Granulomatous Phlebitis of the Hepatic Vein Tributaries after Portal Venous Embolization
Received: 15-May-2015 / Accepted Date: 11-Jun-2015 / Published Date: 14-Jun-2015 DOI: 10.4172/2161-0681.1000233
Abstract
Background & Aims: Granulomatous phlebitis of hepatic vein is a rare disease and its exact etiopathogenesis remains unknown. Recently, we experienced granulomatous phlebitis of small hepatic veins in a patient who underwent hepatic resection for metastatic liver cancer after preoperative portal venous embolization (PVE).
Methods: We surveyed such phlebitis in the patients who underwent PVE (2008 August – 2014 August). A total of 62 patients underwent PVE followed by hepatectomy for hepatocellular carcinoma (HCC), peripheral cholangicarcinoma (pCCA), or metastatic liver tumor (MLT) during this period. As a control, 20 cases of surgically resected livers without a history of preoperative PVE for liver tumors of comparable age and sex distribution in recent 2 years in the same hospital, were used.
Results: Granulomatous phlebitis of small hepatic veins was found in 10 patients with a history of PVE (16.1% of 62 cases) but not in control cases. No microbial agents were identified in these granulomatous lesions. Interestingly, the presence of embolic materials containing sponge gel with inflammation and foreign body reaction in the portal veins in the embolized livers were independently associated with granulomatous phlebitis.
Conclusions: It was suggested that sponge gel used for PVE might have be directly or indirectly related to the occurrence of granulomatous phlebitis. This is the first report of granulomatous phlebitis related to PVE.
Keywords: Preoperative portal venous embolism; Granulomatous phlebitis; Sponge gel; Foreign body reaction; Budd-Chiari syndrome; Hepatic vein.
312776Abbreviations
PVE: Preoperative Portal Venous Embolization; HCC: Hepatocellular Carcinoma; pCCA: Peripheral Cholangiocarcinoma; MLT: Metastatic Liver Tumor; DNA: Deoxyribonucleic Acid; ICG-K: Indocyanine Green Plasma Clearance Rate; IRB: Institutional Review Board; PAS: Periodic Acid Schiff; CD: Cluster of Differentiation; PBC: Primary Biliary Cirrhosis; H&E: Hematoxylin and Eosin.
Introduction
Idiopathic granulomatous phlebitis of the hepatic vein was first reported in an autopsy case by Nakanuma et al, in which epithelioid granulomatous lesions were found in the walls of the small tributaries of the hepatic and portal veins [1]. Subsequently, similar granulomatous phlebitis of the hepatic vein has been reported [2,3]. Although bacterial DNA fragments were detected in these lesions [3], its exact etiopathogenesis remains speculative. In granulomatous liver diseases, particularly in sarcoidosis [4-7], the hepatic vein could be secondarily affected, resulting in granulomatous phlebitis. Both idiopathic and secondary granulomatous phlebitis of the hepatic vein are known to be related with Budd-Chiari syndrome or portal hypertension [2,4-7].
Portal venous embolization (PVE) has been gaining increasing acceptance before major hepatectomy. Although some complications such as hematoma [8-11] were reported in relation with PVE, there has been no report of granulomatous phlebitis of the hepatic vein after PVE. Recently, we experienced granulomatous phlebitis in surgically resected liver after PVE for metastatic liver tumor. Therefore, we retrospectively reviewed the medical records and pathological specimens of the surgically resected livers from the patients who underwent PVE followed by hepatectomy, to clarify the incidence of granulomatous phlebitis after PVE and the causal relation between occurrence of granulomatous phlebitis and PVE. This is the first report on granulomatous phlebitis of the hepatic vein in the surgically resected liver after PVE.
