Research Article Open Access
Growth Performance of Domesticated Prunus africana Population in Muguga, Kiambu County, Kenya
| Nyamai DW1*, Mawia AM1, Wambua FK1, Abdirahman YA1, Osano K1, Lagat R1, Mumbi B1, Arika W1, Juma KK1, Anyango E2, Mulugeta M2, Ngugi MP1, Muchugi A2, Ng’ang’a M1, Burugu MW1and Ramni Jamnadass2 | |
| 1Department of Biochemistry and Biotechnology, School of Pure and Applied Sciences, Kenyatta University, P.O. Box 43844-00100, Nairobi, Kenya | |
| 2Genetics Resource Unit, World Agroforestry Centre, P.O. Box 30677- 00100, Nairobi, Kenya | |
| Corresponding Author : | Nyamai D Wavinya Department of Biochemistry and Biotechnology School of Pure and Applied Sciences, Kenyatta University P.O. Box 43844-00100, Nairobi, Kenya Tel: +254713375320 E-mail: dornyam@gmail.com |
| Received: September 21, 2015; Accepted: October 16, 2015; Published: October 23, 2015 | |
| Citation: Nyamai DW, Mawia AM, Wambua FK, Abdirahman YA, Osano K, et al. (2015) Growth Performance of Domesticated Prunus africana Population in Muguga, Kiambu County, Kenya. Biochem Physiol 4:185. doi: 10.4172/2168- 9652.1000185 | |
| Copyright: © 2015 Nyamai DW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Prunus africana (Hook. f.) is an evergreen tree that grows in African mountains. The species’ bark and bark extracts are used for the treatment of benign prostate hyperplasia. High demand for the bark and bark extracts has led to overexploitation of natural population of the species. P. africana is listed as an endangered species. Conservation of the species can be done through domestication. However, management and growth factors need to be established first to ensure success of on-farm production. The main objective of this study was to evaluate growth characteristics of trees in the domesticated stand at Muguga. Diameter at breast height was measured using a DBH meter, and tree heights were determined using a Suunto meter. The age and ring width of randomly selected trees was measured using TSAPWinTm which was linked to a computer. Height of trees in the domesticated stand at Muguga ranged from 3 meters to 14 meters and diameter at breast height from 0.9 cm to 104.5 cm. Out of the 273 trees in the plantation, 92 (33%) were fruiting at the time of data collection. The age of trees randomly selected from Muguga population was negatively correlated to growth rate (r=-0.0223, p<0.05). There was a non-significant positive correlation between number of cross section growth rings of the selected trees and the diameter at breast height of the trunk (r2=0.0858, r=0.293, p>0.05). The wood density samples from Muguga population was positively correlated to the tree growth rate (r=0.167). Wood density of cross-section samples from Muguga population showed a non-significant negative correlation to the tree diameter at breast height (r=-0.140, p>0.05). The morphological data has important implications in drawing strategies for sustainable harvesting, management and conservation of this species.
