Research Article Open Access
Granulocyte-Macrophage Colony-Stimulating Factor Partially Restores Toll-Like Receptor-Mediated Functional Responses of Monocytes in Septic Shock
Bauer SB1*, Pietropaoli AP1, Georas SN1,2,3, and Williams MA1,2,4*1Department of Medicine, Division of Pulmonary and Critical Care Medicine, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA
2Department of Environmental Medicine, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA
3Department of Microbiology and Immunology, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA
4Lung Biology and Disease Program, School of Medicine and Dentistry, University of Rochester, Rochester, NY, USA
- Corresponding Author:
- Stephen M. Bauer
Department of Biology
Belmont Abbey College
100 Belmont Mt. Holly Road, 28012, Belmont, NC 28012, USA
Tel: 212-410-2094
E-mail: stephenbauer@bac.edu
Received Date: September 20, 2016; Accepted Date: October 19, 2016; Published Date: October 24, 2016
Citation: Bauer SB, Pietropaoli AP, Georas SN, Williams MA (2016) Granulocyte-Macrophage Colony-Stimulating Factor Partially Restores Toll- Like Receptor-Mediated Functional Responses of Monocytes in Septic Shock. J Clin Diagn Res 4:131. doi: 10.4172/2376-0311.1000131
Copyright: © 2016 Bauer SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Clinical Diagnosis and Research
Abstract
Septic shock remains clinically challenging to manage. The dysregulated immune response seen in septic shock contributes to this complexity. However, biological response modifiers including the cytokine recombinant human granulocyte-macrophage colony-stimulating factor (rHuGM-CSF) might be important in managing both septicemia and septic shock. We hypothesized that ex vivo stimulation with rHuGM-CSF could restore functional and phenotypic responses of monocytes. The functional responses of complex whole blood and highly-enriched monocyte cultures were assessed from a panel of n=30 human study subjects (n=23, septic shock and n=7 healthy controls). Cultures were initially primed with rHuGM-CSF then stimulated with relevant toll-like receptor (TLR) ligands for short (4 h) or extended (48 h) time-periods. TLR-specific ligands included lipopolysaccharide (LPS, a surrogate of gram-negative bacterial infection) and double-stranded RNA (dsRNA, a surrogate of a viral infection). Endpoints included measurement of cell surface receptors by flow cytometry and cytokine secretion by multiplex bead-array technology. We found depressed expression of immune cell-surface markers on septic shock monocytes as compared to normal controls. However, a trend of partially restored cell surface expression of functionally important phenotypic markers on septic shock monocytes was associated with restored, or even augmented, cytokine secretion following rHuGM-CSF-priming and secondary stimulation with LPS or dsRNA. A dysregulated pattern of cell activation typically characterizes septic shock and may contribute to inadvertent immune suppression of monocytes. We have showed that rHuGM-CSF priming restored several important aspects of monocyte responsiveness to exogenous bacterial and viral stimuli. This approach could offer therapeutic utility that nonetheless requires empirical proof of concept.
Keywords
Monocyte; Anergy; Biomarkers; Septic shock; Granulocyte-macrophage colony-stimulating factor
Introduction
Septic shock is a leading cause of mortality in critically ill patients and is a concern in post-surgical rehabilitation. Septic shock is complex and characterized by dysregulated immunity and an inability to effectively clear primary microbial infections [1]. The weakened immune state seen in septic shock increases host susceptibility to opportunistic secondary infections, super-infections like those associated with viruses, bacteriophages and bacterial or fungal infections [2]. Hospitalized trauma patients and immunosuppressed patients, such as those presenting with hematologic or solid malignancies, are often at high risk for developing sepsis and septic shock [3,4].
Monocytes serve important roles in the innate immune response to infection by linking innate and acquired immunity [5]. Circulating monocytes coordinate the response of the immune system to eliminate invading pathogens. Infection with gram-negative and gram-positive bacteria or fungal pathogens may lead to septic shock [6,7]. Septic shock involves a heterogeneous response of monocytes to bacterial components, such as lipopolysaccharide (LPS, or endotoxin) [8,9]. The response of monocytes to bacterial cell wall components, such as LPS, forms the basis of the complex and heterogenic responses seen in septic shock, which display both pro-inflammatory and antiinflammatory effects [10].
LPS is an immunogenic component of gram-negative bacterial cell walls and interacts with cell membrane-associated pattern-recognition receptor toll-like receptor 4 (TLR4) and the cell membrane-associated co-receptor CD14 [11]. Occupation of TLR4 transduces a signal via the MyD88 adaptor protein and the mitogen-activated protein kinases p38 MAPK and ERK I/II with subsequent activation of the transcription factors NF-κB p50/p65 and AP-1 [12,13]. This signaling pathway instructs de novo protein synthesis of immunologically important molecules. This includes expression of HLA-DR (MHC Class II), CD14, the co-stimulatory molecules CD40, CD80 and CD86, secretion of pro-inflammatory cytokines such as TNFα, IL-6 and IL-8, and augmented respiratory burst activity, seen as a rapid secretion of reactive oxygen species and hydrogen peroxide, which collectively display potent anti-microbial properties [14-16].
