Research Article Open Access
Green Tea as Biological System for the Synthesis of Silver Nanoparticles
Walaa Fikry Elbossaty*Department of Chemistry, Biochemistry Division, Faculty of Science, Damietta University, Damietta, Egypt
- Corresponding Author:
- Walaa Fikry E
Department of Chemistry
Biochemistry Division, Faculty of Science
Damietta University, Damietta, Egypt
Tel: 011- 23239056
Fax: 011-23210549
E-mail: walaafikry1985@gmail.com
Received date: July 06, 2017; Accepted date: August 21, 2017; Published date: August 28, 2017
Citation: Elbossaty WF (2017) Green Tea as Biological System for the Synthesis of Silver Nanoparticles. J Biotechnol Biomater 7:269. doi:10.4172/2155-952X.1000269
Copyright: © 2017 Elbossaty WF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Silver nanoparticles (Ag-NP) are being used increasingly in wound dressings, catheters and various household products due to their antimicrobial activity. Ag-NP synthesized by different methods, one of them biological method, in which extract from plant can used as reducing agent in synthesis of metal nanoparticles. Synthesis of Ag-NP in fresh green tea extract has been carried out in this work as an environment friendly approach. Ag-NP was obtained by the reduction of Ag+ ions through the action of green tea infusions. Scanning Electron Microscope (SEM) was used for the determination of the average diameter of the Ag-NP which were found of 20 nm in green tea infusion .The growth of nanoparticles is monitored by UV-VIS spectrophotometer and complemented with characterization using Transmission Electron Microscopy and Fourier Transform Infrared Spectroscopy.
Keywords
Green tea extract; Silver nanoparticles; Ag-NP; UV-Vis; Scanning electron microscopy; Transmission electron microscopy
Introduction
Nanoparticles are used in various bio applications included therapeutics, antimicrobial agents, transfection Vectors, and fluorescent labels. The nanoparticles synthesized through chemical route cannot be considered as environment friendly because of their hazardous effect on human health. But recently, the fabrication of nanoparticles through green route has pulled the attention among researchers because of its ecofriendly nature [1-4].
Silver nanoparticles are also extensively studied because of their unique properties such as excellent electrical and thermal conductivity, chemical stability, catalytic activity, non-linear optical behavior and antimicrobial effects. There were different methods in preparation of silver nanoparticles, one of them biological methods [5].
Green chemistry is the design, development, and implementation of chemical products and processes to reduce or eliminate the use and generation of substances that are hazardous to human health and to the environment. Particularly innovative and under great expansion is the topic of green synthesis of Ag-NP using readily available reducing agents and capping agents taken from plants and hence from renewable sources [6].
The use of plant or plant extracts has advantage over the use of microorganisms in synthesized of Ag-NP. The interest on Ag-NP has its roots on the antibacterial and antiviral properties of colloidal silver which is also effective against fungi and protozoa. Ag-NP has gained much popularity recently owing to the broad spectrum of antimicrobial activity [7], Silver impregnated catheters [8] and wound dressings are used in therapeutic applications [9].
Green tea has been the most consumed beverage next to water for many centuries in Asian countries. The beneficial effects of green tea are well known including cancer preventive activity and other medical applications [10].