| Keywords |
| Diameter at breast height; Afromontane; Prunus africana; Cross dating |
| Introduction |
| Prunus africana is an evergreen tree with a height of more than 40 meters and a stem diameter of up to 1 meter [1]. It has shining foliage, blackish-brown bark and greenish or white flowers. P. africana is a geographically widespread species restricted to underlying islands like Sao Tome’ and Madagascar and Afromontane forest islands in Africa (Somalia, South Africa, Kenya, Tanzania, Sudan, Uganda, Malawi, Ethiopia, Zaire, Cameroon, Zimbabwe, Angola and Malawi) which are above 1500 meters altitude [2]. The species grows in the humid midlands and in humid and semi-humid highlands. In Kenya it grows in the slopes of Mt. Elgon, Mt. Kenya, Cherangani Hills, Tugen Hills, Aberdares Range, Mau range and Kakamega, Nandi and Timboroa forests [1]. It is fairly fast growing with a mean annual height increment of 0.6 to 0.8 meters [3]. Populations in the equatorial regions have been reported to have a mean height growth of 1 to 1.9 meters for planted seedlings [4]. |
| In the Equatorial zone, there is no specific flowering season and some individuals of the species flower almost every month of the year [5]. In regions north of 5ºN, flowering takes place as from November to January when the temperatures are low and there is no much rainfall [5]. In regions South of 5ºN, flowering takes place from April to October when the conditions are cool and dry. Fruiting of the species occurs 2-3 months after flowering and is associated with rainfall. In the Northern zones, fruiting takes place in the relatively wet months. In the southern regions, fruiting occurs when the rains are starting or in the second half of the dry season [6]. |
| P. africana tree is used for timber and also in traditional and modern medicine in Africa. In traditional medicine, the bark and leaves are used for treatment of stomachache, fever, chest pains and dressing of wounds [7]. In modern medicine, P. africana bark is used for the production of herbal preparations for treatment of benign prostate hyperplasia [8]. The high demand of the bark extracts of P. africana has caused serious damage to the wild population [8]. More than 3000 tons of bark or bark extracts are exported to Europe per year for the production of herbal preparations for BPH treatment [8]. This high demand causes devastating effect to the wild population of P. africana which is the main source of the bark. Attempts at cultivation of P. africana are underway in Kenya and other countries. The bark of P. africana can regenerate if bark removal does not interfere with the vascular cambium and thus harvesting is sustainable [9]. |
| Despite the resilience of P. africana to debarking, in dry areas, bark re-growth is limited and large scale debarking stresses the tree even when complete re-growth occurs. Limited distribution of the species only in the afromontane islands and increased clearing for agriculture increases the threat for P. africana. The species was included as endangered species in Appendix II of the Convention of International Trade in Endangered Species (CITES) at the ninth conference due to the increasing international demand [10]. The species has also been assigned a vulnerable conservation status on the IUCN Red List. Import of the bark from Cameroon into the European Union was banned from November 2007 to December 2010 when CITES declared the ban lifted but with reduced quota of 150,000 kg for 2010 and 2011. In some African countries, policies have been established aiming at ensuring sustainable management of forests that contain P. africana species. |
| However, control problems and enforcement issues persist and there is need to identify and implement sustainable management options including conservation and domestication measures. In order to optimize sustainable conservation of the vulnerable African cherry, knowledge of the regional variation is essential. World Agroforestry Centre established a P. africana stand at Muguga for determination of the growth performance. The seeds for this stand were obtained from a wild stand at Kobujoi, Nandi. The aim of this study was to evaluate the growth performance of randomly selected trees of P. africana from Muguga. The information from this study showed no significance difference in these characteristics and thus will help in sustainable management of the domesticated species. The data will also act as a guide on the right time of bark harvesting and the conservation strategies of on-farm P. africana. |
| Materials and Methods |
| Study site |