Materials and methods
Patients
Between August 2008 and August 2014, a total of 62 patients who underwent PVE followed by hepatectomy for hepatocellular carcinoma (HCC, 22 patients), peripheral cholangicarcinoma (pCCA, 4 patients), and metastatic liver tumor (MLT, 36 patients) in the Shizuoka Cancer Center, were retrospectively enrolled in this study. Patients who underwent hepatectomy after PVE for biliary cancer were excluded to avoid the influence of biliary inflammation caused by obstructive jaundice. A total of 20 patients who underwent hepatectomy without PVE in the same period, including nine patients with HCC, one patient with pCCA and 10 patients with MLT were selected as a control, considering gender, age, primary tumor, and background liver disease. Patients demographics and preoperative data, including serum albumin, and 15-min indocyanine green retention rate, indocyanine green plasma clearance rate (ICG-K value), estimated future remnant liver volume before and after PVE assessed by computed tomography volumetry, and estimated ICG-K value for the future remnant liver, were retrospectively reviewed in the medical records. ICG-K value for the future remnant liver was calculated using the following formula; ICG K value × future remnant liver volume/total liver volume.
This study was approved by Institutional Review Board (IRB) of Shizuoka Cancer Center (Shizuoka, Japan).
PVE
PVE was indicated, in principle, when a ICG-K value of the future remnant liver was less than 0.05 or when a future remnant liver volume was estimated to be less than 40% of the entire liver and was performed three weeks before the planned hepatectomy by interventional radiologist (T.A.). After local anesthesia with 1.0% lidocaine, one of the portal branches of the future resected liver was punctured under the ultrasonographic guidance (transhepatic ipsilateral technique [12]). Following portography, portal branches of the liver sectors to be resected were embolized with gelatin sponge (Spongel, Astellas, Tokyo, Japan) mixed with an iodinated contrast agent, iopamidol (Iopamirin, Bayer Pharma, Osaka, Japan)
Pathological examination
More than 4 liver specimens including the tumor region and non-tumor region with the area adjacent (proximal area) and not adjacent (distal area) to the tumor, were obtained from surgically resected livers of all of PVE and control cases. For this study, we defined proximal area as the area within 3cm from the tumor margin, while distal area as the area over 3cm from the tumor margin. All of these specimens were fixed in 10% buffered formalin and embedded in paraffin, and more than one thin sections were cut from all specimens for histological diagnosis and more than 5 thin sections were cut from the one representative block from 10 cases of test group in which granulomatous phlebitis was found, were stained for Ziel-Neelsen stain, Gram stain, Grocott’s stain, and periodic acid Schiff (PAS) stain after diastase digestion to detect microbial agents in the granulomatous lesions. In addition, in these selected sections, immunostaining of CD68 to identify macrophages was done to analyze the granulomatous phlebitis.
In these sections, main liver tumor and background liver were pathologically examined with a special attention to the histological features of the tumor and characteristics of background liver including granulomatous phlebitis, portal venous emboli, and arterial sclerosis. We checked the area adjacent and not adjacent to the tumor, separately.
Statistical analysis
Comparisons were performed using the chi-squared test with Yates correction. Independent risk factors of granulomatous phlebitis were calculated using preoperatively measurable factors and pathological features by logistic regression model. All statistical analyses were performed using the Stat View version 5.0. We set the cut-off value of the continuous variables as median value. A p value of<0.05 was considered to be significant.
Results
Main clinicopathological features
The main clinicopathological features and surgical operation processes of these test and control cases were shown in Table 1. Age and sex of the patients in the test and control cases were comparable. In the test cases, duration between PVE and operation was 13~77 days (median; 22.5 days). In the test and control cases, findings strongly suggestive of sarcoidosis or tuberculosis were not obtainable clinically and also pathologically. Histological findings of primary biliary cirrhosis (PBC) such as chronic nonsuppurative destructive cholangitis or bile duct loss or chronic cholestasis were not found, and clinical features were not suggestive of PBC in all of these cases (data not shown).