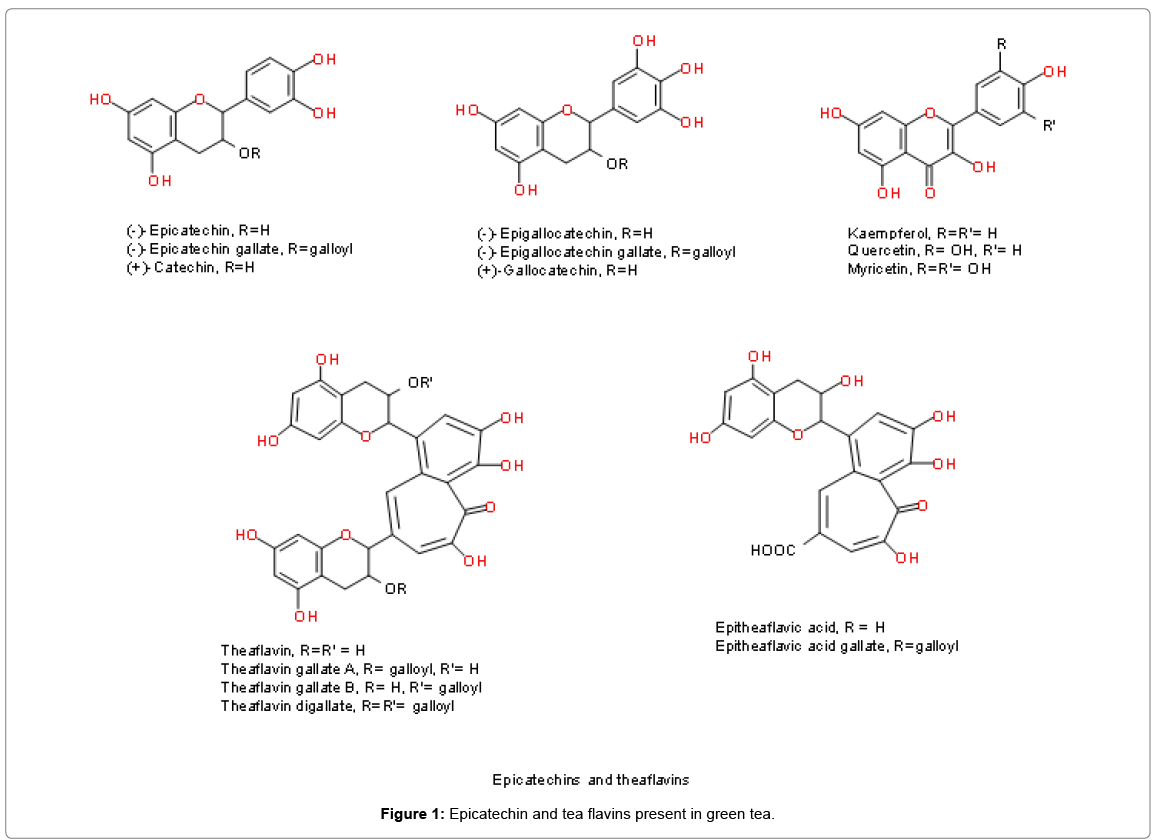
The chemical composition of green tea is complex and incompletely defined. The most abundant components in green tea are polyphenols, in particular flavonoids such as the catechins, catechin gallates and proanthocyanidins. The fresh leaves contain caffeine (approximately 3.5% of the total dry weight, or about 50 mg/cup when brewed), the bromine (0.15-0.2%), theophylline (0.02-0.04%) and other methyl xanthenes, lignin (6.5%), organic acids (1.5%), chlorophyll (0.5%) and free amino acids (1-5.5%), in addition to the unique amino acid theanine (4%); numerous ‘flavor compounds’ are also present in much lower amounts. Many of the biological properties of green tea have been ascribed to the catechin fraction, which constitutes up to 30% of the dry leaf weight (Figure 1) [11].
These potent antioxidants comprise free catechins such as (+)-catechin, (+)-gallocatechin, (−)-epicatechin (EC) and (−)-epigallocatechin (EGC), and the galloyl catechins such as (−)-epicatechin gallate (ECG), (−)-epigallocatechin gallate (EGCg), (−)-catechin gallate (Cg) and (−)-gallocatechin gallate (GCg). EGCg is the most abundant of these, comprising about 50% of the catechin pool; EGC accounts for around 20%, ECG 13% and EC 6% [12].
In this work the preparation of Ag-NP from aqueous green tea extract as one-pot method in which no surfactant, capping agent and/or template were used. The aim of this study preparing the biological silver nanoparticles by using natural product compound, this help in synthesis product ecofriendly and can be used in different fields.
Materials and Methods
Materials
Silver Nitrate (AgNO3) was obtained from Sigma Aldrich and Green tea was collected from the local market of our institute.
Plant extract
1.3 g of green tea bag was left for 20 min in boiling water to get extract. The extract was cooled at room temperature and then filtered by using Whattman filter paper No. 1 (Figure 2).
Silver nitrate solution
Silver nitrate aqueous solution of 1 mM was prepared by dissolving appropriate amount of silver nitrate salt in suitable amount of distilled water and stored in amber color bottle.
