Research Article Open Access
Green Synthesis of Zinc Oxide Nanoparticles Using Parthenium hysterophorus Leaf Extract and Evaluation of their Antibacterial Properties
Arinjoy Datta*, Chandi Patra, Himani Bharadwaj, Sukhjeet Kaur, Neha Dimri and Robinka KhajuriaDepartment of Biotechnology, Lovely Professional University, Phagwara, Punjab 144411, India
- *Corresponding Author:
- Datta A
Department of Biotechnology
Lovely Professional University
Jalandhar-Delhi GT Road (NH-1) Phagwara
Phagwara, Punjab 144411, India
Tel: +7061729181
E-mail: arinjoy07@hotmail.com
Received date: August 15, 2017; Accepted date: September 10, 2017; Published date: September 17, 2017
Citation: Datta A, Patra C, Bharadwaj H, Kaur S, Dimri N, et al. (2017) Green Synthesis of Zinc Oxide Nanoparticles Using Parthenium hysterophorus Leaf Extract and Evaluation of their Antibacterial Properties. J Biotechnol Biomater 7:271. doi:10.4172/2155-952X.1000271
Copyright: © 2017 Datta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Parthenium hysterophorus an annual herbaceous weed, is known for its vigorous growth and as a causative agent for contact dermatitis, hay fever and diarrhea in humans and systemic toxicity in livestock. However, in spite of all the problems associated with this weed, it has been reported to have pharmacological activities. The objective of this study is to evaluate the potential of Parthenium hysterophorus leaf extracts for the synthesis of zinc oxide nanoparticles and their antimicrobial properties. Nanoparticles were extracted with the help of aqueous, methanolic and ethanolic solutions of P. hysterophorus leaves. Since a higher yield of nanoparticles was observed when synthesised using aqueous extract therefore these were used in further experiments. Synthesised nanoparticles were characterised using UV-Vis spectroscopy with maximum absorbance peak at 400 nm. SEM and TEM analysis revealed that the particles were spherical and cylindrical in shape with average particle size ranging from 16-45 nm. The chemical group association and elemental composition of nanoparticles was analysed using FTIR and SEM-EDX. Green synthesized zinc oxide nanoparticles were evaluated for antimicrobial activities against Gram positive and Gram negative bacteria. Zinc nanoparticles exhibited maximum zone of inhibition against Enterobacter aerogenes (36 mm) while least activity was seen against Staphylococcus aureus and Bacillus subtilis.
Keywords
Enterobacter aerogenes, Leaf extract, Parthenium hysterophorus, SEM-EDX, Zone of inhibition
Introduction
Parthenium hysterophorus (Family Asteraceae), commonly known as Congress grassis an aggressive ubiquitous annual herbaceous weed known for its vigorous growth especially in warmer climates.Within the past century it has found its way to Africa, Australia, Asia and Pacific Islands and has now become one of the world’s seven most devastating and hazardous weeds.Due to its high fecundity, a single plant can produce 10,000 to 15,000 viable seeds and these seeds can disperse and germinate to cover large areas. This invasive weed is widely prevalent in India covering approximately two million hectares of land [1,2].
In addition to its rampant growth characteristics, P. hysterophorus has been reported to cause various health hazards ranging from contact dermatitis, skin inflammation, eczema, asthma, allergic rhinitis, hay fever and diarrhea in humans to systemic toxicity in livestock. It is due to the above mentioned problemsassociated with Parthenium, its management has become necessary. However, in spite of all the problems associated with this weed, it can be explored for its beneficial aspects. It has been reported that the decoction of P. hysterophorus has been used in traditional medicine to treat fever, diarrhea, neurologic disorders, urinary tract infections, dysentery and malaria [3]. It has been found to be a pharmacologically active analgesic in muscular rheumatism, therapeutic for neuralgia and as a vermifuge [4]. Parthenin, the major constituent of the plant exhibits significant medicinal attributes including anticancer property [5]. Another probable application of P. hysterophorus could be its utilization for the green synthesis of nanoparticles, thus promoting this noxious weed into a valuable plant for nanotechnology-based industries in future. In the past few years, nanomaterials and specifically metal nanoparticles, have received particular interest due their diverse applications ranging from material science to biotechnology. Methods for synthesis of nanoparticles can be broadly classified into two categories: chemical and physical methods [6]. The major drawbacks of these methods are that they are very costly, labour intensive and time consuming. Furthermore, large quantities of secondary waste are generated resulting from the addition of chemical agents for precipitation and reduction in the processes [7]. Because of these shortcomings, biosynthetic methods employing either microorganisms or plant extracts are rapidly emerging as a simple alternative for nanoparticle synthesis [8]. Due to their amenability to biological functionalization; the naturally synthesized nanoparticles are finding important applications in the field of medicine. The antimicrobial potential of metal based nanoparticles has led to its incorporation in consumer, health-related and industrial products [9].