While the pro-inflammatory response to exogenous microbial “danger signals” like LPS and other antigens is essential for protective host immunity, excessive secretion of pro-inflammatory mediators by monocytes in septic shock is ultimately detrimental [17,18]. In septic shock, the anti-inflammatory response drives monocyte dysfunction and tolerance of the monocyte to further LPS challenge, which may lead to near complete shutdown of the immune competence of monocytes; a state referred to as immune paralysis or immunological anergy of the monocyte [19,20]. In monocyte anergy, cell surface TLR4 expression is lost and expression of both HLA-DR and CD14 is markedly dampened [21,22]. Hyper-responsive monocytes in septic shock may also become immuno-suppressed and subsequently, apoptotic after exhausting metabolic or energetic resources following the initial antigenic challenge, or from sustained stimulation by LPS [23,24].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that is effective in the clinical management of a variety of conditions including complications from infectious diseases and in the management of infections in immuno-compromised patients presenting with hematological or solid tumors. GM-CSF stimulates the production and functional maturation of hematopoietic precursors and developing monocytes both in vivo and in vitro [25]. GM-CSF also reverses monocyte anergy seen in diseases like severe acute pancreatitis [26]. GM-CSF has also seen potential clinical utility in the management of minimal residual disease in human leukemia and other cancers [27-29]. Mouse models of septic shock have reinforced our appreciation that GM-CSF can recover functional responses of monocytes to LPS [30,31]. In addition, GM-CSF may restore monocyte activation in hematological malignancy following high-dose chemotherapy [32,33]. Thus, it seems reasonable that GM-CSF may reconstitute normal immune homeostasis from a position of dysregulated immunity that characterizes septic shock. However, there remains a paucity of mechanistic and functional studies that have explored the ex vivo response of septic shock monocytes to agents capable of restoring functional activity [34,35].
We explored the ability of rHuGM-CSF to partially restore the functional responsiveness of monocytes in septic shock on activation by TLR-dependent signals (i.e., the LPS-TLR4 axis), or viral ligands such as poly I:C, or double-stranded RNA (i.e., the dsRNA-TLR3 axis). We contrasted the responses of monocytes from septic shock and healthy control subjects. By examining a relevant, diverse population of septic shock subjects, our study explored the response of both highly enriched monocyte cultures, and whole blood cultures where the interactions of other cell types and secreted factors were permitted to influence the observed outcomes. The current study sets the foundation for future work detailing observations of restored monocyte responses under the influence of rHuGM-CSF priming ex vivo.
Materials and Methods
Subject recruitment
Thirty subjects were recruited to this study (n=7 healthy controls and n=23 critically ill subjects with septic shock). All septic patients had confirmed or suspected infection and fulfilled the accepted criteria for septic shock at the time [1]. Blood specimens were received within 48 h following diagnosis of septic shock. Investigators were blinded to patient status (septic shock case or healthy control) until completion of all ex vivo laboratory assays of cellular activation. Demographic and clinical parameters of research study subjects are shown in (Table 1a). All subjects or their surrogates provided written informed consent and the study was approved by the University of Rochester Institutional Research Subjects Review Board (RSRB).
| Subject Age | Gender | Primary Infection | Apache Score | Saps II Score Day 1 |
|---|---|---|---|---|
| 71 | Male | Other | 16 | 49 |
| 77 | Male | Pulmonary | 36 | 89 |
| 51 | Male | Pulmonary | 8 | 49 |
| 71 | Male | Pulmonary | 35 | 74 |
| 83 | Male | Pulmonary | 21 | 38 |
| 72 | Male | Pulmonary | 38 | 76 |
| 80 | Male | Pulmonary | 36 | 51 |
| 48 | Male | Pulmonary | 15 | 19 |
| 30 | Male | Pulmonary | 12 | 3 |
| 28 | Male | Pulmonary | 18 | 30 |
| 64 | Male | Pulmonary | 27 | 47 |
| 57 | Male | Intra-abdominal | 9 | 31 |
| 22.6 | 46.3 | |||
| 19.5 | 48 | |||
| 11.3 | 24.7 |
Table 1a: A summary of the demographics of the septic shock.
Ex vivo culture of peripheral blood cells and experimental design
We designed three separate experiments to explore the immune restoring functions of rHuGM-CSF (PeproTech, Rocky Hill, NJ) on activation of monocytes in cultures of whole blood and in highly enriched mononuclear cell cultures ex vivo. In experimental platforms one and two, the objective was to test the response of mononuclear cells in whole blood cultures, which would permit other immune cells to interact and influence the outcome of the measured endpoints. Experimental endpoints included flow cytometric assays of cell surface-expressed function-associated markers and assays of cytokine secretion by multiplex cytokine array (Bio-Rad, Hercules, CA). In the third experimental platform, we wished to test for the ability of highly enriched monocyte cultures to respond to GM-CSF and contrast these outcomes to those obtained from unfractionated blood cultures obtained from platforms one and two. In the third experimental platform, the possible interactions of monocytes with other cellular components of whole blood were excluded, thereby addressing the issue of how an enriched population of mononuclear cells may respond to the immune-stimulating effects of LPS, or dsRNA, with, or without, prior rhGM-CSF-priming.
Short-term culture (4 hrs)/whole blood platform (Experimental Platform 1)
In the first experimental platform, 1-2 ml of peripheral venous blood was anti-coagulated in preservative-free sodium heparin. For these assays, blood specimens were diluted 1:2 in complete culture medium (RPMI-1640 base media, Invitrogen, Carlsbad, CA), supplemented with 20 mM HEPES buffer (Invitrogen, Carlsbad, CA), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 2.5 μg/ml gentamycin sulfate (Invitrogen, Carlsbad, CA), 8% v/v fetal bovine serum (FBS) (Tissue Culture Biologicals, Tulare, CA), 5.5 x 10-2 mM betamercaptoethanol (Gibco, Carlsbad, CA), 1 mM sodium pyruvate (Gibco, Carlsbad, CA), 10-1 mM (each amino acid) MEM nonessential amino acids (Gibco, Carlsbad, CA), and seeded as 0.5 ml volumes into sterile, 12 x 75 mm, pyrogen-free, polypropylene, LP4 tubes (Corning Life Sciences, Lowell, MA). Cells were primed in the presence, or absence, of rHuGM-CSF (50 ng/ml) for 2 h, at 37ºC. Cells were then immediately challenged for an additional 2 h in the presence of no other stimulus (“Resting”), LPS (1 ug/ml, E. coli-derived endotoxin, serotype 055:B5 in endotoxin-free water, Sigma-Aldrich, St. Louis, MO), or dsRNA (100 ng/ml, Poly(I:C), InvivoGen, San Diego, CA), which is a synthetic dsRNA analog reconstituted in endotoxinfree PBS. Four conditions were thus set up: (A) Resting cells without rHuGM-CSF, (B) rHuGM-CSF-primed cells, (C) rHuGM-CSF-primed cells followed by LPS stimulation, and (D) rHuGM-CSF-primed cells followed by dsRNA stimulation. At the conclusion of the assay, monocytes were harvested for quantitation of cell membrane expressed phenotypic markers by flow cytometry as described below.