| Test cases(n=62) | Control(n=20) | |
|---|---|---|
| Gender Male / Female | 46 / 16 | 16 / 4 |
| Age; yeas (median) | 39~82 (65) | 55~85 (71.5) |
| Tumor | ||
| Hepatocellular carcinoma | 23 (35.3%) | 9 (45.0%) |
| Peripheral Cholangiocarcinoma | 4 (6.4%) | 1 (5.0%) |
| Metastatic liver tumor*‡ | 35 (56.4%) | 10 (50.0%) |
| Background liver disease | ||
| Normal liver | 47 (75.8%) | 16 (80.0%) |
| Chronic hepatitis / advanced liver fibrosis / cirrhosis | 15 (24.1%) | 4 (20.0%) |
| HBV related | 6 (9.6%) | 2 (10.0%) |
| HCV related | 7 (11.2%) | 0 (0.0%) |
| Alcoholic | 2 (3.2%) | 0 (0.0%) |
| Non-alcoholic steatohepatitis | 0 (0.0%) | 2 (10.0%) |
| Type of Hepatectomy | ||
| Right hepatectomy | 49 (79.0%) | 1 (5.0%) |
| Left hepatectomy | 2 (3.2%) | 4 (20.0%) |
| Right trisegmentectomy | 3 (4.8%) | 0 (0.0%) |
| Left trisegmentectomy | 5 (8.0%) | 0 (0.0%) |
| Partial hepatectomy | 0 (0.0%) | 10 (50.0%) |
| Other procedures** | 3 (4.8%) | 5 (25.0%) |
| *Metastastic liver tumors of test cases; from 34 colorectal cancer, 1 gastrointestinal stromal tumor and 1 carcinoid tumor, and those of control cases; from 8 colorectal carcinoma, 1 gastric cancer, and 1 gastrointestinal stromal tumor. **Other procedures containing bellow: Test cases; posterior segmentectomy, S1S2S3 segmentectomy, and S5S6S7 segmentectomy. Control; Anteriorsegmentectomy, central bisegmentectomy, lateral segmentectomy. | ||
Table 1: The results of main clinicopathological features.
Occurrence of granulomatous phlebitis
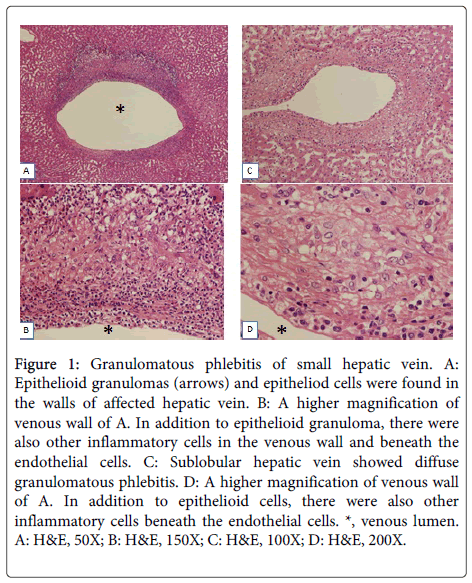
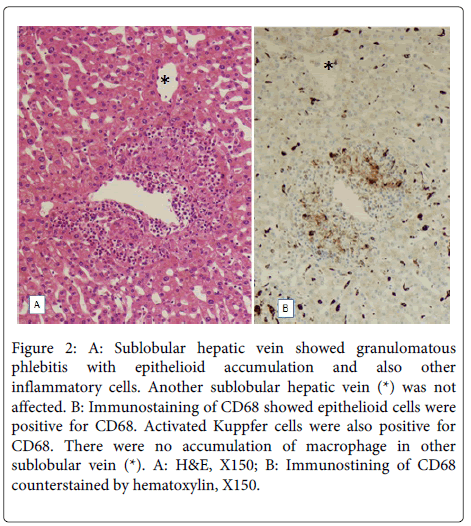
Granulomatous phlebitis of hepatic veins was found in 10 of 62 cases with PVE (16%), while such phlebitis was not found in the control. These lesions were characterized by infiltration of epithelioid cells with focal accumulation appearing epithelioid granuloma in the thickened venous wall, and/or diffuse distributed epithelioid cells in the thickened venous walls (Figure 1). These epithelioid cells were positive for CD68 (Figure 2). In addition to epithelioid cell reaction, there were also infiltration of lymphocytes, eosinophils and occasionally neutrophils in the wall of granulomatous phlebitis. Lymphocytes and eosinophils were also occasionally accumulated beneath of endothelia. However, there were no coagulative necroses or giant cells or fragments of the gelatin sponge. The size of affected hepatic veins were mainly sublobular hepatic veins but hepatic vein branches less than 200 μm in diameter were also affected (small hepatic veins). In these 6 of 10 cases, granulomatous phlebitis was found in more than one third of hepatic veins in the specimens. By special stainings, no bacteria, fungi or mycotic agents were identifiable in these granulomatous lesions.