Synthesis of silver nanoparticles
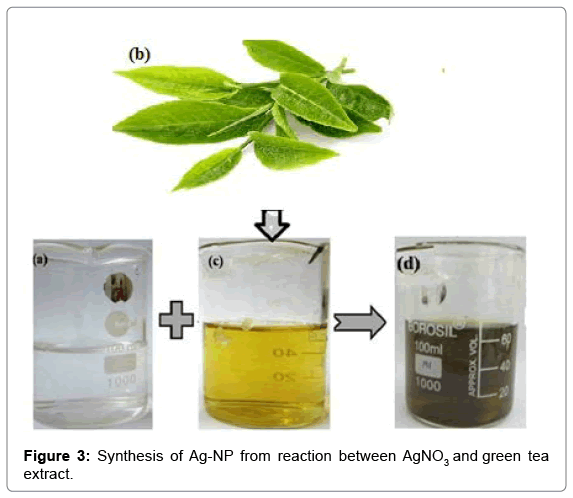
60 ml of green tea extract were taken with a pipette and diluted to 500 ml in a volumetric flask and hand-shaken to homogenize the dilution. The pH of the resulting solution was 6 and was make alkaline by adding a very small portion of potassium carbonate (K2CO3) until a pH=10 was reached. Then 20 ml of the AgNO3 stock solution were added in a single shot (Figure 3).
Characterization
The synthesized silver nanoparticles were characterized by using different techniques including change in color, UV-Visible Spectroscopy, Scanning Electron Microscopy (SEM), Transmission electron microscope (TEM) and Infrared spectroscopy analysis.
Formation of silver nanoparticles: The color change was noted by visual observation in the Erlenmeyer flask which contains AgNO3 solution with green tea extract.
UV-Visible spectroscopy: The reduction of silver ions in the colloidal solution was confirmed by UV-Visible spectroscopy. A small aliquot from Ag NPs was taken in a quartz cuvette and observed for wavelength scanning between 200 to 900 nm with distilled water as a reference. The UV-Vis absorption spectrum of the sample was performed in Perkin Elmer Spectrophotometer, at different time 5, 10, 15, 20, 25 min after addition of green tea extract on AgNO3 solution.
Scanning electron microscopy: Surface morphology of silver nanoparticles was detected by scanning electron microscopy (SEM). The sample was prepared by centrifuging colloidal solution after 6 h of reaction at 14,000 rpm for 4 min. The pellet was redispersed in deionized water and again centrifuged. The process was repeated three times and finally washed with acetone. The purified silver nanoparticles were sonicated for 10 min for making the suspension. Thin films of the sample were prepared by dropping a very small amount of the sample on glass plates and then allowed to dry at room temperature.
The sample was kept under lamp until completely dry. The prepared sample was subjected to SEM analysis by using (SEM) with Zeiss 700 Scanning Electron Microscope (SEM).
Transmission electron microscopy: Shape and size distribution of the synthesized Ag nanoparticles were characterized by transmission electron microscopic (TEM) study. Few drops of Ag nanoparticle solution were dropped onto a TEM grid, and the residue was removed by a filter paper beneath the TEM grid. The prepared sample was subjected to TEM analysis was performed by using a JEOL JEM-2100F (JEOL, Tokyo, Japan) instrument operating at 120 kV, which has a diffraction patterns recorded at selected areas to determine the particle structure and size of silver nanoparticles at a 660 mm camera length.
FTIR spectroscopy analysis: For the Fourier transform infrared (FTIR) spectroscopy analysis, the residual solution was centrifuged at 10,000 rpm for 15 min. To remove any free biomass residue and the resulting suspension was redispersed in 1 mL sterile distilled water. There after the purified suspension was freeze dried to obtain dried powder. Dried Ag NPs were mixed with potassium bromide (KBr) and the spectra were recorded with a Perkin Elmer Spectrum (Perkin Elmer, Germany).
