Research Article Open Access
Graft Materials in Oral Surgery: Revision
Xavier Rosello Labres1, Alvar Rosello Camps1, Enric Jane Salas1, Rui Albuquerque2, Eugenio Velasco Ortega3 and Jose Lopez-Lopez1*1 Department of Stomatology, School of Dentistry, University of Barcelona, Barcelona, Spain
2 School of Dentistry, University of Birmingham, Oral practice in Oral Medicine, Birmingham Dental Hospital, UK
3 Medical and Surgery DoctorIntegratedAdultDentistry, University of Seville, Spain
- Corresponding Author:
- Jose Lopez-Lopez, DDS, PhD
Medical and Surgery Doctor
Doctor Specialist in Stomatology
Professor of Oral Medicine at the School of Dentistry
University of Barcelona, Spain
Tel: 934024270
E-mail: 18575jll@gmail.com
Received date: November 22, 2013; Accepted date: February 06, 2014; Published date: February 13, 2014
Citation: Labres XR, Camps AR, Salas EJ, Albuquerque R, Ortega EV, et al. (2014) Graft Materials in Oral Surgery: Revision. J Biomim Biomater Tissue Eng 19:124. doi:10.4172/1662-100X.1000124
Copyright: © 2014 Labres XR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
Oral implantology is a common procedure in dentistry, especially for fully or partially edentulous patients. The implants must be placed in the best location from both the aesthetic and functional point of view. Because of this it is increasingly more frequent to resort to regeneration techniques that use substitutes of the bone itself, in order to be able to insert the implants in the most appropriate location.
Material and Methodology: A review was performed on the literature from the last ten years based on the following search limitations: “graft materials", "allograft", "xenograft", "autologous graft” and "dentistry”.
Results: 241 works were obtained that after reading their respective summaries, they were reduced to 38, and 9 previous works were included in order to summarize the concepts.
Discussion: Autologous grafts are the "gold standard" of the bone regeneration. They have obvious advantages, but they also have drawbacks. This is why allogeneic and xenogeneic tissues are used. The former because of their clear similarity with the recipient's tissue and the latter due to their wide availability. Given that these grafts also have drawbacks, the industry has developed synthetic materials that have properties similar to those of human bone tissue. However, as of today, the ideal material to substitute human bone has not yet been found. In recent years the tendency has been to combine these synthetic materials with the patient's own bone, which is extracted during drilling in implant placement, with bone marrow aspiration, or with bone morphogenetic proteins. Thus the intention is to equip these substances with the osteogenic capacity.
Conclusions: There is currently no ideal graft material, with the exception of those materials that come directly from the patient. We hope that in the coming years we will have products that will allow us to perform rehabilitations with better results and provide a better quality of life for our patients, especially those who have more complex situations to resolve, like the patients that are operated on for head and neck cancer.
Keywords
Graft materials;Allograft;Xenograft; Autologous graft
Introduction
The use of osseointegrated implants in the rehabilitation of the stomatognathic system is an increasingly more frequent procedure with a very predictable prognosis. However, the success of this technique is not the mere achievement of the osseointegration of the implant in the osseous bed. The ultimate goal must be the restitution of the missing teeth, through the placement of implants in anatomically appropriate positions in order to achieve a good aesthetic and functional result in the long term [1]. This result will depend, in large measure, on the volume of alveolar bone remaining. The loss of volume that the alveolar ridge experiences will vary depending on the time elapsed since the loss of the teeth, the cause of such loss, the previous treatments that were carried out, as well as the patients' intercurrent system factors [2,3].
The loss of teeth leads to atrophy of the alveolar bone, and many times we will face some major technical challenges when placing the implants in the most appropriate positions. Lack of bone volume, coupled with a low quality of the remaining bone, especially in the back sections of the jaw and mandible, forces us to resort to techniques that allow us to increase the volume, both in width and height, and if possible, the quality of the remaining bone, with the purpose of achieving the desired aesthetic and functional results [3].