Various researchers have reported synthesis of silver and gold nanoparticles using different plant materials [10-14]. Though reports are available on the chemical synthesis of Zinc oxide nanoparticles [15- 17] but green synthesis of zinc nanoparticles using plant extracts is an area which has not been explored much yet. Due to the large surface area and high catalytic activity of zinc oxide nanoparticles they are used in many catalytic processes [17]. Various applications of zinc nanoparticles include optoelectronics, nanolasers, solar cells, gas sensors, coating and paints and health related applications [18]. In addition to these applications, studies have demonstrated that zinc oxide nanoparticles have wide range of antibacterial effect on microorganisms which depends upon the size and the presence of visible light [19]. The objective of this study was to evaluate the potential of Parthenium hysterophorus leaf extracts for the production of zinc oxide nanoparticles and to evaluate their antimicrobial potential.
Materials and Methods
Collection of sample
Fresh leaves of Parthenium hysterophorus were collected from fields, near Lovely Professional University, Phagwara, India and were identified by Department of Botany, LPU. The leaves were thoroughly washed for 2-3 times with distilled water and used for further studies.
Preparation of plant extract
Aqueous extract: Aqueous extract of P. hysterophorus was prepared according to the method of Kumar et al. [20]. 10 g of thoroughly washed leaves were immersed in 100 ml of double distilled water at 60°C for 15 minutes. The extract was filtered using Whatman filter paper and stored at 4°C for further use.
Methanolic and ethanolic extract: Extraction was carried out according to method reported by Korbekandi et al. [21]. 15 g of leaves were washed, dried and grinded into fine powder. The powder was mixed in 100 ml ethanol (70%) and left overnight on rotary shaker. The solution was filtered using Whatman Filter No. 1 under aseptic conditions. The protocol was repeated for the preparation of ethanolic extract from leaves.
Biosynthesis of zinc oxide nanoparticles
Plant extract was added to 1 mM zinc nitrate solution in 9:1 ratio and the mixture was incubated for 24 h to facilitate the formation of nanoparticles. Aqueous leaf extract and silver nitrate solution were used as controls throughout the experiments. Change in the colour of the solution indicated the presence of zinc oxide nanoparticles. The samples were purified by centrifugation at 18°C for 15 min and resuspended in 1ml distilled water for further use [20]. The mentioned procedure was followed for aqueous, methanolic and ethanolic plant extracts.
Characterisation of nanoparticles
The bio-reduction of reaction mixture was initially analysed by UVVisible spectroscopy. Spectral analysis was carried out from 300-600 nm. FTIR spectroscopy of the synthesized nanoparticles was recorded in the range of 300-4000 cm-1. The particle shape was identified using Scanning Electron Microscopy. For the preparation of sample, 500 μl samples was coated on a copper metal stub to form a thin smear which was then subjected to SEM analysis by using JEOL/EO electron microscope. Further confirmation of elemental composition of zinc nanoparticles was obtained from the EDS imaging. Surface morphology and size of the synthesised nanoparticles was studied by transmission electron microscope (TECNAI G2, Netherlands). Sample was mounted on a carbon-coated copper grid and TEM was operated at 200 kv.