Results
Effects of addition of green tea extract on synthesis of silver nanoparticles
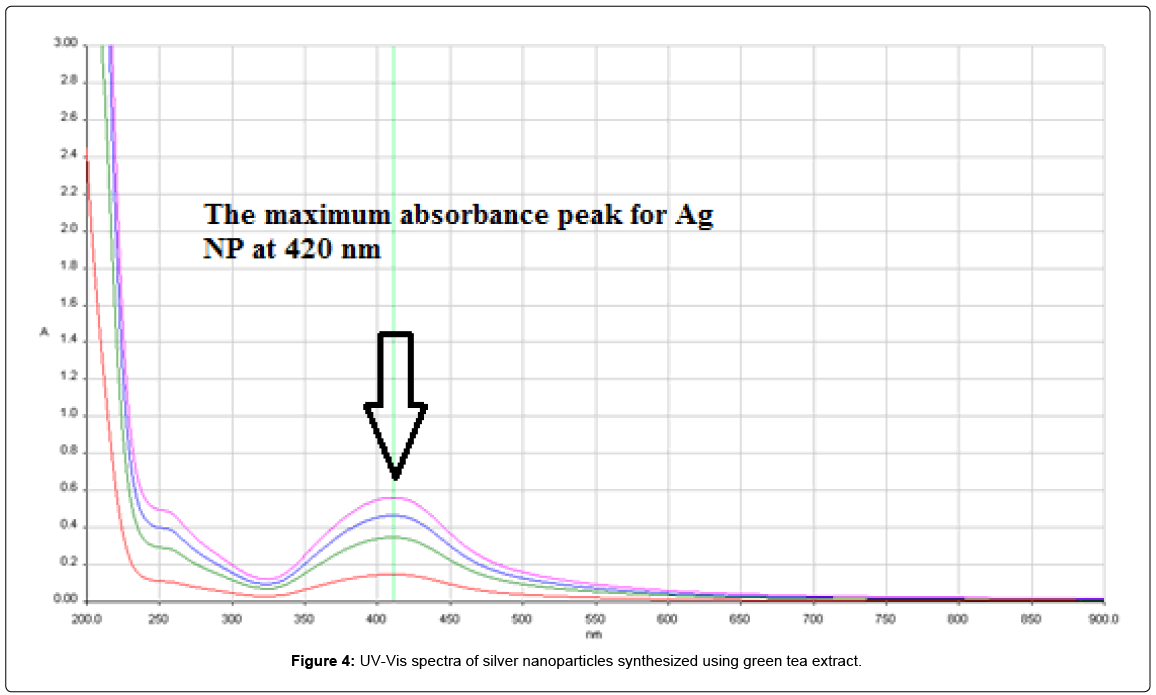
After the addition of the green tea extract to the aqueous AgNO3 solution, light yellow brown colors slowly appeared in the mixture, indicating the formation of Ag-NP and finally turn into dark brown color Figure 3. The characterization absorption spectra was the important properties of the Ag NPs, and the UV-Vis spectra was a good method for characterization of the formation and growth of Ag NPs. Absorption peaks of silver nanoparticles synthesized using green tea extract solutions were obtained at wavelength of 420 nm (Figure 4).
Scanning electron microscopy investigation
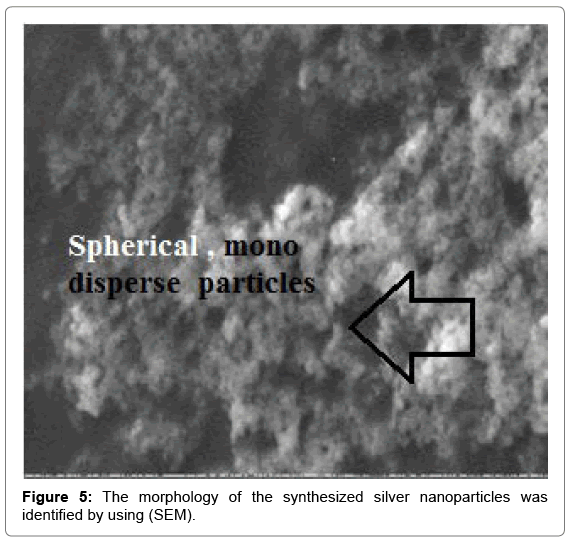
Scanning electron microscopy (SEM) was used to investigate the morphology and size of the synthesized Ag-NP. The Ag NPs obtained with the green tea reduction method were predominantly spherical with diameters 20 nm. This image further indicates that the silver nanoparticles are not aggregated i.e., mono disperse in nature due to repulsion forces (Figure 5).