Numerous surgical techniques have been developed for the reconstruction of the alveolar ridge and in these techniques various graft materials are used, all of which have advantages and disadvantages. Given that thus far no technique has been described that could be considered ideal and suitable for all circumstances, the surgeon must select and use the technique and graft material that allow for the best implant insertion in each particular case [4]. The materials that we can use as graft material can have diverse origins, which in turn condition their different properties and behaviors. Depending on their ability to interact with the surrounding bone, they can be classified as bioinert or bioactive materials. Bioactive materials are capable of stimulating the formation of bone tissue, by directly joining itself to the bone, thus forming a strong and unique interface of bone-biomaterial [4]. Graft material, on the other hand, should be biocompatible and it should be reabsorbed in order to be integrated into the neoformed bone, which is structurally similar to bone, osteoconductive and if possible also, osteoinductive and osteogenic (Table 1) [5,6]. When we act on the bone regeneration process we must also take its structure into consideration. We know that bone tissue is porous and depending on the size and the interconnection of the pores, it allows for vascularization, the diffusion of nutrients, and ultimately the growth of the tissue. It is this type of architecture and its composition that allows for the migration of the cells of adhesion, their proliferation and differentiation, thus contributing to the bone formation and its repair and regeneration [7].
| Osteoconduction |
| It is the property through which the bone graft material serves as scaffolding for the growth of new bone. The osteoblasts move from the margins of the defect that we want to repair, using the bone graft material as a framework through which it can diffuse and generate new bone, they will continue to incorporate the graft into this new bone, until it completely disappears. All bone graft material must at least be osteoconductive. |
| Osteoinduction |
| This is the stimulating capacity of the osteoprogenitor cells so that they differentiate into osteoblasts. By promoting the formation of new osteoblasts, faster integration of the graft is achieved. This property depends on the bone morphogenetic proteins (BMP) and the osteogenic proteins (collagen, osteonectin, osteopontin and bone sialoprotein) present in the extracellular matrix. This property is that which allows the bone to repair its fractures and to regenerate itself on its own. |
| Osteogenesis |
| It is the production of bone. A material is osteogenic when it has the intrinsic capacity to stimulate the formation of new bone. This is only possible when there are stem cells or vital osteoblasts in the graft material that we put in place. |
Table 1: Concepts of osteoconduction, osteoinduction and osteogenesis [5,6].
The objective of the present study is to review the different graft materials that we can use in oral surgery with the aim of increasing or remodeling the bone volume in order to place implants.
Material and Methodology
Search strategy
The PubMed database was searched by using the keywords “graft materials”, “allograft”, “xenograft”, “autologous graft" and "dentistry".
Selection and inclusion criteria
The first search with a span of up to ten years provided us with 241 works. After reading their abstracts we included 47 of them and 9 previous studies that were relevant to this revision: graft materials AND allograft AND dentistry: 91; graft materials AND xenograft AND dentistry: 31; and graft materials AND autologous graft AND dentistry: 119.
Discussion
Below we will review the most relevant aspects found in the literature with regard to the autologous grafts, allogeneic grafts, xenografts, alloplastic grafts and bone morphogenetic proteins.
Autologous grafts
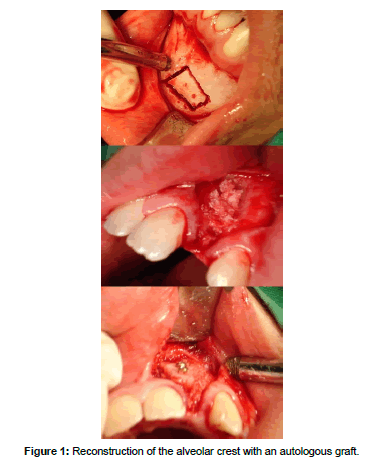
The current reference standard in bone grafts is the autograft, with tissue obtained from the patient himself/herself, which is called an autologous graft (Table 2). This material can be obtained from donor areas near the one on which we are going to intervene, fundamentally the chin, the retromolar space and the ascending ramus of the mandible. It can also be obtained from distant areas such as the iliac crest or the cranial vault, which can cause a less comfortable postoperative experience for the patient (Figure 1). From the intraoral donor sites we are able to obtain a block of monocortical bone tissue, that if necessary, we will be able to divide. If what we need is a particulate tissue, it would be best to resort to the upper maxillary tuberosity area by using a trephine or a chisel. A true fact is that many times the tissue that we are able to obtain from the donor sites is insufficient for the needs of the receptor site. Inorder to try to solve this problem, techniques have been developed such as the RIA system (Reamer/Irrigator/Aspirator), which has been designed to obtain bone tissue and bone marrow as well as eliminate the necrotic and/or infected bone tissue. The tissue that we are going to graft is an osteoconductive, osteoinductive (it contains growth factors) and osteogenic material, which means that it contains living cells that will be transferred to the recipient bed, provided that the time between the extraction and the placement does not exceed two hours and it is kept in an isotonic saline solution [8]. If we process the obtained tissue in order to transform it into small particles, we will destroy a significant percentage of the living cells, therefore reducing its osteogenic capacity [9]. Another aspect to consider is that the tissue is not antigenic.
