
| Age | -6.6 ± 1.6 | -0.46 | 0.002 | |||
| MIP: maximal inspiratory pressure; ISWT: incremental shuttle walk test; HGS: handgrip strength. Model adjusted by age, sex and body mass index. *p<0.05. | ||||||
Table 2: Results of the linear regression analysis (n=36).
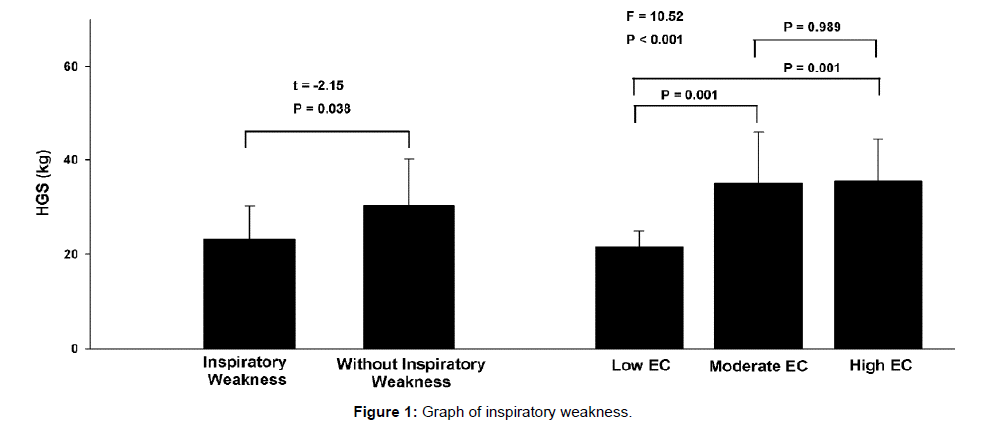
The HGS was able to stratify the sample by MIP and EC level (Figure 1). Stratifying the sample by MIP, patients with IMW have lower HGS than patients with normal MIP with a difference of 8.6 kg (95% CI 0.5–16.7). The cut-off points of distance walked during ISWT in each EC level were: low EC (tertile 1) <278.3 m; moderate EC (tertile 2) between 278.3–531.7 m; and high EC (tertile 3)>531.7 m. Patients with low EC showed lower HG than patients with moderate EC [mean difference of 13.4 kg (95% CI 4.7–22.1)] and high EC [mean difference of 13.9 kg (95% CI 5.2–22.6)]. There were no differences between moderate and high EC.
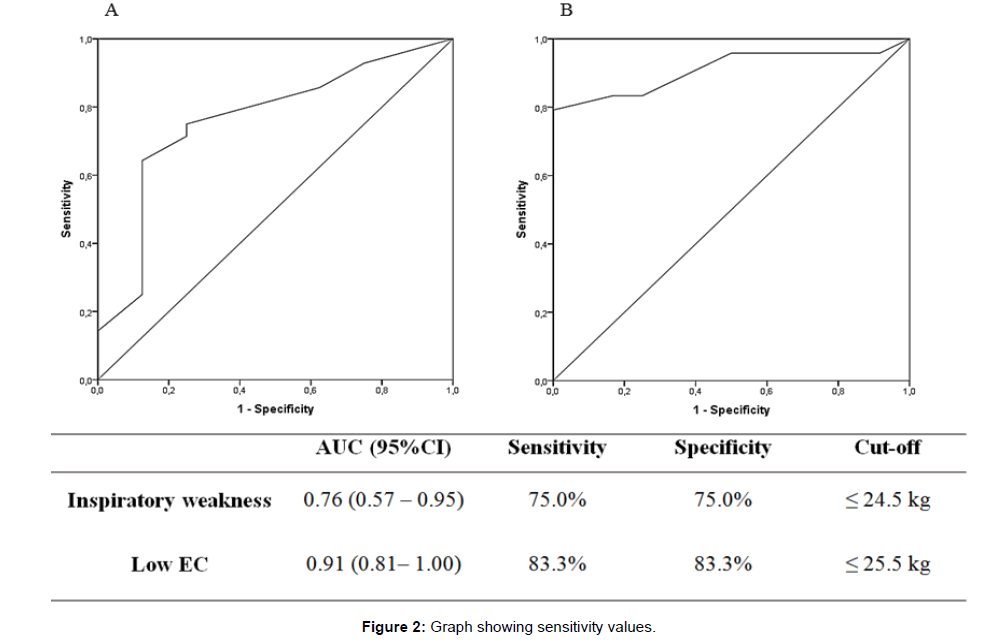
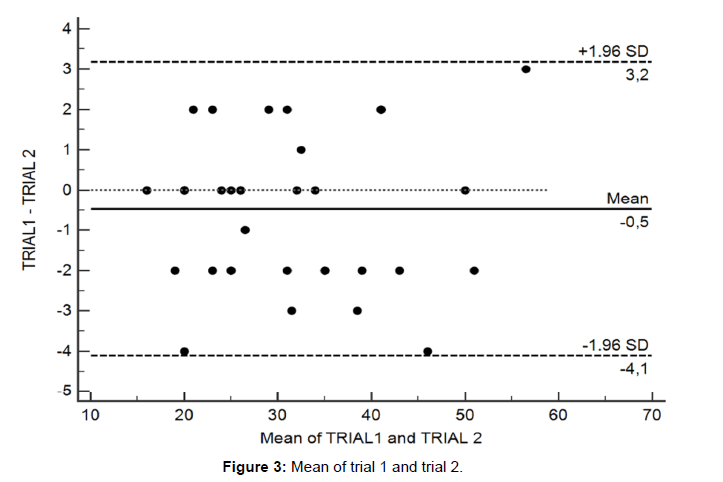
The results of the ROC analysis are shown in Figure 2. The HGS cutoff value of 24.5 kg had sensitivity and specificity of 75.0% in predicting IMW. The area under the curve for this analysis was 0.76. The HGS cut-off of 25.5 kg had a sensitivity and specificity of 83.3% in predicting low EC, with an accuracy of 91.0%. Table 3 presents the results of testretests reliability (ICC) and absolute reliability data (SEM and MDC90). There were no differences between trial 1 and 2. The HGS presented high test-retest reliability (ICC > 0.90). Figure 3 show Bland–Altman