| The Prunus africana stand was established by the World Agroforestry Centre at Muguga, Kenya. Muguga Regional Research Centre is situated at Kiambu County, 1º14’ S, 36º38’ E. Muguga is located approximately 2150 meters above sea level and has an average annual rainfall of 1200 mm. |
| Sample collection |
| Morphological characteristics evaluated included stem shape, diameter at breast height (DBH), tree heights and fruition condition. Stem shape was given as a score of one to five while tree heights were determined and recorded in meters. DBH is the diameter of a tree at a height between 1.3 and 2 meters. The assessment of the domesticated P. africana growth rate involved the measurement of the diameter at breast height and tree height. Tree age was determined using the ring method where ring widths were measured using TSAP-WinTm. The diameter at breast height (DBH) was determined using a DBH meter and recorded in centimeters. In this study the DBH was taken at a height of 1.3 meters above the ground. The height of individual trees was determined using a Suunto optical height meter. Morphological data was grouped based on tree heights. The assumption was that the trees were planted at the same time and thus were of same age. |
| Preparation of tree rings for age determination |
| Tree ring samples were collected by cutting cross sections from trees that were fell during thinning of Muguga population. Cross sections were obtained at a height of 1.3 meters using a chain saw and all ring samples were labeled. A height of 1.3 meters was used to maximize the estimation of tree age as the number of rings decrease with increase in height. The samples were dried and an orbital power sander was used to prepare each sample surface and to buff out the scratches. Hand sanding of the surfaces was then done using progressively finer sand paper (80, 120, 180, 240 and 360 grit) to increase visibility of the growth rings. The rings were counted from the outermost to the innermost and each ring boundary was marked. Ring width was visualized using a binocular microscope and measured using TSAP-WinTm which was linked to a computer. Samples were continuously adjusted to ensure ring width was measured perpendicularly to avoid under or over estimation of ring width. The ring width measurements were taken along two randomly selected radii. |
| Visual cross dating |
| Cross dating is the process or activity of establishing the exact year in which a ring was formed [11,12]. Correct cross dating indicates the effect of external factors like environmental factors on the growth pattern of trees [13]. Visual cross dating of samples was done by matching patterns of narrow and wide rings using the skeleton plot method [11]. One vertical line represents one ring on the skeleton plotr and each ring was assigned a value on a scale of 1-10 based on the width previously marked. Each ring was compared to the neighboring ring and the narrowest rings which mainly indicate the dry season were assigned high values close to 10. The wider ring was marked with a B and rings with average width were not scored [11]. The patterns of the wide and narrow widths were aligned in order to get the composite skeleton plot that allowed dating the rings to the exact year they were formed [11]. After ring width measurement and visual cross dating, ring width data was then stored in the computer in a raw image format ready for analysis. |
| Determination of tree density |
| Samples for the density determination were obtained from cross sections cut at the DBH of randomly selected trees at Muguga. The fresh weight of each sample was measured using an electronic balance. The wet volume was determined by use of the displacement method with water as the liquid. The samples were then dried in an oven at 100°C for 24 hours and the dry weight and volume determined immediately. The density of the samples was then determined using the dry weight and dry volume using Archimedes principle. |
| Statistical analysis |
| The ring width data was analyzed using COFECHA software to help identify the problem segments and verify cross-dating before tree growth curves were combined into a chronology [14]. The data collected for Muguga domesticated population was analyzed using Minitab version 17.0 software. Means for the morphological parameters for Muguga population were calculated to establish an overview of relationships. The means were separated using Tukey’s studentized honestly significant difference at 5% level of significance. Intercorrelations of different parameters and correlation with tree heights were also calculated. ANOVA was used to test for parameter differences between the groups in Muguga population. A Pearson’s correlation analysis was carried out to determine the relationship between tree ages and their growth rates, wood density and DBH and ring number and DBH. |