| AUTOLOGOUS MATERIALS | |
|---|---|
| INTRAORAL ORIGIN | EXTRAORAL ORIGIN |
| CHIN MANDIBULAR RAMUS MAXILLARY TUBEROSITY EXOSTOSIS BIOLOGICAL DRILLING |
ILIAC CREST FIBULA CRANIAL VAULT RIBS TIBIA |
| ALLOGENEIC MATERIALS | |
| FRESH FROZEN FREEZE-DRIED DEMINERALIZED FREEZE-DRIED |
|
| XENOGRAFTS | |
| BOVINE PORCINE EQUINE |
|
| ALLOPLASTIC MATERIALS | |
| CALCIUM SULPHATE CALCIUM PHOSPHATE BETA-CALCIUM TRIPHOSPHATE CORAL HYDROXYAPATITE CERAMICS BIOACTIVE CRYSTALS SYNTHETIC POLYMERS COLLAGEN MATRICES COMPOSITES |
|
| BONE MORPHOGENETIC PROTEINS | |
Table 2: Materials available to regenerate bone in oral surgery.
Autologous grafts can be derived from cortical bone, medullary bone, or a mixture of both. Cortical grafts are fundamentally osteoconductive and grant greater structural support. The spongy ones have greater cellularity, and therefore are more osteogenic, although they confer lesser resistance [10].
The surgeon must consider the use of autologous bone as graft material as a first option, but if this is not possible he/she must then search for alternative materials. In this case the most logical option would be to resort to the tissue that is most similar to the autologous bone, that being bones tissue from the same species, i.e. an allograft.
Allogeneic grafts
According to Russell and Leighton allografts represent 35% of the bone grafts that are carried out [11]. This type of transplant is being performed 25 times more than kidney transplants and 100 times more than heart transplants. This kind of material is closely monitored to ensure maximum safety with respect to its use. The control and monitoring in Spain correspond to the National Transplant Organization (Organización Nacional de Transplantes, ONT) [12] and in the USA to the American Association of Tissue Banks (AATB). Both follow the norms of the Center for Biologics Evaluation and Research (CBER) [13], an agency created under the U.S. Food and Drug Administration (FDA). These organizations require the tissue banks to comply with strict regulations with regard to the selection of donors, protocols for obtaining tissues, and tissue treatments, in order to minimize the possibility of disease transmission [14]. It is therefore necessary, that if we use this type of material we must know its provenance and if the distributor obtains it from a duly authorized tissue bank. Although the bone tissue could come from living donors, it is not usually the case and most commonly it comes from donors who have died.
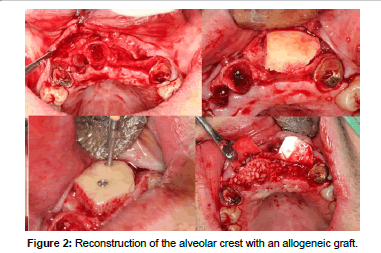
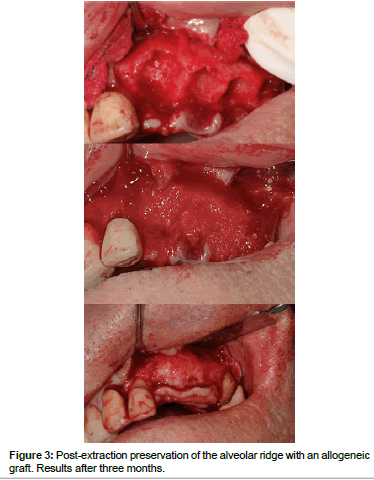
Allografts (Figures 2 and 3) are available in multiple presentations. They are generally used in our field in a particulate form, either in cortical bone, spongy bone, or a mixture of both and also in the form of blocks. The properties of the allografts are limited by the processes of preparation and sterilization that they undergo [15]. These procedures are aimed at reducing the antigenicity of the graft so that it doesn't trigger a graft-versus-host reaction, and to eliminate the risk of disease transmission. The risk of disease transmission, although theoretically possible, is described as less than the risk that exists in a blood transfusion [6,16-19].