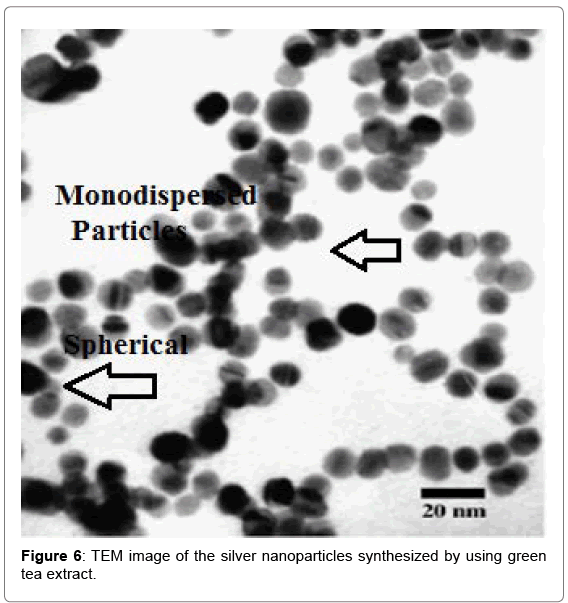
Transmission electron microscopy
Shape and size distribution of the synthesized Ag-NP were characterized by transmission electron microscopic (TEM) study. The particles are predominantly spherical shape with smooth surface morphology and highly monodispersed with an average diameter of 20 nm (Figure 6).
FTIR spectroscopy characteristics
FTIR measurement was carried out to identify the possible biomolecules responsible for capping and reducing agent for the Ag-NP synthesized by green tea extract. Capping of green tea on silver nanoparticle has been examined by FTIR spectroscopy.
The FT-IR absorption spectra of the samples were shown in Figure 7 with resolution of 4 cm-1, which was performed in Spectrum BX series. The peak at 3424 cm-1 of the FTIR spectra contains O-H stretching in amides, the peak around 2931-2925 cm-1 due to C–H stretching in alkanes, peak around 1390-1384 cm-1 characteristic of hydroxyl groups at phenolic hydroxyl, the peak at 1051-1031 cm-1 due to C-stretching as ether groups.
The FT-IR analysis indicated the involvement of amides, carboxyl, amino groups and poly phenols in the synthesized Ag-NP. The organic compounds in tea extract could attribute to the reduction of AgNO3 and the stabilization of Ag-NP by the surface bound by the organic compounds. The medium intense band at 1617 cm-1 arises from the C=O stretching mode in amine I group which is commonly found in the protein.
Discussion
The simple and viable technique in photosynthetic method became an alternative to chemical and physical methods. Such consistent and environmental friendly technologies help to boost the synthesis and application of nanoparticles which are good for. In green synthesis, it was believed that the plant extract acts as reducing and stabilizing agent for the production of metal nanoparticles [1].
Green silver nanoparticles have been synthesized using various natural products like green tea (Camellia sinensis) [13], starch [14], lemongrass leaves extract, leguminous shrub (Sesbania drummondii) [15].
Silver nanoparticles can be prepared in a size range of 10-40 nm with fewer amounts of leaf extract and without adding chemicals and physical steps like centrifugation, sonication, annealing [2]
In this work, we described a simple one step method for the synthesis of Ag-NP by the reduction of aqueous silver ions using green tea extract at room temperature without using any additive protecting the silver nanoparticles from aggregation. Green tea capped silver nanoparticles have been prepared through the reduction of silver ions. When the aqueous extract was mixed with silver nitrate solution its color started to change. It was previously known that Ag- NP in aqueous solution exhibit dark brown color. The appearance of a yellowish-brown color in the reaction vessels suggested the formation of silver nanoparticles [16].
A change in color occurred because of excitation in surface Plasmon resonance wherein, it can be an indication of the formation of Ag-NP [14]. The UV-Vis spectra showed maximum absorbance at 420 nm, which increased with time of incubation of silver nitrate with the plants extract. The observation indicated that the reduction of the Ag+ ions took place extracellular. It was reported earlier that absorbance at around 430 nm for silver is a characteristic of these noble metal particles [17].
SEM determination of the brown color stable samples showed the formation of silver nanoparticles and well dispersed nanoparticles could be seen in the samples treated with silver nitrate. One can understand that since the silver colloidal particles possessed a negative charge due to the adsorbed ions, a repulsive force worked along particles and prevented aggregation.