plots with bias and limits of agreement (at 95%CI) for the trials. It was possible to verify the agreement between trials 1 and 2, with a bias of 0.5 kg, representing a difference lower than 1.5% between then (see S1 Table Original data from HGS).
| Trial 1 | Trial 2 | Difference | p | ICC (95%CI) | SEM | MDC90 | |
|---|---|---|---|---|---|---|---|
| HGS (kg) | 30.7 (27.2 – 34.3) | 31.2 (27.7 – 34.7) | 0.6 (-6.1 – 4.8) | 0.82 | 0.98 (0.97 – 0.99) | 1.3 | 3.1 |
| Data represented as mean (95% CI). HGS: handgrip strength; ICC: intraclass correlation coefficient; SEM: standard error for repeated measures; MDC90: minimal detectable change scores at individual level at 90% CI. |
Table 3: Relative and absolute reliability of the handgrip strength (n=36).
Discussion
To the best of our knowledge, this is the first study to demonstrate that the reductions of the peripheral muscle strength are associated with the inspiratory muscle strength and EC in hemodialysis patients. In addition, the HGS is able to identify patients with IMW and low EC. The main findings of the present study were: (1) the association between HGS and MIP and between HGS and EC; (2) HGS cut-off values to identify patients with IMW and with low EC; (3) the high reliability of the HGS. These results have important clinical meaning, as HGS is an easy-to-perform method with known prognostic values and important for the nutritional evaluation of this population [14]. Moreover, can also be used on a large scale for screening, risk stratification and functional assessment in the hemodialysis units.