Tissue banks act on three levels to ensure the safety of the products that they distribute. First of all, they control the selection of donors, so that they don't use tissue from high-risk donors. Once the donor is accepted, they proceed to perform a battery of serological tests in order to rule out communicable diseases. If the serological results are correct, they proceed to carrying out cultures of the tissue in order to determine the bacterial load. If the results show a high degree of contamination, the tissue will be rejected. Finally, that tissue will undergo an additional process of sterilization in order to minimize the maximum risk [19,20]. Several protocols for treatment and subsequent sterilization have been described, usually through gamma rays, such as BioCleanse®, Tutoplast® or Clearant®, that are able to reduce the risk of infection to 1/106. For the specific case of HIV, the risk can be reduced to 1 in 8,000,000, much lower than the risk of a blood transfusion, which is 1/450,000. From the technical point of view, it has been achieved by applying certain radioprotectors to tissues, they can be irradiated under certain conditions with a dose of 50 kGy and there is no reduction in the structural properties of the tissue [17,21]. In spite of all of this, the risk of "zero" does not exist and some cases of disease transmission from donor to recipient have been reported [21-24]. While this is true, companies such as Tutogen Medical GmbH or RTI Surgical™ (Berlin, Germany) that have been distributing these kinds of grafts for over 30 years, and with millions of surgical procedures performed with their products, do not have any documented case of disease transmission to any recipient [24].
The structural properties of the tissue can be affected by the processes that it undergoes. Depending on their method of conservation the allografts may be fresh, frozen or freeze-dried (Table 3). Fresh allografts are highly antigenic and have little time available for the purposes of carrying out serological tests and detecting possible diseases from the donor, therefore they are no longer used. The frozen allografts will induce less of an antigenic response than the fresh ones; their biomechanical properties are well preserved. Their storage time may be up to one year if they are preserved at -20°C and up to 5 years if we keep them at temperatures of -70°C. The freeze-dried preparations are even less antigenic and may be stored indefinitely at room temperature, although they are given an expiration date of 5 years [18].
| Origen | Osteo conduction | Osteo induction | Osteo genesis | Structural resistance | Antigenicity | Risk of disease transmission | ||
|---|---|---|---|---|---|---|---|---|
| Autologous materials | Cortical | Natural | +++ | ++ | ++ | +++ | - | - |
| Spongy | Natural | +++ | +++ | +++ | - | - | - | |
| Allogeneic materials | Fresh | Natural | +++ | ++ | + | +++ | *** | *** |
| Frozen | Natural | +++ | + | - | +++ | ** | * | |
| Freeze-dried | Natural | +++ | + | - | ++ | * | * | |
| Demineralized | Natural | + | ++ | - | - | - | * | |
| Xenografts | Bovine / porcine / equine | Natural | +++ | - | - | +++ | * | * |
| Alloplastic materials | Calcium phosphate | Synthetic | + | - | - | - | - | - |
| Calcium sulphate | Synthetic | + | - | - | - | - | - | |
| Beta-calcium triphosphate | Synthetic | +++ | - | - | - | - | - | |
| Coral | Natural | +++ | - | - | ++ | - | - | |
| Hydroxyapatite | Synthetic | + | - | - | - | - | - | |
| Collagen | Natural | ++ | - | - | - | - | - | |
| Synthetic polymers | Synthetic | ++ | - | - | ++ | - | - | |
| Bioactive crystals | Synthetic | ++ | - | - | ++ | - | - | |
| Bone morphogenetic proteins | Synthetic | - | +++ | - | - | - | - |
Score: - (none), + (low), ++ (medium), +++ (excellent) Score: - (none), * (low), *** (high)
Table 3: Different materials used as bone graft in oral surgery and their most important features.