Long-term culture (48 hrs)/whole blood platform (Experimental Platform 2)
In this experiment, 2 ml of peripheral blood was anti-coagulated in sodium citrate to test the effect of long-term exposure (48 h in culture) of whole blood monocytes to rHuGM-CSF stimulated with/without TLR ligands (LPS-TLR4 axis and dsRNA-TLR3 axis). Each blood sample was diluted in complete RPMI1640 culture medium at a 1:3 ratio, and seeded into a 12-well culture dish (Corning Life Sciences, Lowell, MA) at 1 ml/well. Cultures were primed with/without rHuGMCSF (50 ng/ml) for 4 h, at 37ºC prior to secondary stimulation with/ without, LPS (1 ug/ml), or dsRNA (100 ng/ml) for 48 h. In this way, six conditions were set up: (A) "Resting" cells without rHuGM-CSF, (B) Cells stimulated with LPS alone, (C) Cells stimulated with dsRNA alone, (D) rHuGM-CSF-primed cells, (E) rHuGM-CSF-primed cells and LPS stimulation, and (F) rHuGM-CSF-primed cells and dsRNA stimulation. At the conclusion of the assay, monocytes were analyzed for cell surface expression of various immune markers by flow cytometry as described below. Culture supernatants were aliquotted in duplicate and stored at -80ºC until assayed for cytokine secretion by multiplex array analysis as described below.
Long-term culture (48 hrs)/purified monocyte platform (Experimental Platform 3)
In this experiment, a monocyte-enriched fraction was prepared from 1-2 ml K3. EDTA anti-coagulated blood by centrifugal separation over a discontinuous solution of 13.5% w/v iodixanol (Metrizamide, Sigma-Aldrich, St. Louis, MO) cell separation medium. Briefly, whole blood was diluted 1:2 with sterile divalent cation-free phosphate buffered saline (PBS) that was supplemented with 2% v/v fetal bovine serum (F-PBS), and layered over an equal volume of 13.5% w/v iodixanol to enrich for peripheral blood mononuclear cells containing monocytes. Samples were centrifuged at 800 x g, 30 min, at 20ºC, without brakes off and at the lowest acceleration. The low-density cells were harvested from the iodixanol/F-PBS interface, washed three times in warm complete RPMI1640 following which, cells were resuspended in complete culture medium, counted and seeded into a 6-well plate at 1 ml/well, or 106 cells/well.
Flow cytometry of whole blood cultured cells
Flow cytometric analysis of whole blood cultured cells included assessment of cell surface function and activation-associated molecules, or receptors. These included the following anti-human monoclonal antibodies conjugated to either a fluorescein isothiocyanate (FITC), or phycoerythrin (PE) fluorochrome: MHC Class II (HLA-DR) (FITC; BD Biosciences, San Jose, CA), CD14 (FITC; BD Biosciences, San Jose, CA), CD71 (PE; Immunotech, Fullerton, CA), CD80 (PE; BD Biosciences, San Jose, CA), CD86 (PE; BD Biosciences, San Jose, CA), TLR2 (PE; Santa Cruz, Santa Cruz, CA), TLR4 (PE; Santa Cruz, Santa Cruz, CA), Tissue Factor (FITC; American Diagnostics, Hauppauge, NY), CD11b (PE; BD Biosciences, San Jose, CA), and CD54 (FITC; Immunotech, Fullerton, CA). Samples were stained at 4ºC, for 30 min, in the dark. Samples were next lysed in BD FACS lysis solution (BD Biosciences, San Jose, CA) in the dark for 10 min, at room temperature, then centrifuged at 400 x g, 4ºC, for 6 min, washed once in FACS wash buffer (divalent cation-free PBS supplemented with 2% v/v FBS and 0.2% w/v sodium azide), centrifuged at 400 x g, 4ºC, for 6 min, and finally, each sample was resuspended and fixed in 250 μL of 2% w/v paraformaldehyde in FACS wash buffer prior to subsequent analysis using a FACScaliber flow cytometer (Becton Dickinson, Franklin, NJ) and CellQuest 3.1 software (Becton Dickinson, Franklin, NJ). In conjunction, the appropriate murine IgG isotypic controls were used for cell surface marker analysis (PE and FITC; BD Biosciences, San Jose, CA) to control against non-specific Fc-gamma receptor-mediated, nonspecific binding of the antibodies. Data was acquired in real-time as geometric mean fluorescence intensity (MFI) and percent fluorescent positive cells (% positive). The instrument had a standard optical filter configuration with band pass filters of 530/30-nm and 585/44-nm for FL1 (FITC-conjugated antibodies) and FL2 (PE-conjugated antibodies) data acquisition, respectively. For the analysis of forwardangle light scatter, side-angle light scatter, and cell surface receptor expression, data was acquired in real-time. The instrument was standardized prior to phenotypic analysis with calibration beads (FluoroSpheres 6-Peak; Dako Cytomation, Carpinteria, CA) and cleaned with sequential washes of distilled water, 10% v/v hypochlorite, and distilled water before data acquisition.
Quantitation of inflammatory cytokines by multiplex cytokine array assay
The Bio-Plex 200 (Bio-Rad, Hercules, CA) was used to assess the amount of human cytokines in the supernatants of the long-term whole blood and whole blood-purified monocyte cultures; 50 μL of each undiluted supernatant was run. The cytokine bead standards were diluted in RPMI-base complete culture medium to correlate with the supernatant base matrix. A minimum of one blank (complete culture media alone) was run on each Bio-Plex 200 ninety-six well plate.