Many researchers have stated the biosynthesis of nanoparticles with plants extract for biosynthesis reaction. Synthesis of spherical silver nanoparticles using purified compound, extracted from henna leaf at ambient conditions [18].
Using green tea, C. sinensis extract as reducing and stabilizing agents gold nanoparticles and silver nanostructures could be produced in aqueous solution at ambient conditions [17].
Conclusion
We have proposed an ecofriendly approach for the synthesis of silver nanoparticles using green tea extracts. This simple, efficient and rapid green synthesis of Ag-NP can be used in various biomedical and biotechnological applications. The result showed that green tea extract can be used AgNP synthesis. The formation of nanoparticles is confirmed by UV-Vis spectroscopy, while crystalline nature of the nanoparticles and average particle size is determined by SEM and TEM show clearly the formation of nanoparticles without any aggregation Silver nanoparticles were synthesized by applying environmentally safe method which minimizes the addition of hazardous wastes in the environment. The synthesized nanoparticles were spherical, 20 nm in size, crystal in nature and showed absorption spectrum at 420 nm characterized by using different techniques. The current study could be a helpful contribution to the following fields: health, environment, energy, information technology and cosmetics. Moreover, silver nanoparticles may be proven safer in medical field as board spectrum antibiotics because of their non-resistant nature in comparison to conventional antibiotics.
References
- Seyed H, Masoud D (2014) Crystalography, morphology and optical properties of silver nanoparticles synthesized by extract of black pepper as a green method. Optics 3: 19-23.
- Yoon K, Hoon B, Park J, Hwang J (2007) Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373: 572-575.
- Tan W, Jiang S, Zhang Y (2007) Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 28: 1565-1571.
- Su J, Zhang J, Liu L, Huang Y, Mason R (2008) Exploring feasibility of multicolored cdte quantum dots for in vitro and in vivo fluorescent imaging. J Nanosci Nanotechnol 8: 1174-1177.
- Soo-Hwan K, Lee HS, Ryo DS, Choi SJ, Lee DS (2011) Antibacterial activity of silver-nanoparticles against Staphylococcus aurous and Escherichia coli. Korean J Microbial.Biotechnol 39: 77-85.
- Zhao D, Liub T, Park JG, Zhang M, Chen JM, et al. (2012) Conductivity enhancement of aerosol-jet printed electronics by silver nanoparticles ink with carbon nanotubes. Microelectron Eng 96: 71-75.
- Lok CN, Ho CM, Chen R, He QY, Yu WY, et al. (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5: 916-924.
- Samuel U, Guggenbichler J (2004) Prevention of catheter related infections: The potential of a new nano-silver impregnated catheter. Int J Antimicrobial Agents 23: S75-S78.
- Chen J, Han CM, Lin XW, Tang ZJ, Su SJ (2006) Effect of silver nanoparticle dressing on second degree burn wound. Zhonghua Wai Ke Za Zhi 44: 50-52.
- Zaveri NT (2006) Green tea and its polyphenolic catechins: Medicinal uses in cancer and non-cancer applications. Life Sci 78: 2073-2080.
- Graham HN (1992) Green tea composition, consumption and polyphenol chemistry. Prev Med 21: 334-350.
- Hui H (1992) Encyclopedia of Food Science and Technology, Wiley, New York.
- Vilchis-Nestor A, Sanchez-Mendieta V, Camacho-Lopez M (2008) Solvent less synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett 62: 3103-3105.
- Vigneshwaran N, Ashtaputre N, Varadarajan P, Nachane R, Paralikar K (2007) Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 61: 1413-1418.
- Sharma N, Sahi S, Nath S, Parsons J, Gardea-Torresdey J (2007) Synthesis of plant mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol 41: 5137-5142.
- Ahmad P, Mukherjee S, Senapati D, Mandal M, Khan R (2002) Extracellular biosynthesis of silver nanoparticles using the Fungus Fusarium oxysporum. Colloids Surf B 28: 313-318.
- Nestor A, Mendieta V, Lopez M, Espinosa R, Lopez M (2008) Solvent less synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett 62: 3103-3105.
- Kasthuri J, Veerapandian S, Rajendiran N (2009) Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces 68: 55-60.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 8894
- [From(publication date):
September-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 7387
- PDF downloads : 1507