As previously reported, changes in the characteristic of muscle structure and function of people with ESRD may adversely affect muscle strength and endurance [35]. Reduction of the oxidative capacity, increase of protein depletion, vitamin D deficiency and chronic inflammation are cause of worse of muscular function [36]. Thus, the reduction of muscular strength in these patients may also be manifested by the weakness of the respiratory muscles. The strongest association between MIP and HGS suggests a worse of global muscular function in affected patients, a common consequence of ESRD [3].
It is known that the strengthening of the upper trunk portion is decisive for increasing the strength of the upper limbs, as well as the MIP [37]. Correlations between upper limb strength and respiratory muscle strength have been demonstrated in populations with a functional impairment [18,19,38,39], similar results to those observed in the present study. In addition, we observed that HGS was different between individuals with and without IMW, which demonstrates the ability of the HGS, as a measure of muscle function capable of identifying hemodialysis patients with a reduction in MIP, later confirmed by the ROC curve analysis. IMW has been related to cardiac complications in cardiovascular diseases [40,41] and predictor of functional impairment in the ESRD [11]. Therefore, the early identification of affected individuals may also be important in the setting of ESRD, although the relationship between MIP and clinical outcomes is still unknown. In hemodialysis patients, the association between peripheral and respiratory muscles function has been demonstrated through the correlation between MIP and lower limb strength [8].
Another important finding of the current study was the association observed between HGS and EC, evaluated by the ISWT. The reduction of EC causes a series of negative changes in the patient’s life, such as the ability to perform activities of daily living and communication with society, as well as affect the independence of the patient. Moreover, is an important predictor for the death in this population. Our results showed that the HGS strength was able to differentiate patients with low EC which is clinically relevant. In spite of the lower limb strength is more important for walking speed, the HGS represents the global musculature strength and is also related to mobility parameters [42].
In ESRD, HGS measurement has been widely recommended for nutritional assessment and as a measure of muscle function [43]. Although the association between HGS and functional parameters has not been detailed, our results are agreeing with those observed in other health conditions [44,45]. These data reinforce the importance of muscle function in EC in hemodialysis patients, as well as the need to obtain methods that allow a routine of evaluation in large-scale in dialysis centers. Our analysis demonstrated that HGS, a simple and fast execution method could identify individuals with IMW and low EC. Based on these results, we evaluated different cut-off points that could identify the individuals affected by the ROC curve. HGS values greater than 24.5 kg reject the hypothesis of IMW with good sensitivity and specificity. Regarding the EC, the cut-off point of 25.5 kg showed sensitivity and specificity above to 80% to identify the impaired individuals. We believe that the use of the 24 kg and 26 kg, respectively, would be a good reference values for such stratification which makes it easy to use them in clinical routine. Therefore, this finding suggests that individuals on hemodialysis with HGS values greater than 26 kg do not present IMW or significant EC impairment. Individuals with HGS less than 24.0 kg are doubly affected and individuals with values of HGS between 24 and 26 kg may present EC compromise without significant changes in MIP. As a consequence, this information may stimulate the use of HGS measurement as a useful tool for functional evaluation and development of specific rehabilitation strategies for these individuals.
Finally we evaluated the relative and absolute reliability of the HGS in this population. The HGS presented high test-retest reliability having ICC above the minimal acceptable reliability for a clinical test (0.90) and an excellent agreement between trials. The ICC found was similar to observed previously in hemodialysis patients (ICC of 0.96 and 0.95 for dominant and nondominant arms, respectively) [46].
Based on SEM showed in Table 3 (1.3 kg), there is a 68% probability that a repeated measure of the test will be within 1 SEM and there is a 96% probability that a repeated test will be within 2 SEM (2.6 kg). This information is extremely useful in clinical practice.