Statistical analysis
Demographic data of all subjects are contained in Table 1b. Phenotypic markers and cytokine measures were evaluated for distributional properties and transformed into the logarithm scale to meet model assumptions. Linear mixed models utilizing geometric mean data for phenotypic markers and observed cytokine values were used to assess each parameter as a function of condition (healthy vs. septic shock), treatment (± rHuGM-CSF and/or ± LPS, or dsRNA), and interaction of condition and treatment in short-term (4 hrs), or long-term (48 hrs) in vitro cell culture assays. Comparisons of the treatment groups with baseline within and over the conditions were carried out subsequent to the overall analyses. All analyses were carried out using SAS 9.2 (Copyright © 2009 SAS Institute Inc., Cary, North Carolina 27513, USA) on a Windows Vista Enterprise 32-bit platform. An alpha value of p<0.05 was considered a statistically significant difference between pairs or groups of data, unless otherwise stated.
| Subject Age | Gender | Primary Infection | Apache Score | Saps II Score Day 1 |
|---|---|---|---|---|
| 49 | Female | Blood | 47 | 10 |
| 66 | Female | Other | 39 | 10 |
| 39 | Female | Pulmonary | 60 | 11 |
| 44 | Female | Intra-abdominal | 22 | 7 |
| 74 | Female | Pulmonary | 97 | 14 |
| 65 | Female | Intra-abdominal | 72 | 9 |
| 88 | Female | Blood | 30 | 5 |
| 54 | Female | Pulmonary | 53 | 8 |
| 62 | Female | Intra-abdominal | 51 | 7 |
| 47 | Female | Pulmonary | 51 | 14 |
| 51 | Female | Pulmonary | 27 | 4 |
| 23.4 | 49.9 | |||
| 24 | 51 | |||
| 8.7 | 21.6 |
Table 1b: A summary of the demographics of the healthy subjects that were a part of this study.
Results
Phenotypic characterization of whole blood cultures, either 4 h (short-term) or 48 h (long-term) was performed as described above in Materials & Methods. Phenotypic characterization of whole blood purified monocyte cultures (third experimental platform) was not assessed due to an insufficient number of cells, which was accounted for in the initial experimental platform. However, cytokine secretion, a functional measurement, was assessed for the 48 h whole blood cultures and 48 h whole blood-purified monocyte cultures. The cumulative data was obtained from a blinded study of 30 individuals that when uncoded, revealed 23 septic shock subjects and 7 healthy subjects. Demographic data of all subjects are contained in Table 1b.
Initial assessment of septic shock whole blood and wholeblood purified monocytes
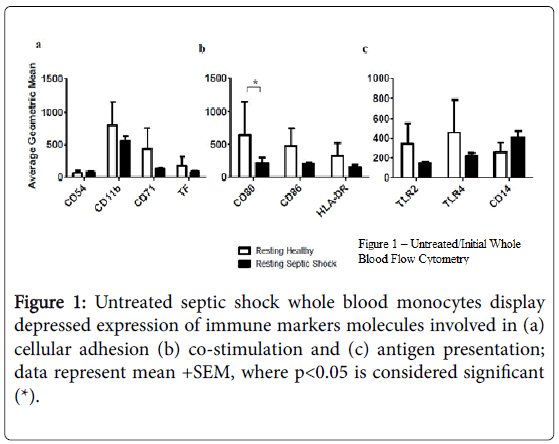
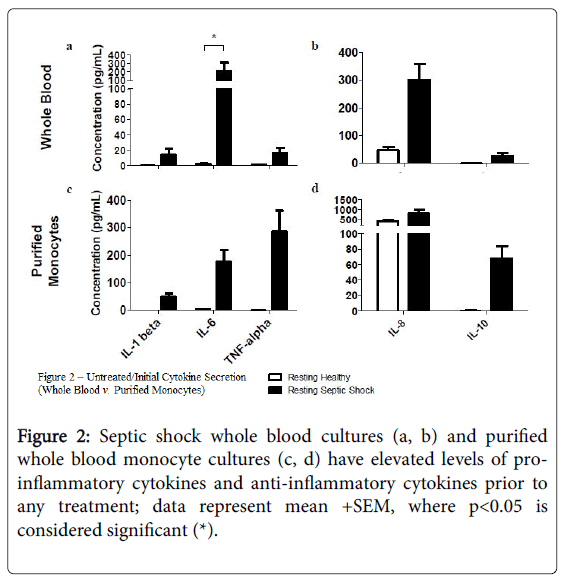
Using flow cytometry, we found that cell surface expression of several markers was reduced on monocytes from subjects with septic shock compared with healthy controls, including TLR2 and TLR4, as well as CD11b, CD71, CD80 (p<0.05), CD86, and HLA-DR (Figure 1). In addition, the supernatants from resting, septic shock, whole blood cultures, from which these monocytes were examined, had elevated average levels of IL-1 beta, IL-6 (p<0.05), TNF-alpha, IL-8, and IL-10.
Figures 2a and 2b, when compared to the resting, healthy, whole blood cultures; a similar trend was found in resting, septic shock, whole blood-purified monocyte cultures Figures 2c and 2d.
Assessment of monocytes in short-term whole blood cultures (Experimental Platform 1)
rHuGM-CSF alone was unable to significantly enhance the expression of any of the monocyte markers examined (data not shown). Furthermore, in short-term 4 h cultures ex vivo , rHuGM-CSF had an almost negligable impact on the septic shock monocyte phenotypic response to TLR agonists, LPS, or dsRNA (data not shown). Healthy whole blood monocytes were used for comparison. Cell culture supernatants were not assessed for cytokine levels in this experimental platform, due to the short duration of the experiment, which is not applicable to cytokine detection.