Effect of incubation time: The reaction mixture in a ratio of 9:1 was incubated for different time intervals (0-72 h). Samples were withdrawn after regular intervals of 3 h. The absorbance of the solution was measured via spectrophotometric analysis at 400 nm to identify optimum incubation time.
Effects of plant extract concentration: The effect of change in P. hysterophorus leaf filtrate concentration with respect to 1 mM zinc nitrate solution was analysed for determining the rapid synthesis of metal nanoparticles. Plant extract and metal salt solution (1 mM) were prepared in different ratios viz., 9:1, 4:1, 7:3, 3:2 and 1:1, respectively. The absorbance of the solution was measured using a spectrophotometer at 400 nm.
Antimicrobial activity of zinc nanoparticles
The synthesized nanoparticles were tested for antibacterial activity by agar well diffusion method against different bacteria viz., the gram positive bacteria Staphylococcus aureus and Bacillus subtilis and the gram negative Klebsiella pneumonia, Escherichia coli and Enterobacter aerogens. Norfloxacin, a broad-spectrum antibiotic was used as control.
Results and Discussion
Characterization of nanoparticles
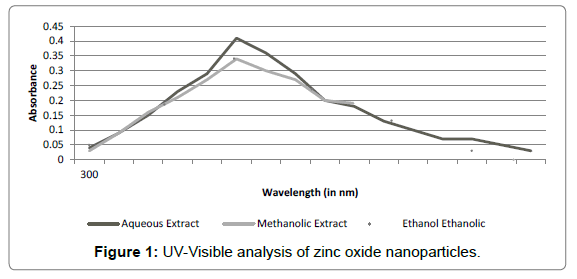
UV-visible spectral analysis: Confirmation of presence of zinc nanoparticles in colloidal solution was done using UV-Spectral analysis. Preliminary confirmation of nanoparticles synthesis was indicated by the visual change in the colour of the colloidal solution from brownish green to white. This change is caused due to coherent oscillation of electron gas at the surface of nanoparticles which results in Surface Plasmon Resonance [20]. The samples were then analysed by UVspectral analysis (300-600 nm). It was observed that all the three sample extracts showed an optical absorption band peaking at 400 nm and gradually decreasing at higher wavelengths (Figure 1). No other peak was observed in the spectrum, confirming that the synthesized product was ZnO only. However, maximum absorption peak was observed in case of aqueous extract sample. Therefore, further experimentation was carried out using aqueous extract of P. hysterophorus leaves. Zinc oxide nanoparticles have been reported to exhibit a characteristic broad absorption peak between 330-460 nm [7]. Thus optical absorption band peaking at 400 nm confirms the synthesis of zinc nanoparticles by P. hysterophorus leaf extracts. It can be deduced from the above results that the biomolecules present in the plant extract induce the reduction of Zinc ions into zinc nanoparticles (ZnONP). This process of reduction is extracellular, fast and thus can be developed into an easy method for nanoparticle synthesis.
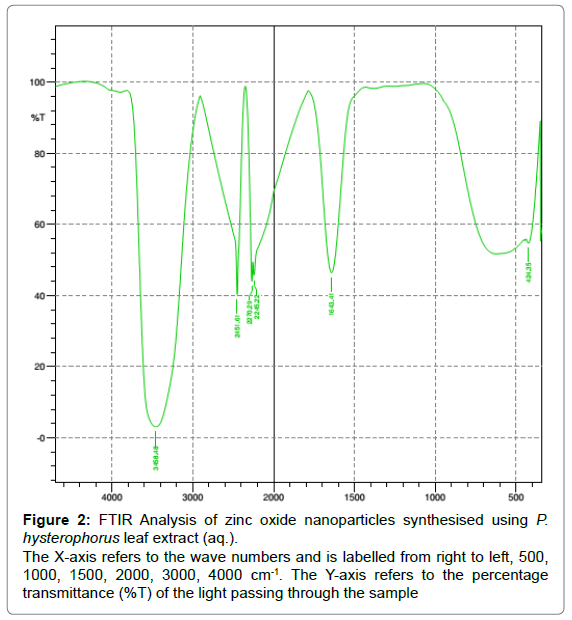
Fourier transform infrared spectroscopy (FTIR): FTIR analysis of the ZnONP revealed the presence of peaks at 3458, 2451, 2270, 2245, 1643 and 424 cm-1 (Figure 2). The appearance of an intense broadened band at 3458.48 cm-1 denotes the presence of stretched OH bond. The bands at 1643.51 cm-1 confirm the presence of primary amines and the peak at 2245.22cm-1 denotes the presence of nitriles (C (triple bond) N stretch) and alkyls (C (triple bond) C– stretch). FTIR analysis of the ZnONP reveals the appearance of bonds that confirms the presence of certain enzymes that are responsible for the reduction, capping and stability of the nanoparticles. It can be stated that the nanoparticles are shielded by the enzymes and the proteins present in the extract so as to help prevent the formation of clusters (Figure 3) [8].