Figure 1: Granulomatous phlebitis of small hepatic vein. A: Epithelioid granulomas (arrows) and epitheliod cells were found in the walls of affected hepatic vein. B: A higher magnification of venous wall of A. In addition to epithelioid granuloma, there were also other inflammatory cells in the venous wall and beneath the endothelial cells. C: Sublobular hepatic vein showed diffuse granulomatous phlebitis. D: A higher magnification of venous wall of A. In addition to epithelioid cells, there were also other inflammatory cells beneath the endothelial cells. *, venous lumen. A: H&E, 50X; B: H&E, 150X; C: H&E, 100X; D: H&E, 200X.
Figure 2: A: Sublobular hepatic vein showed granulomatous phlebitis with epithelioid accumulation and also other inflammatory cells. Another sublobular hepatic vein (*) was not affected. B: Immunostaining of CD68 showed epithelioid cells were positive for CD68. Activated Kuppfer cells were also positive for CD68. There were no accumulation of macrophage in other sublobular vein (*). A: H&E, X150; B: Immunostining of CD68 counterstained by hematoxylin, X150.
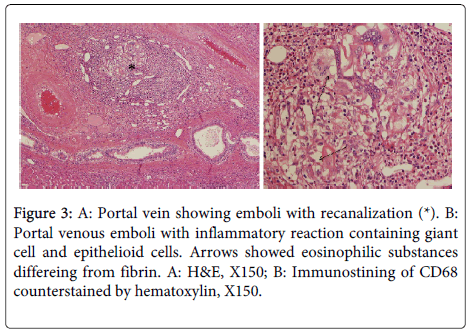
In the portal tracts, portal veins were not infrequently obstructed by organized thrombi or emboli containing sponge gel (eosinophilic substances differing from fibrin) and also inflammatory changes including giant cells of the foreign body type, eosinophils and occasionally epithelioid cells (Figure 3). However, the hepatic parenchyma did not show granulomatous changes or foreign body reaction, while Kupffer cells were swollen and lymphoid cells were found in the sinusoids. In control cases, granulomatous changes were not found at all, and portal tract and portal venous changes found in the cases PVE were not found.
Comparison of clinical factors and background livers
Univariate analyses revealed that the history of hypertension (p=0.028), presence of arterial sclerosis in the proximal and/or distal area from the tumor (p=0.031), and presence of embolic materials in the portal veins in the area proximal from the tumor but not in distal area from the tumor (p<0.001) were associated with granulomatous phlebitis (Table 2). Logistic regression analysis revealed that presence of arterial sclerosis in the proximal and/or distal area from the tumor (p=0.021, odds rate=24.000) and presence of embolic materials in the portal veins in the area proximal from the tumor but not in distal area from the tumor (p<0.001, odds rate=64.000) was independently associated with the presence of granulomatous phlebitis.
| No | GPh (+) (n=10) | GPh (-) (n=52) | Univariate Analysis P | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | |||||
| Gender | 0.383 | |||||
| Male | 47 | 6 | 41 | |||
| Female | 15 | 4 | 11 | |||
| Age | 0.815 | |||||
| < 65 | 30 | 4 | 26 | |||
| > 65 | 32 | 6 | 26 | |||
| Hypertension | 0.028 | 0.980 | ||||
| Absent | 35 | 2 | 33 | 1 | ||
| Present | 27 | 8 | 19 | 1.031(0.87,12.239) | ||
| Diabetes mellitus | 0.8416 | |||||
| Absent | 48 | 7 | 41 | |||
| Present | 14 | 3 | 11 | |||
| Serum albumin | 0.494 | |||||
| < 4.3 g/dl | 28 | 6 | 22 | |||
| > 4.3 g/dl | 34 | 4 | 30 | |||
| ICG R15 | 0.480 | |||||
| < 12 % | 28 | 3 | 25 | |||
| >12 % | 34 | 7 | 27 | |||
| Remnant K value | 0.647 | |||||
| < 0.05 /min | 32 | 4 | 28 | |||
| > 0.05 /min | 30 | 6 | 24 | |||
| HBV infection | >0.999 | |||||
| Absent | 56 | 9 | 47 | |||
| Present | 6 | 1 | 5 | |||
| HCV infection | 0.458 | |||||
| Absent | 47 | 9 | 38 | |||
| Present | 15 | 1 | 14 | |||
| History of chemotherapy | >0.999 | |||||
| Absent | 28 | 5 | 23 | |||
| Present | 34 | 5 | 29 | |||
| Arterial sclerosis (proximal and/or distal area) | 0.031 | 0.021 | ||||
| Absent | 57 | 7 | 50 | 1 | ||
| Present | 5 | 3 | 2 | 24.000(1.615, 356.729) | ||
| Embolic agents (proximal area) | <0.001 | <0.001 | ||||
| Absent | 57 | 6 | 51 | 1 | ||
| Present | 5 | 4 | 1 | 64.000(3.248, 1261.015) | ||
| Embolic agents (distal area) | 0.196 | |||||
| Absent | 27 | 2 | 25 | |||
| Present | 35 | 8 | 27 | |||
| CI: confidence interval; PVE: portal vein embolization; Proximal area, area within 3cm from the tumor margin; Distal area, area over 3cm from the tumor margin; ICG R15, 15-min indocyanine green retention rate; ICG K, indocyanine green plasma clearance rate;Remnant K, estimated ICG-K value for the future remnant liver | ||||||
Table 2: The results of the univariate analyses of clinicopathological factors associated with granulomatous phlebitis.