Assessment of monocytes in long-term whole blood cultures (Experimental Platform 2)
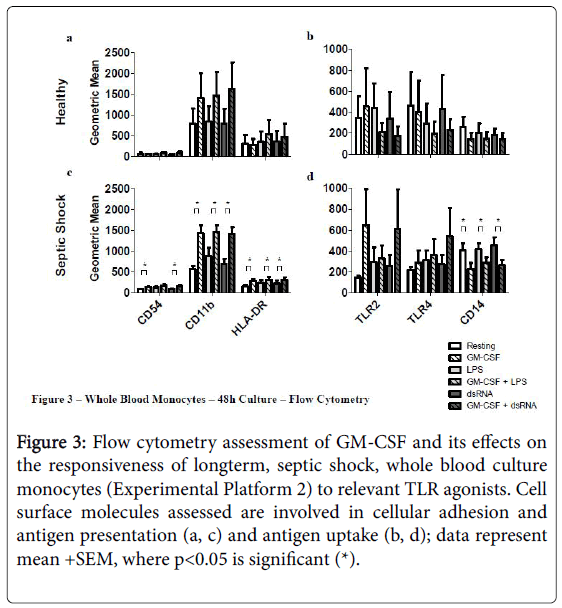
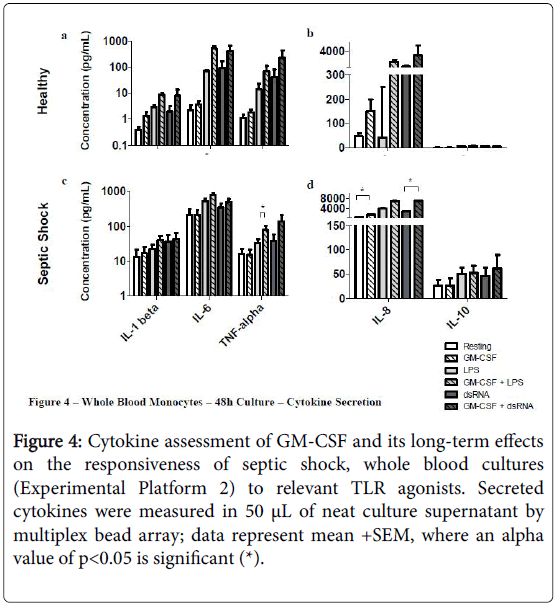
Priming septic shock whole blood cultures with rHuGM-CSF for 48 h had a stronger impact than in the short-term 4 hr cultures (Experimental Platform 1; Figures 3 and 4).
Figure 3: Flow cytometry assessment of GM-CSF and its effects on the responsiveness of longterm, septic shock, whole blood culture monocytes (Experimental Platform 2) to relevant TLR agonists. Cell surface molecules assessed are involved in cellular adhesion and antigen presentation (a, c) and antigen uptake (b, d); data represent mean +SEM, where p<0.05 is significant (*).
Figure 4: Cytokine assessment of GM-CSF and its long-term effects on the responsiveness of septic shock, whole blood cultures (Experimental Platform 2) to relevant TLR agonists. Secreted cytokines were measured in 50 μL of neat culture supernatant by multiplex bead array; data represent mean +SEM, where an alpha value of p<0.05 is significant (*).
For example, long-term culture with rHuGM-CSF alone was able to significantly-enhance (p<0.05) septic shock monocyte levels of CD54, CD11b and HLA-DR, while also noticeably enhancing TLR2 and TLR4. Furthermore, prior to LPS activation, rHuGM-CSF-priming significantly-enhanced whole blood septic shock monocyte levels of CD11b and HLA-DR (p<0.05), and noticeably enhanced the expression levels of CD54, TLR2, and TLR. Moreover, in whole blood cultures that were primed with rHuGM-CSF alone or with LPS activation following rHuGM-CSF priming, CD14 expression levels were significantly decreased (p<0.05). rHuGM-CSF-priming also significantly-enhanced the levels of CD54, CD11b, and HLA-DR on whole blood septic shock monocytes, in response to dsRNA; TLR2 and TLR4 were also enhanced, while CD14 was significantly decreased (p<0.05). Whole blood healthy monocytes are provided for a phenotypic comparison. Monocyte phenotypic changes facilitated by rHuGM-CSF priming of septic shock whole blood, resulted in culture supernatant cytokine level changes (Figure 4). rHuGM-CSF alone was able to significantly-enhance septic shock whole blood levels of IL-8, while also enhancing IL-1 beta. Furthermore, rHuGM-CSF-priming significantly-enhanced the TNF-alpha septic shock whole blood culture responses to LPS; IL-1 beta, IL-6, and IL-8 were also enhanced. rHuGM-CSF-priming significantly-enhanced the septic shock whole blood culture IL-8 response to dsRNA, while also enhancing the IL-1 beta, IL-6, TNF-alpha, and IL-10 responses to this TLR3 agonist.
Assessment of monocytes in long-term cultures (Experimental Platform 3)
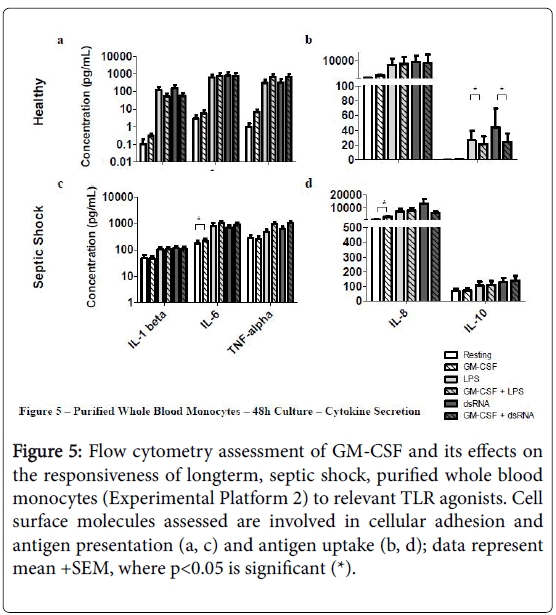
rHuGM-CSF-priming of purified septic shock monocyte cultures allowed for significant-enhancement of IL-6 and IL-8 secretion, while also enhancing secretion of IL-6 and TNF-alpha, in response to LPS, or dsRNA (Figure 5). Due to the amount of patient blood received, a phenotypic assessment could not be performed in this experimental platform.