Figure 2: FTIR Analysis of zinc oxide nanoparticles synthesised using P.
hysterophorus leaf extract (aq.).
The X-axis refers to the wave numbers and is labelled from right to left, 500,
1000, 1500, 2000, 3000, 4000 cm-1. The Y-axis refers to the percentage
transmittance (%T) of the light passing through the sample
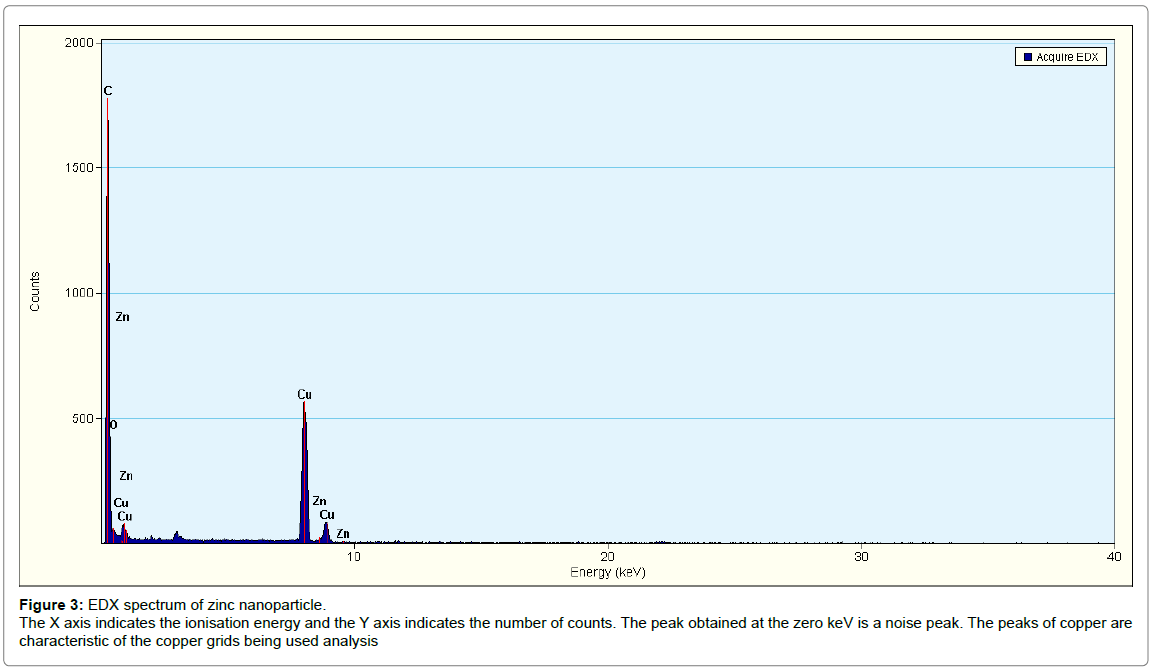
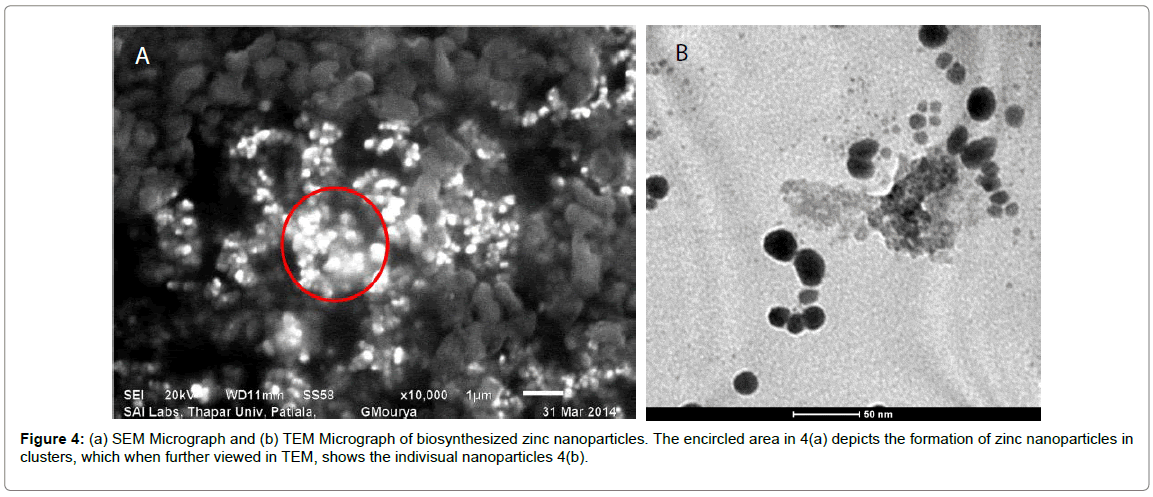
SEM and EDX analysis: Typical SEM micrographs (Figure 4a) of the ZnONPs obtained by the biosynthesis method revealed that the zinc oxide particles were quasi-spherical, radial and cylindrical in shape with different sizes. They were also seen to be present in small aggregated/ clustered forms. Table 1 shows the EDS analysis of ZnONP’s with 97.6% of zinc and 2.4% of Oxides which confirms the elemental composition of zinc oxide nanoparticles. EDX spectrum (Figure 3) shows four peaks which were identified as zinc (97.6%) and oxygen (2.4%). The presence of copper peaks in the spectrum can be attributed to the use of copper grids for sample loading or to the fact that P. hysterophorus is known to bioaccumulate copper [22].