A somewhat different from of the allograft is the Demineralized Bone Matrix (DBM). It is obtained from cortical bone that undergoes a decalcification process. The cells and minerals are removed from the tissue by subjecting it to an acid solution. Initially a solution of 0.5N of HCl was used for this purpose. Once it was processed, no viable cells were found in the tissue, and therefore by definition this product would be an aloimplant [25].
On the other hand, the activity of the growth factors varies depending on the tissue bank that it comes from, and evens the batch. Although it can have a certain amount of potential for osteoinduction due to the growth factors that it presents, it essentially behaves as an osteoconductor [16,18,26]. It is used for the filling in of bone defects that affect one or two walls. It can also be used as an autograft expander in order to obtain greater volume. Its use in situations that involve load support is contraindicated because it will collapse since it doesn't have sufficient structural rigidity [13,23].
Xenografts
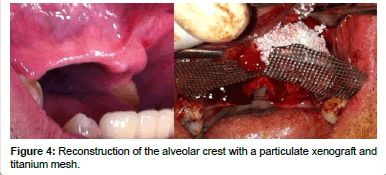
The FDA defines a xenotransplant as the use of living cells, tissues or organs from an animal, non-human source, that are transplanted into a human being, or used in ex vivo contact with human bodily fluids, cells, tissues or organs, that will later be placed in a human [27,28] (Figure 4). In dentistry the use of graft materials from bones of bovine origin is very common. In the last few decades the bovine bone has become a common source for the preparation of human bone substitutes, since these bones have a good osteoconductive capacity. The non-human origin of these materials may generate some aversion due to the risk that they might produce some type of disease transmission from the donor to the recipient, as can happen in the case of allografts. This concern is clearer after the episodes of the Bovine Spongiform Encephalopathy (BSE), which made the industry increase their efforts with respect to obtaining sanitarily safe materials [29].
During the manufacturing process the bovine bone undergoes thermal processes to eliminate the proteins present in the tissue, which may reach, in some cases, temperatures close to 1000ºC for several hours. This produces ceramming of the tissue which alters its porosity, therefore losing part of its osteoconductive capacity. Additionally this ceramming can hinder the proper reabsorption of the material and the integration into the neoformed bone [29]. These tissues are also subject to the action of a strong alkaline solution, NaOH 1-2molar solution, for two hours, which results in a decrease in the risk of infectivity of the tissue to a minimum of 1/105. BSE prions, if they were to exist, could be considered practically inactivated. Additionally, the tissue will undergo a treatment with organic solvents which will give rise to the removal of fat. These solvents will destroy the three-dimensional structure of proteins due to the hydrophobic nature of the organic solvents [29,30] The distribution of xenografts in the USA is also controlled by the FDA and they are considered and regulated as medical devices [31]. In Europe, in order to minimize the risk of BSE transmission to humans through these products, the German Federal Ministry of Health published health requirements that the products derived from bovine, caprine, or sheep tissue must meet [32,33]
Xenografts are an efficient material and are regarded as sanitarily safe products. However we must comment on the work of Kim who carry out a comprehensive review in which they identify studies published about the presence and infectivity of the BSE prions in bovine bone, the inactivation of these prions during the process of conditioning and the sterilization of the products for bone grafts [34]. The authors conclude that these biomaterials involve a risk of transmission of BSE prions to the patients, although the risk cannot be quantified based on the information that is currently available. Even when this risk exists, the strict controls in the selection of the donor animals and the processes that the tissues must undergo have ensured that there are no documented cases of transmission to the recipients.
Alloplastic grafts
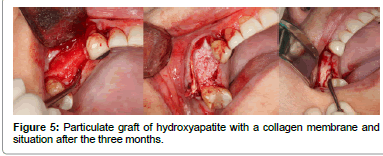
Finally, among the grafts, we have the strictly synthetic materials left to comment on, these constitute the alloplastic grafts (Figure 5). They are osteoconductors and they can be bioactive or inert. They are available in multiple presentations. While bioactive materials chemically interact with the bone of the recipient, and thus are able to induce bone neoformation, the inert materials don't join in any way to the recipient bone and they only establish intimate contact between them that will allow for the transmission of forces [7]. We have various types of materials available depending on their composition: Compounds of calcium phosphate, calcium sulfate, coral, Hydroxyapatite, collagen matrices, synthetic polymers, bioactive crystals and calcium triphosphate, in addition to the osteoinductive proteins, which, although they are a synthetic product, their mechanism of action is completely different [35]. These materials have some obvious advantages since they cannot transmit diseases, and in principle, they have unlimited availability.