Figure 5: Flow cytometry assessment of GM-CSF and its effects on the responsiveness of longterm, septic shock, purified whole blood monocytes (Experimental Platform 2) to relevant TLR agonists. Cell surface molecules assessed are involved in cellular adhesion and antigen presentation (a, c) and antigen uptake (b, d); data represent mean +SEM, where p<0.05 is significant (*).
Discussion
Septic shock mortality remains at a consistently high level in hospital intensive care units [36]. As such, considerably more clinical and animal research must be done to understand the complex nature of this condition. Our clinical study adds valuable data to the field, not only in its examination of modulating the response of septic shock subjects’ whole bloods to relevant toll-like receptor (TLR) ligands by the hematopoietic growth factor, GM-CSF, but in its focused effort to examine the rHuGM-CSF-modulation of a key cell in these responses, the monocyte. Known to possess immunomodulating properties, GMCSF, was studied for its ability to enhance the monocyte response to relevant TLR ligands, such as LPS and dsRNA, in whole-blood and whole blood-purified monocyte ex vivo cell cultures.
Our initial examination of resting whole blood and whole bloodpurified septic shock monocytes revealed depressed expression of CD80 (p<0.05), as well as, depressed expression of CD11b, CD71, CD86, HLA-DR, TLR2, and TLR4, when compared to healthy monocyte controls. The reduced expression of these important monocyte immune markers has been previously shown to be a characteristic of septic shock. Furthermore, the enhanced levels of proinflammatory cytokines, such as IL-6 (p<0.05), IL-8, TNF-alpha and of IL-10, an anti-inflammatory cytokine, support the septic shock diagnosis of these blood samples. Monocytes examined in our wholeblood purified monocyte cultures displayed a similar cytokine secretion pattern.
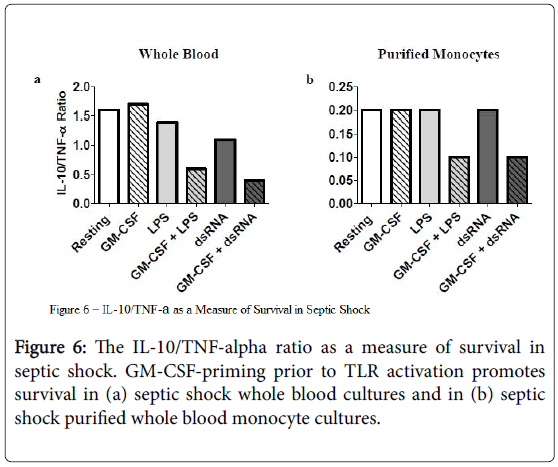
IL-10/TNF-alpha ratio as a measure of responsiveness to rHuGM-CSF
The ratio of the anti-inflammatory cytokine IL-10, in relation to the pro-inflammatory cytokine TNF-alpha, decreased under conditions where rHuGM-CSF-priming occurred before stimulation with LPS or dsRNA (Figure 6). This trend was consistent when studying culture supernatants derived from both septic shock whole blood and their counterpart purified monocyte cultures and supports the potential immune-enhancing effect of rHuGM-CSF priming on septic shock monocytes prior to TLR activation [43].
In the 4 h experimental platform, we observed no significant phenotypic changes in healthy or septic shock whole blood monocytes that were cultured with rHuGM-CSF alone, or together with LPS or dsRNA. There are two reasonable explanations for this. First, it is possible that although there were no effects of rHuGM-CSF on healthy or septic shock whole blood monocytes in the short-term culture platform, other cells in the blood may have been affected by the rHuGM-CSF. GM-CSF is a multi-functional biological response modifier that is produced by many cell types and has effects on the functional activities of various lymphocytes [37]. Second, a 2 h priming period may not be sufficient to see an average effect of rHuGM-CSF on monocytes. For instance, Brock, et al. found that enhanced leukotiene synthesis in alveolar macrophages required a minimum of 6 h GM-CSF pre-treatment before subsequent stimulation with a calcium ionophore [38].
GM-CSF, like another proposed septic shock therapy, IFN-gamma, targets the alteration of cellular activity, a broad-spectrum effect. Other septic shock therapies aimed at specific molecular targets, such as anti- TNF-alpha, have been ineffective in clinical trials [39]. Studies supporting the use of GM-CSF instead of IFN-gamma in septic shock treatment include those where IFN-gamma treatment of monocytes did not reconstitute defective pro-inflammatory cytokine production in sepsis after major visceral surgery [40]. Antibiotics, routinely used in treatment against bacterial-based septic shock, have the potential of exacerbating the inflammatory condition, as they provide “fuel for the fire” by destroying bacteria, thus releasing more LPS into an already dysregulated system [41].