| Results |
| Morphological characterization of Prunus africana stand trees at Muguga |
| For this stand, morphological data was grouped based on tree heights and analyzed as shown in Table 1. It was assumed that the trees were planted at the same time and thus were of the same age. Two trees in the population had heights below five and half meters tall. There were 146 trees with a height of between 5.6 to 10.5 meters. The third group had 124 trees which had a height range of 11.6 to 15 meters tall. The mean height of group one were significantly different from the other groups in Muguga stand (p<0.05). There was no significant difference in the stem shapes among the three groups. The mean DBH of the three groups was positively correlated to the mean height of the groups. The tree diameter increased with increase in tree height. DBH can be used as a measure of growth rate and so is height. |
| The mean DBH of the three groups ranged from 9.45 to 54.85 centimeters. Group one had significantly lower mean diameter at breast height compared to the other two groups which showed no significance difference in diameter size (p<0.05). Fruition condition was expressed as percentages of the total number of trees in a particular group. The two trees in group one was not in the fruition stage. Out of the 146 trees in group 2, 40 (27.4%) of them were in the fruiting stage while 106 (72.6%) trees in this group had no fruits. In group 3 41.9% of the 124 trees in this group were in the fruiting period. |
| Visual and statistical cross dating |
| In this study visual cross dating was first done using the skeleton plot method [11]. The cross dating process was important as it enables one detect presence of false or missing rings in each tree cross section. The process was carried out using two radii on the cross sections to enable detection of wedging rings. Fifteen out of twenty three trees were successfully cross-dated and their age ranged from seventeen to twenty six years. |
| Relationship between age and growth rate in Muguga population |
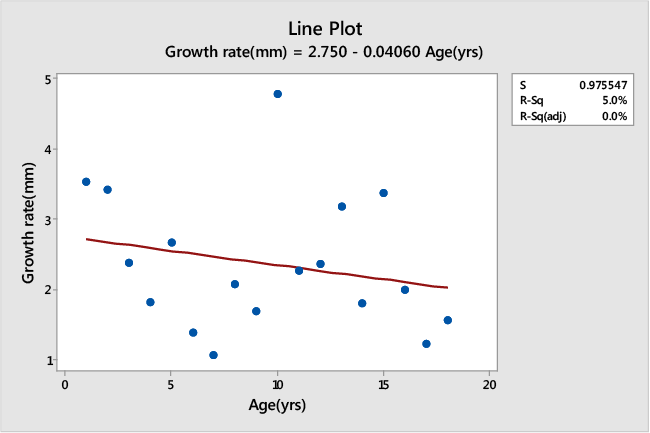
| The age and growth rate in millimeters of seven randomly selected trees from Muguga is tabulated in Table 2. A Pearson’s correlation analysis for age and average growth rate of trees randomly selected from Muguga population showed a non-significant relationship (t=-0.916, DF=16, r2=0.0498, p>0.05). In addition, age was negatively correlated to the growth rate (r=-0.223), thus as tree age increases the growth rate decreases (Figure 1). |
| Relationship between ring number and diameter at breast height of trees from Muguga population |
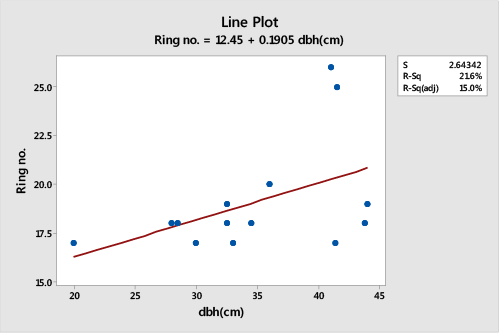
| A regression and Pearson’s correlation analysis of trunk cross section ring number and DBH of randomly selected tress from Muguga population showed a non-significant relationship (DF=14, r2=0.0858, p>0.05), (Figure 2). The number of growth rings in the trunk cross section of the selected trees were positively correlated to the DBH of the tree (r=0.293). The number of rings increased with increase in diameter size. |
| Relationship between wood density and DBH of trees from Muguga population |
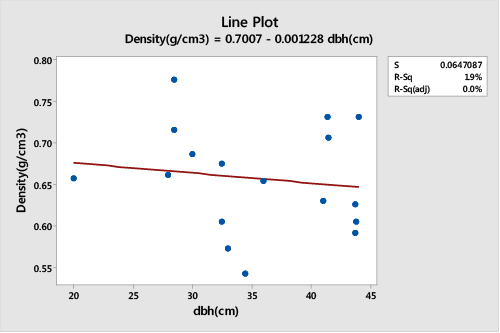
| A Pearson correlation and regression analysis of the wood sample density and DBH of trees randomly selected from Muguga population showed a non-significant relationship (DF=16, r2=0.0196, p>0.05), (Figure 3). The density of wood sections samples from Muguga population was negatively correlated to the tree DBH thus trees with larger diameter had lower densities (r=-0.140). |
| Relationship between wood density (g/cm3) and growth rate (mm) |
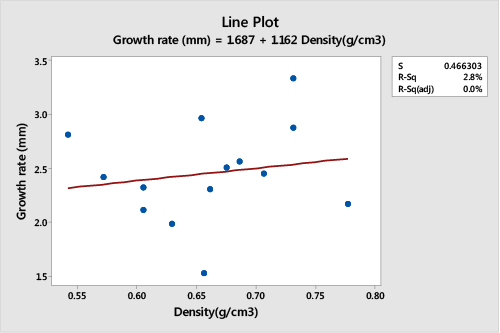
| A Pearson correlation and regression analysis of the wood sample density and growth rate of trees randomly selected from Muguga population showed a non-significant relationship (DF=14, r2=0.0279, p>0.05), (Figure 4). The density of wood sections samples from Muguga population was positively correlated to the tree growth rate (r=0.167). Wood density of trees in this stand was shown to increase with increase in average tree growth rate. |
| Discussion |
| The differences in height and DBH among trees in Muguga P. africana stand was probably due to differences in shading periods within the population. It was also observed that trees that were in the eastern side of the plantation were shorter than trees on the opposite side. Competition for sunlight for the trees away from the sunlight may explain this difference in heights. Light is an environmental factor that influences plant growth as it is a crucial requirement for photosynthesis [15,16]. Light gradients can differ both within plant canopies and within the crowns of individual plants thus, all plants are exposed to some degree of shade in their lifetime. High or low sunlight can limit growth characteristics of a plant but sunlight must be present for photosynthesis to take place [17]. |