During the manufacturing process, they can be modified to be particle-sized, pore-sized, to have adequate porousness, contact area, and a chemical structure that makes the material an ideal osteoconductor. Their structural properties are high but they are fragile materials with little resistance to traction, and therefore they cannot be used in areas subject to loads of weight. Their mechanical properties are closely correlated to porosity and pore size. The larger the size of the pore, the better cell permeability they will have, however there is less structural resistance. The manufacturing process will also determine its reabsorption time. The time that it takes to reabsorb the synthetic material is important, while an adequate volume is maintained, this material may be able to be substituted by bone, if disappears too quickly, there won't be enough time for the bone to be formed [35,36].
If we consider the different graft products (Table 2), the use of materials made up of calcium sulfate began to appear in dentistry as early as the 70’s. They were developed as bone substitution materials because of their similarity in composition to that of bone. This similarity grants them similar behavior to that of human bone. They are used in cement forms in an aqueous solution or as titanium surface coatings. They can be used as an abrasive instead of alumina [7]. When they are used in cement forms, they can be applied directly to the bone defect or through the use of a syringe. Once placed, the hardening process of the material occurs in situ, due to the precipitation of the calcium phosphate particles. Not all of the materials of this type can be used in the oral cavity, since some of them quickly dissolve in an aqueous medium [36]. Tricalcium phosphate, in its beta phase, is marketed in the form of granules and is reabsorbed almost in its entirety in a few months, at the same time that it is forming new bone in whose metabolism it does not interfere. It is a biocompatible material that is obtained, just like Hydroxyapatite, through a heat treatment [37]. Hydroxyapatite can be obtained naturally from the exoskeletons of the coral reef. Through a variety of processes, such as chemical and thermal ones, the coral becomes Hydroxyapatite. The porous structure of the coral remains intact, providing an ideal matrix through which new bone tissue can grow. It is possible to obtain it through industrial processes and there are no differences in the properties of one or the other [37]. Their process of reabsorption is much slower than that of calcium phosphate [38]. Ceramics are made up of Hydroxyapatite crystals and beta Tricalcium phosphate. They are used as a framework to facilitate bone regeneration and they are good osteoconductors. Osteoid substance is deposited directly on the ceramic surface in order to continue forming new bone that will be remodeled by the action of osteoblasts and osteoclasts [39]. The bioactive crystals are amorphous materials, made up of oxides and silica. They constitute a three-dimensional porous network which promotes the growth of bone tissue. The synthetic polymers used as graft materials are polylactic acid and polyglycolic acid. The latter is reabsorbed more quickly than the former. To improve their features of resistance and radiopacity, they are mixed with calcium phosphate [40]. The search for the ideal bone substitute has led to the development of new materials that are made up of several components and are called composites. They generally consist of an osteoconductive matrix that will promote the growth of new bone, osteoinductive proteins that induce the differentiation of stem cells and osteoblasts, or their precursors, that will lead to the formation of new bone [36].
Currently the option of using these materials as containers or carriers of various drugs is also being studied. These drugs include antibiotics, growth factors or the same bone morphogenetic proteins, which would make these materials osteogenic [41-43].
Morphogenetic proteins (BMP or rhBMP)
They are obtained through recombinant DNA techniques that allow a bacterium to produce a human protein. RhBMP-2 was approved by the FDA for its use in certain surgeries of the spine. In March of 2007 authorization was obtained to use it in sinus lifting techniques, postextraction preservation of the dental alveolus, and augmentation of the alveolar crest [44]. It is presented in the form of a freeze-dried powder that must be reconstituted and it is applied after being soaked in a reabsorbable collagen sponge. The most effective product concentration is 1.5 mg/cc. The sponge keeps the protein in the desired place and it provides a support so that the new bone can grow. While the graft is progressing, the sponge is being reabsorbed and it is replaced by the neoformed bone. Wikesjo et al. [45] posed the hypothesis that if dental implants coated with this protein are used, they could stimulate local bone formation and improve the osteointegration in areas of poor bone quality or areas where it is necessary to perform augmentation of the alveolar crest. In the study that they carried out, they obtained good results [45]. Very recent aspects on these issues have been addressed by Díaz-Sánchez et al. and Kühl et al. [46,47].