Discussion
The findings obtained in this study are summarized as follows: i) granulomatous phlebitis of small hepatic veins was not infrequent in surgically resected livers with PVE, ii) granulomatous phlebitis of hepatic veins was frequently associated with the portal venous emboli containing sponge gel, and iii) sclerosis of hepatic arteries were associated with occurrence of granulomatous phlebitis.
PVE has become a widely accepted practice before major hepatectomy [8-11]. The embolized liver part(s), in which liver tumor(s) were located, became atrophic and the non-embolized liver parts hypertrophic. So far, there have been reported some complications such as portal thrombosis, embolization of nontarget vessels, liver hematoma, infection/abscess, mesenterioportal venous thrombosis, intra-abdominal bile leakage and so on [9,11]. To our knowledge, previous authors had never reported about the granulomatous phlebitis as an associated lesion of PVE.
Since the first case reported in 1980, only several cases of idiopathic granulomatous phlebitis of the hepatic vein have been reported, and their exact etiologies still have been unclear [1-3]. Interestingly, it was found in this study that 10 of 62 surgically resected livers with preoperative PVE showed granulomatous phlebitis of small hepatic vein in embolized livers. These patients did not present any preceding hepatobiliary diseases associated with hepatic granulomatous reaction such as PBC, tuberculosis or sarcoidosis. They did not present any signs of infection through their clinical course either, and in fact, there were no microbial agents detectable in the affected veins of the liver. Though the etiology of idiopathic granulomatous phlebitis remains speculative, the present study suggests that pathophysiological features of PVE may be related to the pathogenesis of granulomatous phlebitis.
It is known that epithelioid granulomas are etiologically related to bacterial infection, and in fact, some cases of granulomatous phlebitis might have been related to bacterial components [2]. The prolonged presence of foreign substances can also cause granulomatous inflammation [13,14]. In our institutions, gelatin sponge mixed with Iopamidol have been routinely used for PVE. Interestingly, Ishikura et al reported that trans-arterial gelatin sponge embolization produced granulomatous arteritis with massive infiltration by eosinophilic leucocytes and histiocytes (13), and they suggested that a hypersensitivity reaction against the intra-arterial gelatin sponge could be responsible for granulomatous arteritis. Kawano et al. suggested that absorbable gelatin sponge usually induces a foreign body granuloma reaction [14]. In the present study, it seems plausible that gelatin sponges or its ingredients used for PVE might have caused granulomatous phlebitis of the small hepatic veins. In fact, inflammatory changes including foreign body reaction and eosinophils was found in the emboli in the portal veins. Furthermore, the presence of embolic agents in the portal veins of the embolized liver was independently associated with the granulomatous phlebitis. However, gelatin sponge or foreign body reaction was not seen in granulomatous phlebitis of the small hepatic veins and there were no granulomatous or foreign body reactions in the hepatic parenchyma, suggesting an indirect inflammatory or hypersensitivity mechanism(s) may be operative in the hepatic veins.