Transmission electron microscope analysis: In order to determine the structure and size of the nanoparticles, transmission electron microscopy (TEM) was used. The synthesized zinc nanoparticles were quasi-spherical, radial and cylindrical in shape. They were polydispersed with the particle size ranging from 16-45 nm (Figure 4b). Studies have shown that the smaller the size of zinc nanoparticles, the greater is the efficacy in inhibiting the growth of bacteria [23]. The toxicity of zinc nanoparticles depends on the fact these NPs can locally change microenvironments near the bacteria and produce ROS or increase the NPs solubility, which can induce bacterial damage [24]. Therefore smaller the size of the particle, better will be the nanoparticle accumulation and therefore better antimicrobial activity. The size of the nanoparticles synthesised using P. hysterophorus leaf extract ranged from 16-45 which could have a potential effect on the antimicrobial properties of the particles.
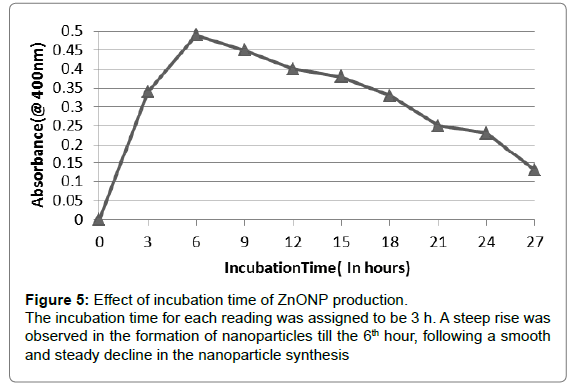
Effect of incubation time
Effect of incubation on the synthesis of zinc nanoparticles was studied by incubating the reactions mixture (9:1) from 0 to 72 h. The absorbance of the sample was taken at a regular interval of three hours at 400 nm. It was observed that the synthesis of the nanoparticles increased from 0 to 6th hour, peaking at the sixth hour followed by a gradual decrease with an increase in incubation period (Figure 5).