In the second experimental platform, 4 h of rHuGM-CSF-priming enhanced the septic shock whole blood monocyte response to LPS, observed by an increase in monocyte expression of CD54, CD71, CD11b (p<0.05), TLR2, TLR4, and HLA-DR (p<0.05) after 48 h in culture. The enhanced monocyte immune phenotype supports the observed enhanced levels of IL-1 beta, IL-6, TNF-alpha (p<0.05), and IL-8 found with rHuGM-CSF-priming prior to LPS activation; IL-10 levels remained stable whether or not rHuGM-CSF was given prior to LPS activation. rHuGM-CSF-priming enhanced the septic shock whole blood monocyte response to dsRNA as well, with enhanced CD54 (p<0.05), CD11b (p<0.05), TLR2, TLR4, CD80, and HLA-DR (p<0.05). The enhanced monocyte immune phenotype supported the increased secretion of IL-1 beta, IL-6, TNF-alpha, and IL-8 (p<0.05) that were observed if rHuGM-CSF priming occurred prior to dsRNA activation; IL-10 levels did not significantly increase. Interestingly, septic shock monocyte CD14 levels significantly decreased when rHuGM-CSF was used to prime the cultures before activation with LPS or dsRNA. This may seem contradictory, as CD14 cooperates with TLR4 in the monocyte immune response to LPS and GM-CSF has been shown to activate mature macrophages and granulocytes. However, the findings of Kruger, et al. suggest that this CD14 downregulation, facilitated by GM-CSF, may represent an initial responsiveness to further monocyte differentiation to prepare for responsiveness to TLR ligands, such as LPS [42].
Experimental platform three sought to directly assess the effects of rHuGM-CSF, if any, on septic shock monocytes, where monocytes were isolated from the whole blood and put into culture. Whole blood contains a variety of cell types and serum components that could alter monocyte activity, making it important to confirm the effects of rHuGM-CSF on monocytes.
Due to a limiting amount of patient blood, we were only able to examine cell culture supernatants from whole blood purified monocyte cultures in this platform. Healthy whole blood culture control data is provided for comparison. rHuGM-CSF-priming resulted in a marginal enhancement of IL-8 alone (p<0.05), as well as in TNF-alpha in response to LPS, or dsRNA, with no change in IL-10 production. This further supports the importance of monocyte responsiveness to TLRagonists, such as LPS and dsRNA.
Further support for beneficial effects of GM-CSF on septic shock monocytes is shown by Figure 6. It has been shown that the ratio of IL-10/TNF-alpha is a predictor of survival. The average IL-10/TNFalpha ratio is higher in patients who die from septic shock [43]. In our studies, we found that the average IL-10/TNF-alpha ratio decreased when rhGM-CSF-priming was utilized prior to LPS or dsRNA activation of cell cultures of both septic shock whole blood and whole blood-purified monocytes. This would indicate a trend whereby GMCSF is promoting survival in septic shock by promoting monocyte immune responsiveness.
In conclusion, our data trends with this clinical study group support our earlier studies and a thus further investigation of the biological response modifier, GM-CSF, in septic shock therapy. Our resting whole blood phenotypic data supports monocyte immunosuppression, while our phenotypic and cytokine data support rHuGM-CSF’s ability to enhance septic shock monocyte responses to relevant toll-like receptor ligands, such as LPS and dsRNA.
We acknowledge that the effect of GM-CSF on immunological restoration of functional monocytes deserves to be further investigated in a much larger parallel study, which might ideally also incorporate other potential immuno-stimulating compounds in combination with rHuGM-CSF. Furthermore, the timing of GM-CSF treatment in the clinic, genetic factors, and stage of disease will likely determine the effectiveness of GM-CSF in septic shock patients and is another area of research that still needs further study. This information will be useful in properly assessing the differential advantages or disadvantages of GM-CSF therapy. Finally, it is also important to study the effects of GM-CSF on septic shock in combination with other immune enhancing agents; possibly utilizing vitamin D analogs, which are regulators of myeloid cell development and differentiation.
Acknowledgements
We would like to thank Saria Awadalla for assistance with the biometric/statistical analysis of the data, Judith Stewart for assistance with the clinical samples, and Dr. Mary Ann Morgan for assisting with the provision of venous blood samples. The Department of Medicine, the University of Rochester School of Medicine and Dentistry, NY, NIH K23 HL080077 and NIH/NHLBI T32 HL66988 supported this work.
References
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644-1655.
- Martin GS (2012) Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Exp Rev Anti-infective Therapy 10: 701-706.
- Chai J, Zhi-yong S, Hong-ming Y, Dai-feng H, Chuan-an S, et al. (2007) Treatment strategies for mass burn casualties. Chin Med J (Engl) 120: 1783-1787.
- Pène F, Percheron S, Lemiale V, Viallon V, Claessens YE, et al. (2008) Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med 36: 690-696.
- Schlitzer A, McGovern N, Ginhoux F (2015) Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin Cell Dev Biol 41: 9-22.
- Seneca H. (1977) Gram-negative sepsis: part I. Pathogenesis. Compr Ther 3: 49-56.
- Kim YJ, Kim SI, Kim YR, Park YM, Park YJ, et al. (2007) Successful Treatment of Septic Shock with Purpura Fulminans Caused by Trichosporon asahii in an Immunocompetent Patient. Annals of Clinical & Laboratory Science 37: 366-369.
- Parant M, Parant F, Chedid L (1977) Inheritance of lipopolysaccharide-enhanced nonspecific resistance to infection and of susceptibility to endotoxic shock in lipopolysaccharide low-responder mice. Infect Immun 16: 432-438.
- Schöffel U, Shiga J, Mittermayer C (1982) The proliferation-inhibiting effect of endotoxin on human endothelial cells in culture and its possible implication in states of shock. Circ Shock 9: 499-508.
- Gentile LF, Cuenca AG, Efron PA, Ang D, McKinley BA, et al. (2012) Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72: 1491-1501.
- Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, et al. (1988) The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science 239: 497-500.
- Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, et al. (2000) Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol 12: 113-117.
- Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, et al. (1999) Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem 274: 7611-7614.
- Ou XM, Feng YL, Wen FQ, Wang K, Yang J, et al. (2008) Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-kappaB. Respirology 13: 63-72.
- Klinke DJ, Ustyugova IV, Brundage KM, Barnett JB (2008) Modulating temporal control of NF-kappaB activation: implications for therapeutic and assay selection. Biophys J 94: 4249-4259.