We also calculated the MDC90, a score used to differentiate a true change from an individual variation in the test. Clinically is used to determine whether a single patient has made a real improvement. The value of the MDC90 showed for HGS (3.1 kg) was close to 3.4 kg found in a previously study [46]. Segura-Ortí and Martínez-Olmos (2011) evaluated the highest HGS value in both dominant and nondominan arms in older patients and we evaluated the highest value in the arm without arteriovenous fistula in younger individuals. In addition the time frame of the test–retest assessment was longer in our study (6-8 weeks). Despite the differences pointed out in the HGS assessment protocols and in the sample characteristics between our study and the previous study, excellent reliability was presented in both. This demonstrates the high reliability of the HGS in this population.
Some limitations needed to be addressed. The sample of the present study was composed of younger individuals with less morbidity than those observed in other studies. One possible explanation is that this study was conducted in a region with a low human development index, where cardiovascular diseases, such as hypertension and diabetes are early manifested. In addition, in this region, specialized health services (nephrology) are difficult to reach the population, delaying the diagnosis and clinical management of renal disease. Many individuals, especially the elderly, die before starting dialysis. The HGS values of the present study sample were close to those observed by other authors who studied individuals with similar age [16]. However, we believe that the characteristics of our sample may limit external validation. Finally, the criteria used for EC classification was based on tertiles of the ISWT. Because the ISWT is a functional test with a high correlation with the cardiopulmonary exercise test and that individuals can achieve values of peak oxygen uptake similar to those obtained in the maximal exercise test at ISWT, we believe that this limitation did not influence the results.
Conclusion
Based on these results, we can conclude that HGS is a reliable outcome measures and is directly related to the inspiratory muscle strength and EC of hemodialysis patients. Being a simples and easy to perform test, the HGS can be applied in large scale in the hemodialysis units. In this context, the HGS measurement becomes a useful tool for functional evaluation and monitoring of this population. Consequently, allowing early detection of functional impairment and contributing to the planning of therapeutic strategies for the rehabilitation.
Acknowledgment
The Laboratório de Reabilitação Cardiovascular, Laboratório de Inflamação e Metabolismo, and Laboratório de Fisiologia do Exercício of Universidade Federal dos Vales do Jequitinhonha e Mucuri, Diamantina, Brazil.
References
- Li PKT, Lui SL, Ng JKC, Cai GY, Chan CT, et al. (2017) Addressing the burden of dialysis around the world: A summary of the roundtable discussion on dialysis economics at the First International Congress of Chinese Nephrologists 2015. Nephrology 22: 3-8.
- Chen Lin SH, Chen JS, Hsu YJ (2013) Muscle wasting in hemodialysis patients: new therapeutic strategies for resolving an old problem. Scientific World Journal 2013: 643954.
- Rhee CM, Kalantar-Zadeh K (2014) Resistance exercise: an effective strategy to reverse muscle wasting in hemodialysis patients? J Cachexia Sarcopenia Muscle 5: 177-180.
- Johansen KL, Kaysen GA, Dalrymple LS, Grimes BA, Glidden DV, et al. (2012) Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol 8: 248-253.
- Fassbinder TRC, Winkelmann ER, Schneider J, Wendland J, Oliveira OB (2015) Functional capacity and quality of life in patients with chronic kidney disease in pre-dialytic treatment and on hemodialysis-a cross sectional study. J Bras Nefrol 37: 47-54.
- Matsuzawa R, Matsunaga A, Wang G, Kutsuna T, Ishii A, et al. (2012) Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 7: 2010-2016.
- Dipp T, Silva AMVd, Signori LU, Strimban TM, Nicolodi G, et al. (2010) Respiratory muscle strength and functional capacity in end-stage renal disease (ESRD). Revista Brasileira de Medicina do Esporte 16: 246-249.
- Pomidori L, Lamberti N, Malagoni AM, Manfredini F, Pozzato E, et al. (2016) Respiratory muscle impairment in dialysis patients: can minimal dose of exercise limit the damage? A Preliminary study in a sample of patients enrolled in the EXCITE trial. J Nephrol 29: 863-869.