Finally we can state that the ideal substitute of human bone in oral surgery does not exist, and that the surgeon must choose the most appropriate material for each situation among the many materials that are available. We hope that with the investigations that are under way, in the next few years we will have a synthetic material that possesses all of the characteristics of human bone and that allows us to eliminate all of the disadvantages that autologous and allogeneic grafts present.
References
- Kutkut A, Andreana S, Kim H, Monaco E Jr (2012) Extraction socket preservation graft before implant placement with calcium sulfate hemihydrate and platelet-rich plasma: a clinical and histomorphometric study in humans. J Periodontol83: 401–409.
- Misch CE (1990) Density of bone: Effect on treatment plans surgical approach healing and progressive bone loading. Int J Oral Implantol 6: 23-31.
- Misch CM, Misch CE (1995) The repair of localized severe ridge defects for implant placement using mandibular bone grafts. Implant Dent 4: 261- 267.
- Pietrzak WS, Eppley BL (2008) In Orthopedic Biology and Medicine: Musculoskeletal Tissue Regeneration: Biological Materials and Methods. Humana Press Inc 153.
- KlokkevoldPR, Jovanovic SA (2002) Advanced Implant Surgery and Bone Grafting Techniques. Clinical Periodontology (9th edn) Philadelphia 907–908.
- Blokhuis TJ, Chris Arts JJ (2011) Bioactive and osteoinductive bone graft substitutes: Definitions facts and myths. Injury Int J Care Injured 42:S26–S29
- LeGeros RZ (2008) Calcium phosphate-based osteoinductive materials. Chem Rev 108:4742-4753.
- Samartzis D, Shen FH, Goldberg EJ, An HS (2005) Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine Phila Pa 30:1756-1761.
- Beaman FD, Bancroft LW, Peterson JJ, Kransdorf MJ (2006) Bone graft materials and synthetic substitutes. Radiol Clin North Am 44:451-461.
- Gazdag AR, Lane JM, Glaser D, Forster RA (1995) Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg 3:1–8.
- Russell TA, Leighton RK (2008) Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am 90:2057–2061.
- Editorial (2013) Organización Nacional de Trasplantes.
- Editorial (2013) US Food and Drug Administration.
- Editorial (2013) Vaccines Blood and Biologics. US Food and Drug Administration.
- Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, et al. (2008) Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank 9:289-298.
- Zimmermann G, Moghaddam A (2011) Allograft bone matrix versus synthetic bone graft substitutes. Injury Int J Care Injured 42: S16–S21.
- Grieb TA, Forng RY, Bogdansky S, Ronholdt C, Parks B, et al (2006) High-Dose Gamma Irradiation for Soft Tissue Allografts: High Margin of Safety with Biomechanical Integrity. J Orthop Res 24:1011–1018.
- Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: An update. Injury Int J Care Injured 36S: S20-S27.
- Schoepf C (2006) Allograft safety: Efficacy of the Tutoplast® Process. International Magazine of Oral Implantology 1:10-15.
- Editorial (2013) RTI SurgicalTissue Safety.
- Boyce T, Edwards J, Scarborough N (1999) Allograft bone: the influence of processing on safety and performance. OrthopClin North Am 30:571-581.
- Simonds RJ, Holmberg SD, Hurwitz RL, Coleman TR, Conley LJ, et al. (1992) Transmission of human immunodeficiency virus type I from a seronegative organ and tissue donor. N Engl J Med 326:726-732.
- Tomford WW (1995) Transmission of disease through transplantation of musculo-skeletal allografts. J Bone Joint Surg Am 77:1742-1754.
- Centers for Disease Control Prevention (CDC) (2003) Invasive Streptococcus pyogenes after allograft implantationColorado. MMWR Morb Mortal Wkly Rep 52:1174–1176.
- Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84: 454–464.
- Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO (2012) Demineralized bone matrix in bone repair: History and use. Advanced Drug Delivery Reviews 64: 1063–1077.
- Jacobsen G, Easter D (2011) Allograft vs. Xenograft:Practical Considerations in Biologic Scaffolds. An online monograph edited by Garth Jacobsen. A CME home-study sponsored by University of California. San Diego. School of Medicine.
- Editorial (2013) US Food and Drug Administration. Guidance for Industry Public Health Issues Posed by the Use of Nonhuman Primate Xenografts in Humans.
- Wenz B, Oesch B, Horst M (2001) Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 22:1599-1606.
- Calasans-Maia M, Resende R, Fernandes G, Calasans-Maia J, Alves AT, et al. (2013) A randomized controlled clinical trial to evaluate a new xenograft for alveolar socket preservation. Clin Oral Impl Res
- Editorial (2013) Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans. US Food and Drug Administration.
- Bundesgesundheitsamt (1994) Bekanntmachung der Sicherheitsanforderungen an Arzneimittel aus KoK rperbestandteilen von Rind, Schaf oder Ziege zur Vemeidung des Risikos einer UG bertragung von BSE bzw. Scrapie. Bundesanzeiger 40:1851-1855.
- Bundesgesundheitsamt (1996) Bekanntmachung uKber die Zulassung und Registrierung von Arzneimitteln. Bundesanzeiger 67:4158-4162.
- Kim Y, Nowzari H, Rich SK (2013) Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin Implant Dent Res 15:645-653.
- Precheur HV (2007) Bone Graft Materials. Dent Clin N Am 51: 729–746.
- Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, et al. (2012) Current trends and future perspectives of bone substitute materials - From space holders to innovative biomaterials. J CranioMaxillSurg 40:706-718.
- Hannink G, Arts JJ (2011) Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration. InjuryInt J Care Injured 42: S22-S25.
- Kamitakahara M, Ohtsuki C, Miyazaki T (2008) Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J BiomaterAppl 23: 197-212.
- Horch HH, Sader R, Pautke C, Neff A, Deppe H, et al. (2006). Synthetic pure-phase betatricalcium phosphate ceramic granules (Cerasorb) for bone regeneration in the reconstructive surgery of the jaws. Int J Oral MaxillofacSurg 35:708-713.
- Schnurer SM, Gopp U, Kuhn KD, Breusch SJ (2003) Bone substitutes Orthopade 32: 2-10.
- Coquelin L, Fialaire-Legendre A, Roux S, Poignard A, Bierling P, et al. (2012) In vivo and in vitro comparison of three different allografts vitalized with human mesenchymal stromal cells. Tissue Eng Part A 18:1921-1931.
- Larsso S, Hannink G (2011) Injectable bone-graft substitutes: Current products, their characteristics and indications, and new developments.Int J Care Injured 42: S30–S34.
- Zhang J, Zhou H, Yang K, Yuan Y, Liu C (2013) RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials 34: 9381-9392.
- McKay W, Peckham S, Badura J (2007) A comprehensive clinical review of recombinant human bone morphogenetic protein-2 INFUSE® Bone Graft. IntOrthop 31:729–734.
- Wikesjo UME, Qahash M, Huang YH, Xiropaidis A, Polimeni G, et al. (2009) Bone morphogenetic proteins for periodontal and alveolar indicationsbiological observations – clinical implications. OrthodCraniofac Res 12:263–270.
- Diaz-Sanchez RM, Yanez-Vico RM, Fernandez-Olavarria A, Mosquera-Perez R, Iglesias-Linares A, et al. (2013) Current approaches of bone morphogenetic proteins in dentistry. J Oral Implantol.
- Kuhl S, Payer M, Kirmeier R, Wildburger A, Acham S, et al. (2013) The Influence of ParticulatedAutogenous Bone on the Early Volume Stability of Maxillary Sinus Grafts with Biphasic Calcium Phosphate: A Randomized Clinical Trial.Clin Implant Dent Relat Res.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14694
- [From(publication date):
February-2014 - Apr 06, 2025] - Breakdown by view type
- HTML page views : 10001
- PDF downloads : 4693