- Barbera M, Caputo A, Zampiron A, Gobbi S, Rampa A, et al. (2008) The ability of coumarin-, flavanon- and flavonol-analogues of flavone acetic acid to stimulate human monocytes. Oncol Rep 19: 187-196.
- Ito K, Goto N, Futamura K, Okada M, Yamamoto T, et al. (2015) Death and kidney allograft dysfunction after bacteremia. Clin Exp Nephrol 20: 309-15.
- Tsujimoto H, Ono S, Majima T, Efron PA, Kinoshita M, et al. (2006) Differential toll-like receptor expression after ex vivo lipopolysaccharide exposure in patients with sepsis and following surgical stress. Clin Immunol 119: 180-187.
- Brandtzaeg P, Osnes L, Ovstebø R, Joø GB, Westvik AB, et al. (1996) Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med 184: 51-60.
- Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, et al. (1995) Downregulation of pro-inflammatory cytokine release in whole blood from septic patients. Blood 85: 1341-1347.
- Williams MA, White SA, Miller JJ, Toner C, Withington S, et al. (1998) Granulocyte-macrophage colony-stimulating factor induces activation and restores respiratory burst activity in monocytes from septic patients. J Infect Dis 177: 107-115.
- Arens C, Bajwa SA, Koch C, Siegler BH, Schneck E, et al. (2016) Sepsis-induced long-term immune paralysis-results of a descriptive, explorative study. Crit Care 20: 93.
- Shao R, Fang Y, Yu H, Zhao L, Jiang Z, et al. (2016) Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care 20: 124.
- Hotchkiss RS, Nicholson DW (2006) Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813-822.
- Schriber JR, Negrin RS (1993) Use and toxicity of the colony-stimulating factors. Drug Saf 8: 457-468.
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, et al. (2015) Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33: 1325-1333.
- Hutzschenreuter F, Monsef I, Kreuzer KA, Engert A, Skoetz N (2016) Granulocyte and granulocyte-macrophage colony stimulating factors for newly diagnosed patients with myelodysplastic syndromes. Cochrane Database Syst Rev 2: CD009310.
- Clifton GT, Gail V, Peoples GE, Mittendorf EA (2016) Clinical development of the E75 vaccine in breast cancer. Breast Care (Basel) 11: 116-21.
- Zeidner JF, Gladstone DE, Zahurak M, Matsui WH, Gocke C, et al. (2014) Granulocyte-macrophage colony-stimulating factor (GM-CSF) enhances the clinicalresponses to interferon- α (IFN) in newly diagnosed chronic myeloid leukemia (CML). Leuk Res 38: 886-90.
- Bleiburg I, Riklis I, Fabian I (1990) Enhanced resistance of bone barrow transplanted mice to bacterial infection induced by recombinant granulocyte-macrophage colony-stimulating factor. Blood 75: 1262-1266.
- Bermudez LE, Martinelli JC, Gascon R, Wu M, Young LS (1990) Protection against gram-negative bacteremia in neutropenic mice with recombinant granulocyte-macrophage colony-stimulating factor. Cytokine 2: 287-93.
- Williams MA, Kouroumoussis I, Syndercombe-Court D, Hendry L, Newland AC, et al. (1995) Administration of recombinant human granulocyte-macrophage colony-stimulating factor after chemotherapy regulates the expression and secretion of monocyte tumor necrosis factor (TNF) and TNF receptors. Blood 86: 4234-4242.
- Williams MA, Kelsey SM, Collins PW, Gutteridge CN, Newland AC (1995) Administration of rHuGM-CSF activates monocyte reactive oxygen species secretion and adhesion molecule expression in vivo in patients following high-dose chemotherapy. Br J Haematol 90: 31-40.
- Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, et al. (2009) Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 180: 640-648.
- Mathias B, Szpila BE, Moore FA, Efron PA, Moldawer LL (2015) A review of GM-CSF therapy in sepsis. Medicine 94: 1-10.
- Cohen J, Opal S, Calandra T (2012) Sepsis studies need new direction. Lancet Infect Dis 12: 503-05.
- Shi Y, Liu CH, Roberts AI, Das J, Xu G, et al. (2006) Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Research 16: 126-133.
- Brock TG, McNish RW, Coffey MJ, Ojo TC, Phare SM, et al. (1996) Effect of Granulocyte-Colony Stimulating-Factor on Eicosanoid Production by Mononuclear Phagocytes. J. Immunol 156: 2522-2527.
- Reinhart K, Wiegand-Löhnert C, Grimminger F, Kaul M, Withington S, et al. (1996) Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med 24: 733-742.
- Weighardt H, Heidecke CD, Emmanuilidis K, Maier S, Bartels H, et al. (2000) Sepsis after major visceral surgery is associated with sustained and interferon-gamma-resistant defects of monocyte cytokine production. Surgery 127: 309-315.
- Kumar A (2009) Optimizing antimicrobial therapy in sepsis and septic shock. Crit Care Clin 25: 733-751, viii.
- Kruger M, van de Winkel GJ, De Wit TPM, Coorevits L, Ceuppens JL (1996) Granulocyte-Macrophage Colony-Stimulating Factor Down-regulates CD14 Expression on Monocytes. Immunology 89: 89-95.
- Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181: 176-180.
Relevant Topics
- Back Pain Diagnosis
- Cardiovascular Diagnosis
- Clinical Diagnosis
- Clinical Echocardiography
- COPD Diagnosis
- Diabetes Diagnosis
- Diagnosis Methods
- Diagnosis of cancer
- Diagnosis of CNS
- Diagnosis of Diabetes
- Diagnostic Products
- Diagnostics Market Analysis
- Heart diagnosis
- Immuno Diagnosis
- Infertility Diagnosis
- Medical Diagnostic Tools
- Preimplementation Genetic Diagnosis
- Prenatal Diagnostics
- Ultrasonography
Recommended Journals
Article Tools
Article Usage
- Total views: 4669
- [From(publication date):
December-2016 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 3720
- PDF downloads : 949