- Silva VG, Amaral C, Monteiro MB, Nascimento DM, Boschetti JR (2011) Effects of inspiratory muscle training in hemodialysis patients. J Bras Nefrol 33: 62-68.
- Figueiredo PHS, Lima MMO, Costa HS, Gomes RT, Neves CDC, et al. (2017) The role of the inspiratory muscle weakness in functional capacity in hemodialysis patients. PloS one 12: e0173159.
- Bohannon RW (2001) Dynamometer measurements of hand-grip strength predict multiple outcomes. Percept Mot Skills 93: 323-328.
- BuÄar Pajek M, LeskoÅ¡ek B, Vivoda T, Svilan K, Cuk I, et al. (2016) Integrative examination of motor abilities in dialysis patients and selection of tests for a standardized physical function assessment. Ther Apher Dial 20: 286-294.
- Leal VO, Stockler-Pinto MB, Farage NE, Aranha LN, Fouque D, et al. (2011) Handgrip strength and its dialysis determinants in hemodialysis patients. Nutrition 27: 1125-1129.
- Lopes MB, Silva LF, Dantas MA, Matos CM, Lopes GB, et al. (2018) Sex-age-specific handgrip strength and mortality in an incident hemodialysis cohort: The risk explained by nutrition and comorbidities. Int J Artif Organs 41: 825-832.
- Matos Silva LF, Santana LD, Santos LS, Protásio BM, Rocha MT, et al. (2014) Handgrip strength at baseline and mortality risk in a cohort of women and men on hemodialysis: a 4-year study. J Ren Nutr 24: 157-162.
- Vogt BP, Borges MCC, de Goés CR, Caramori JCT (2016) Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clinical Nutrition 35: 1429-1433.
- Efstathiou ID, Mavrou IP, Grigoriadis KE (2016) Correlation between maximum inspiratory pressure and hand-grip force in healthy young and middle-age individuals. Respir Care 61: 925-929.
- Opasich C, Ambrosino N, Felicetti G, Febo O, Traversi E, et al. (1993) Skeletal and respiratory muscle strength in chronic heart failure. G Ital Cardiol 23: 759-766.
- Wilkinson TJ, Xenophontos S, Gould DW, Vogt BP, Viana JL, et al. (2018) Test–retest reliability, validation, and “minimal detectable change†scores for frequently reported tests of objective physical function in patients with non-dialysis chronic kidney disease. Physiother Theory Pract 35: 565-576.
- Silva Matos CM, Lopes GB, Martins MTS, Martins MS, Arias LU, et al. (2011) Handgrip strength as a simple indicator of possible malnutrition and inflammation in men and women on maintenance hemodialysis. J Ren Nutr 21: 235-245.
- Schlüssel MM, dos Anjos LA, de Vasconcellos MTL, Kac G (2008) Reference values of handgrip dynamometry of healthy adults: a population-based study. Clin Nutr 27: 601-607.
- American Thoracic Society/European Respiratory Society (2002) ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 166: 518-624.
- Neder JA, Andreoni S, Lerario M, Nery L (1999) Reference values for lung function tests: II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res 32: 719-727.
- Singh SJ, Morgan M, Scott S, Walters D, Hardman AE (1992) Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 47: 1019-1024.
- Probst VS, Hernandes NA, Teixeira DC, Felcar JM, Mesquita RB, et al. (2012) Reference values for the incremental shuttle walking test. Respir Med 106: 243-248.
- da Cunha-Filho IT, Pereira DAG, de Carvalho AMB, Campedeli L, Soares M, et al. (2007) The reliability of walking tests in people with claudication. Am J Phys Med Rehabil 86: 574-582.
- Figueiredo PHS, Lima MMO, Costa HS, Martins JB, Flecha OD, et al. (2018) Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial. PloS one 13: e0200727.