| In the Equatorial region, P. africana species has no specific flowering and some individuals of the species flower almost every month of the year [5]. This wide range of flowering period leads to different fruiting conditions observed in the current study. Fruiting of the species occurs 2-3 months after flowering and is associated with rainfall. Growth rate of plant species over short time periods do not strongly correlate with single environmental factors indicating that the growth rates are determined by complex environment-plant interactions [18]. Age was negatively correlated to the average growth rate of randomly selected P. africana individuals in Muguga stand. This observation was also recorded in Pinus contorta and Pinus ponderosa [19]. This negative relationship between age and growth rate can be attributed to reduced rate of photosynthesis as age of the species increases [19]. |
| Change in seasons lead to variation of cambial activity and thus differences in growth ring widths and other phonological patterns of trees. There are no clear seasons in the tropics [20], and this may lead to indistinct ring boundaries, however dendrochronology techniques have been used to identify ring boundaries and to determine tree ages. Variation or ring width is due to changes in seasons. Narrow ring indicate the dry seasons while wide rings are an indication of rainy seasons [21,22]. P. africana does not interrupt the dormancy of cambium activity especially in the short rains but it reactivates cambium activity when the long rains begin [23]. Increase in ring width may occur even at periods with no precipitation as reported in Podocarpus falcatus mainly due to secondary cell enlargement and thickening even when no cambium activity takes place [24]. |
| Wood density is used to describe wood quality especially dimensional stability, workability, and mechanical timber properties. Wood density was negatively correlated to the DBH of trees. This negative relationship was also reported in Picea mariana species [25]. There was a positive relationship between growth ring number and DBH of tree at Muguga population. This can be attributed to the increase in cambium activity with increase in age. In this study, wood density was positively correlated to growth rate. Previous studies show that there is no relationship between wood density and growth rate of diffuse-porous hardwoods [26,27]. The same observation was reported in Betula pendula and Prunus serotine [28,29]. Some work on Populus species reported a weak negative correlation between growth rate and wood density [30,31] while some studies showed absence of correlation of the two parameters [32,33]. |
| Conclusion |
| Shading periods may have an effect on the growth rate of P. africana hence variation in tree height and DBH of trees growing in same habitat. P. africana species had varied flowering periods even for individuals growing in the same habitat leading to difference in fruiting seasons. The growth rate of P. africana is negatively correlated to age. From the study, I conclude that P. africana wood density is negatively correlated to the diameter at breast height but positively correlated to growth rate (mm). |
| Acknowledgements |
| My gratitude goes to my research supervisors Dr. Marion Burugu, Dr. Margaret Ng’ang’a and Dr. Alice Muchugi for their guidance, encouragement and scholarly suggestions throughout the research. |
| My heartfelt thanks goes to the World Agroforestry Centre- Genetics Resource Unit for funding the project and to International Centre for Insect Physiology and Ecology through Prof. Baldwyn Torto for allowing me to use their facilities for this research. My gratitude goes to Kenya Forestry Research Institute more-so the Seed Center for providing me with facilities to carry out my field work. |
| Special thanks to all the staff of Biochemistry and Biotechnology Department, Kenyatta University for their support and cooperation during this research. |
| My special thanks goes to my friends especially Robert Kariba, Samuel Manthi, Xavier Cheseto and Agnes Were for their support towards the completion of this research. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15451
- [From(publication date):
December-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10843
- PDF downloads : 4608