Generally, biosynthetic methods are considered as time consuming when compared with chemical methods. However as per the results obtained, the prime reaction time for the formation of plant mediated ZnO nanoparticles is 6 h, which results in the maximum synthesis. Due to the continuous synthesis of the nanoparticles, the reaction mixture got exhausted over time which is clearly visible as a declining graph.
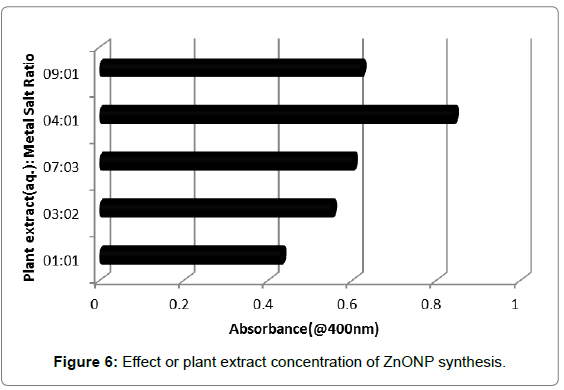
Effects of plant extract concentration
Different ratios of aqueous plant extract-metal salt solutions were taken to evaluate the effect of plant extract concentration on nanoparticle synthesis. The reaction mixtures were incubated at optimum incubation period of 6 h and analyse during UV-visible spectroscope at 400 nm. As shown in Figure 6, it was observed that with an increase in the concentration of plant extract with respect to metal salt concentration in the reaction mixture, there was a gradual increase in the nanoparticle synthesis with maximum absorbance exhibited at plant extract: metal salt solution ratio of 4:1. With a further increase in plant extract concentration, a decrease in the nanoparticle synthesis was observed.
Antimicrobial activity of zinc nanoparticles
Antibacterial activity of synthesised zinc oxide nanoparticles were investigated against gram positive bacteria such Staphylococcus aureus and Bacillus subtilis and gram negative Klebsiella pneumonia, Escherichia Coli and Enterobacter aerogenes with respect to Norfloxacin. Maximum zone of inhibition (36 mm) was observed against Enterobacter aerogenes while least activity was seen against S. aureus and B. subtilis (Table 2). It was observed that nanoparticles exhibited higher antibacterial activity against gram-negative bacteria as compared to gram-positive bacteria with a maximum activity against E. aerogenes followed by E. coli. It is important to identify the key physicochemical properties of nano-metal oxides that govern antibacterial activity and cytotoxicity to mammalian cells also needs to be evaluated. However, these results exhibit that P.hysterophorus can be used for the synthesis of nanoparticles, providing a new platform to this noxious plant and making it a value added weed for the green synthesis of nanoparticles.
Conclusion
A method for the preparation of ZnONPs was developed using the leaf extract of Parthenium hysterophorus. Aqueous, ethanolic and methanolic extracts of P. hysterophorus leaves were used for the synthesis and among the three extracts, maximum synthesis was observed in case of aqueous extract which was used for further experiments. Synthesised nanoparticles were characterised using UV-Vis spectroscopy with maximum absorbance peak at 400 nm. SEM and TEM analysis revealed that the particles were spherical and cylindrical in shape with average particle size ranging from 16-45 nm. Zinc oxide nanoparticles exhibited maximum zone of inhibition against Enterobacter aerogenes (36 mm) while least activity was seen against Staphylococcus aureus and Bacillus subtilis.
Conflict of Interest
All the authors hereby declare that they do not have any conflict of interest.
References
- Singh RK, Kumar S, Kumar S, Kumar A (2008) Development of parthenium based activated carbon and its utilization for adsorptive removal of p-cresol from aqueous solution. J Hazard Mater 155: 523-535.