- Dourado VZ, Guerra RLF, Tanni SE, de Oliveira Antunes LC, Godoy I (2013) Valores de referência para o teste de caminhada com carga progressiva em indivÃduos saudáveis: da distância percorrida à s respostas fisiológicas. Jornal Brasileiro de Pneumologia 39: 190-197.
- Portney L, Watkins M (1993) Foundations of clinical research: application to practice. Stamford, USA: Appleton & Lange.
- Fernandes-Andrade AA, Britto RR, Soares DC, Velloso M, Pereira DA (2017) Evaluation of the Glittre-ADL test as an instrument for classifying functional capacity of individuals with cardiovascular diseases. Braz J Phys Ther 21: 321-328.
- McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, et al. (2011) Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol 57: 962-970.
- Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment 6: 284-290.
- Stratford PW (2004) Estimating the standard error of measurement from reliability studies. Physiother Can 56: 27-30.
- Ikizler TA, Pupim LB, Brouillette JR, Levenhagen DK, Farmer K, et al. (2002) Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab 282: E107-E116.
- Carrero, Stenvinkel P (2010) Inflammation in Endâ€Stage Renal Disease-What Have We Learned in 10 Years? Semin Dial 23: 498-509.
- Moreno M, Silva E, Gonçalves M (2005) The effect of proprioceptive neuromuscular facilitation techniques—Kabat Method—on maximum respiratory pressure. Fisioter Mov 18: 53-61.
- Iee Shin H, Kim DK, Seo KM, Kang SH, Lee SY, et al. (2017) Relation between respiratory muscle strength and skeletal muscle mass and hand grip strength in the healthy elderly. Ann Rehabil Med 41: 686-692.
- Parentoni AN, Lustosa LP, Santos KDd, Sá LF, Ferreira FO, et al. (2013) Comparison of respiratory muscle strength between fragility subgroups in community elderly. Fisioterapia e Pesquisa 20: 361-366.
- Kaminski DM, Schaan BDA, da Silva AMV, Soares PP, Plentz RDM, et al. (2011) Inspiratory muscle weakness is associated with autonomic cardiovascular dysfunction in patients with type 2 diabetes mellitus. Clin Auton Res 21: 29-35.
- Reis MS, Arena R, Archiza B, Toledo CF, Catai AM, et al. (2014) Deep breathing heart rate variability is associated with inspiratory muscle weakness in chronic heart failure. Physiother Res Int 19: 16-24.
- Alley DE, Shardell MD, Peters KW, McLean RR, Dam TTL, et al. (2014) Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 69: 559-566.
- Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, et al. (2016) Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 90: 53-66.
- Kaymaz D, Candemir IC, Ergun P, Demir N, Tasdemir F, et al. (2018) Relation between upper-limb muscle strength with exercise capacity, quality of life and dyspnea in patients with severe chronic obstructive pulmonary disease. Clin Respir J 12: 1257-1263.
- Ro HJ, Kim DK, Lee SY, Seo KM, Kang SH, et al. (2015) Relationship Between Respiratory Muscle Strength and Conventional Sarcopenic Indices in Young Adults: A Preliminary Study. Ann Rehabil Med 39: 880-887.
- Segura-Ortà E, MartÃnez-Olmos FJ (2011) Test-retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther 91: 1244-1252.
Citation: Figueiredo PHS, Nominato GA, Santos JM, Lima MMO, Alves FL, et al. (2019) Handgrip Strength is a Reliable Outcome Measure and is Associated with Inspiratory Muscle Strength and Exercise Capacity in Hemodialysis Patients. J Nov Physiother 9:417. DOI: 10.4172/2165-7025.1000417
Copyright: © 2019 Figueiredo PHS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2761
- [From(publication date): 0-2019 - Apr 29, 2025]
- Breakdown by view type
- HTML page views: 1971
- PDF downloads: 790