In addition, presence of sclerosis of hepatic arterial branches was independently associated with granulomatous phlebitis. Hepatic arterial sclerosis may lead to a compensatory increase of portal venous inflow in the affected areas, raising a possibility that the relatively increased amounts of portal emboli might have arrived in the areas with hepatic arterial sclerosis. Hypertension in clinical history may be related to hepatic arterial sclerosis. Taken together, sufficient distribution of embolic agents into the portal veins may have association with the pathogenesis of granulomatous phlebitis of hepatic vein.
So far, granulomatous phlebitis of the hepatic veins has not been described in the surgically resected livers with PVE [8-11]. The reasons why such phlebitis has not been described or reported, may be oversight of the lesion because this lesion is more or less subtle in H&E stained sections or because in embolized livers, the portal venous emboli with inflammation might have overshadowed this hepatic venous lesion.
In conclusion, granulomatous phlebitis is not an infrequent associated lesion of preoperative PVE in the embolized liver, and material(s) related to or included in emboli for PVE could be directly or indirectly related to the pathogenesis of granulomatous phlebitis.
Acknowledgement
We thank Mr. Shogo Fujii and Ms. Asuka Fukushima for their technical assistance.
References
- Nakanuma Y, Ohta G, Doishita K, Maki H (1980) Granulomatous liver disease in the small hepatic and portal veins. Arch Pathol Lab Med 104: 456-458.
- Young ID1, Clark RN, Manley PN, Groll A, Simon JB (1988) Response to steroids in Budd-Chiari syndrome caused by idiopathic granulomatous venulitis. Gastroenterology 94: 503-507.
- Saito T, Harada K, Nakanuma Y (2002) Granulomatous phlebitis of small hepatic vein. J GastroenterolHepatol 17: 1334-1339.
- Tanaka M, Wanless IR (1998) Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology 27: 488-496.
- Russi EW, Bansky G, Pfaltz M, Spinas G, Hammer B, et al. (1986) Budd-Chiari syndrome in sarcoidosis. Am J Gastroenterol 81: 71-75.
- Wanless IR, Huang WY. Vascular disorders (2007) In: MacSween’s Pathology of the liver. Editors; A Burt, BCPortmnn, L Ferrell. 6th edition, Churchill Livingstone Elsevier pp: 601-643.
- Yamazaki Y, Doishita K, Nakanuma Y, Haba T, Takeshita H, et al. (1983) [Sarcoidosis with the development of portal hypertension during a long-term observation: an autopsy study]. Nihon ShokakibyoGakkaiZasshi 80: 1212-1216.
- Lim C, Farges O (2012) Portal vein occlusion before major hepatectomy in patients with colorectal liver metastases: rationale, indications, technical aspects, complications and outcome. J ViscSurg 149:86-96
- Liu H, Zhu S (2009) Present status and future perspectives of preoperative portal vein embolization. Am J Surg 197: 686-690.
- Shindoh J, Tzeng CW, Aloia TA, Curley SA, Huang SY, et al. (2014) Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J GastrointestSurg 18: 45-51.
- van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, et al. (2013) Portal vein embolization before liver resection: a systematic review. CardiovascInterventRadiol 36: 25-34.
- Nagino M, Nimura Y, Kamiya J, Kondo S, Kanai M (1996) Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology 200:559-563.
- Ishikura H, Sotozaki Y, Adachi H, Sato M, Yoshiki T (1991) Granulomatous arteritis with massive eosinophilic leukocyte infiltration and transient peripheral eosinophilia subsequent to transarterial embolization therapy with a gelatin sponge. ActaPatholJpn 41:618-622.
- Kawano H, Arakawa S, Satoh O, Matsumoto Y, Hayano M, et al. (2010)Foreign body granulomatous change from absorbable gelatin sponge and microcoil embolization after a guidewire-induced perforation in the distal coronary artery. Intern Med 49:1871-1874.
Citation: Kohga A, Kakuda Y, Sugiura T, Okamura Y, Ito T et al. (2015) Granulomatous Phlebitis of the Hepatic Vein Tributaries after Portal Venous Embolization. J Clin Exp Pathol 5:233. DOI: 10.4172/2161-0681.1000233
Copyright: © 2015 Nakanuma Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16482
- [From(publication date): 8-2015 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 12045
- PDF downloads: 4437