- Dwivedi P, Vivekanand V, Ganguly R, Singh RP (2009) Parthenium sp. as a plant biomass for the production of alkalitolerantxylanase from mutant Penicillium oxalicum SAUE-3.510 in submerged fermentation. Biomass Energy 33: 581-588.
- Patel S (2011) Harmful and beneficial aspects of Parthenium hysterophorus: An update. Biotech 1: 1-9.
- Maishi AI, Ali PKS, Chaghtai SA, Khan G (1998) A proving of Parthenium hysterophorus. Br Homoeopathic J 87: 17-21.
- Venkataiah B, Ramesh C, Ravindranath N, Das B (2003) Charminarone, a seco-pseudoguaianolide from Parthenium hysterophorus. Phytochem 63: 383-386.
- Ghorbani HR, Safekordi AA, Attar A, Sorkhabadi SMR (2011) Biological and non-biological methods for silver nanoparticles synthesis. Chem Biochem Eng 25: 17-326.
- Nagarajan S, Kuppusamy KA (2013) Extracellular synthesis of zinc oxide nanoparticle using seaweeds of Gulf of Mannar, India. J Nanobiotechnol 11: 1-11
- Sindhura KS, Prasad TNVKV, Selvam PP, Hussain OM (2013) Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl Nanosci 1: 1-9.
- Salam HA, Rajiv P, Kamaraj M, Jagadeeswaran P, Gunalan S, et al. (2012) Plants: Green route for nanoparticle synthesis. Inte Res J Biol Sci 1: 85-90.
- Sharma NC, Sahi SV, Nath S, Parsons J, Gardea-Torresdey JL, Pal T (2007) Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol 41: 5137-5142.
- Narayanan KB, Sakthivel N (2008) Coriander leaf mediated biosynthesis of gold nanoparticles. Mat Lett 62: 4588-4590.
- Daisy P (2010) Green synthesis of gold and silver nanoparticles using Hibiscus rosasinensis. Physica E 42: 1417-1424.
- Daisy P (2011) Mangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochimi Acta Part A Mol Biomol Spectrosc 78: 327-331.
- Awwad AM, Salem NM, Abdeen AO (2013) Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem 4: 29-34
- Meruvu H, Vangalapati M, Chippada SC, BammidI SR (2011) Synthesis and characterization of zinc oxide nanoparticles and its antimicrobial activity against Bacillus subtilis and Escherichia coli. Rasayan Journal of Chemistry 4: 217-222.
- Talam S, Karumuri SR, Gunnam N (2012) Synthesis, characterization and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnology.
- Kumar SS, Venkateswarlu P, Rao VR, Rao GN (2013) Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int Nan Lett 3: 1-6.
- Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Cluster bean (Cyamopsis tetragonoloba L.). Agri Res 2: 48-57.
- Jones N, Ray B, Ranjit KT, Manna AC (2007) Antibacterial activity of ZnO nanoparticles suspension on a board spectrum of microorganism. Microbiol Lett 279: 71-76.
- Kumar DA (2012) Rapid and Green synthesis of silver nanoparticles using the leaf extracts of Parthenium hysterophorus: A novel biological approach. Int Res J Pharm 3: 169-173.
- Korbekandi H, Asghari G, Jalayer SS, Jalayer MS, Bandegani M (2013) Nanosilver particle production using Juglans regia L. (Walnut) leaf extract. Jundishapur J Nat Pharm Prod 8: 20-26.
- Biradar DP, Shivakumar KS, Prakash SS, Pujar BT (2006) Bionutrient potentiality of Parthenium hysterophorus and its utility as green manure in rice ecosystem. Karnataka Journal of Agricultural Sciences 19: 256-263.
- Azam A (2012) Antimicrobial activity of metal oxide nanoparticles against gram-positive and gram negative bacteria? A comparative study. Int J Nanomedicine 7: 6003-6009.
- Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, de Larramendi IR, et al. (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30: 499-511.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 10035
- [From(publication date):
September-2017 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 8117
- PDF downloads : 1918