Gonadotropin-Releasing Hormone Receptor is expressed in Retinoblastoma
Received: 04-Feb-2015 / Accepted Date: 03-Jun-2015 / Published Date: 05-Jun-2015 DOI: 10.4172/2161-0681.1000231
Abstract
Background: Despite the fact that retinoblastoma treatment has dramatically increased the survival and vision preservation in these patients, it is still important to pursue new therapeutic targets to minimize the side-effects of current therapies. Gonadotropin releasing hormone (GnRH) has been shown to exert a direct anti-proliferative effect on many types of malignancies, such as breast cancer, skin melanoma, and glioblastoma. The aim of this study is to describe the presence of GnRH receptor (GnRHR) in retinoblastoma in order to identify a possible new therapeutic target for this disease. Methods: Protein expression of GnRHR was studied by immunohistochemistry in 32 eyes with retinoblastoma and in the Y79 retinoblastoma cell line. Expression was scored according to intensity (1-3) and distribution (1-4), which were multiplied to generate an immunoreactive score (IRS). A score of 1 to 6 was considered low, while scores higher than 6 were considered high. The Fisher’s exact test was used to compare GnRHR IRS with presence or absence of each morphological high-risk feature. Results: GnRHR was expressed in all retinoblastoma cases and in the Y79 cell line. High, and low expression according to IRS score was evident in 62.5% and 37.5% of cases. There were no differences in GnRH IRS with respect to uni- versus multi-focal tumors, type of growth (mixed/endophytic), rosette formation, choroidal invasion, extraocular extension orextension to the sclera, or optic nerve invasion. Conclusion: GnRHR is expressed in varying degrees in all retinoblastomas. Therefore, GnRHR may be a novel therapeutic target for the treatment of retinoblastoma. Moreover, GnRHR expression could be a biomarker for the responders. Nevertheless, GnRHR expression did not correlate with morphological prognostic factors in this series. Further studies with a larger number of cases and follow up are needed to confirm our results.
Keywords: Retinoblastoma; Gonadotropin release hormone receptor; AN-152
312746Background
Retinoblastoma (RB) is the most common primary intraocular malignant tumor in childhood [1]. It has an incidence of only 1 in 15,000 to 20,000 live births [2], making it a relatively infrequent disease. However, as an early childhood disease, the unfortunate consequent deterioration in the quality of life affects the entire family. Moreover, there are severe comorbidities associated with RB treatment.
RB is a worldwide disease that shows no preference for race or gender. The incidence of RB diminishes with age, and the mean age at diagnosis varies depending on the country and presentation. In the UK, the mean age of diagnose is 9 months (range 2-37) for bilateral tumors and 24 months (2-102) for unilateral tumors [3], while in Kenya the mean age is 25 months (SD 16.8) for bilateral and 36 months (SD 21.4) for unilateral RB[4].
The relationship between RB and RB1, a tumor suppressor gene, located on 13q14, is well known. Biallelic mutation of RB1 is necessary to develop tumor growth. In heritable cases (40%), which are usually bilateral, the first mutation is constitutional, predisposing a retinal tumor. In non-heritable RB (60%), which are always unilateral, both mutations are somatic [5].
Leukocoria is the most frequent presentation of this tumor (54%), which can be detected early by flash photography. Other important forms of presentations include strabismus (19%), patients with positive family history who request early clinical surveillance (5%), red eye (5%), and decreased vision (4%) [6]. Due to its rapid and invasive growth, early diagnosis and treatment are crucial.
Multiple therapeutic modalities are available for RB, such as photocoagulation, radiotherapy, enucleation, and chemotherapy. Photocoagulation and radiotheraphy are the treatment of choice for small tumors. Enucleation usually indicated for large tumors (>50 percent of globe volume) with no hope of useful vision, painful eyes, tumors that extend to the optic nerve, choroid, or orbit, and eyes that failed “globe-conserving” treatments. Furthermore, eyes with secondary glaucoma, anterior chamber invasion or vitreous seeding are also usually managed with this modality [7]. Chemotherapy in retinoblastoma is a systemic combination-type therapy using Etoposid, Carboplatin, and Vyncristin [8].
Gonadotropin releasing hormone (GnRH; also known as luteinizing releasing hormone) is the hypophysiotropic hormone that regulates gonadotropin secretion through the activation of specific GnRH receptors present in pituitary gonadotroph cells [9]. In addition, GnRH is known to play a physiological role in the regulation of the hypothalamus-pituitary-gonadal axis, and GnRH has been shown to exert direct anti-proliferative effects on many malignant neoplasias of the reproductive tissues where GnRH receptors (GnRHR) are found, including breast [10], ovary, endometrial [11], and prostate cancer [12]. Moreover, this receptor has also been found in non-hormone-related malignant neoplasia such as melanoma [13], glioblastoma, and renal cell carcinoma [14], where it exhibits similar antiproliferative functions. Moreover, studies have shown the anticancer effects in vitro using a cytotoxic analog of GnRH in melanoma [15] and glioblastoma.
Despite the fact that RB treatments have dramatically increased survival and vision preservation in these patients, it is still important to pursue new therapeutic targets to minimize the side-effects of current therapies. The aim of this study is to analyze the expression of GnRHR in RB samples and in one RB cell line.
Methods
Patients and tissue samples: Pathologic data from 32 patients with RB were collected from the archives of the Henry C. Witelson Ocular Pathology Laboratory, McGill University (Montreal, Quebec, Canada). The following morphological features were re-examined: presence of multifocal tumor (unifocal/multiple), type of growth (endophytic/exophytic/diffuse), differentiation of the tumor estimated as presence of rosette formation type (Homer Wright, Flexner-Wintersteiner and fleurettes), choroid invasion (focal (<3 mm)/massive (>3 mm)) [16], invasion of anterior chamber structures, extraocular extension, extension to the sclera (including vortex vessels), optic nerve invasion (head or prelaminar, laminar, and postlamellar invasion), necrosis, calcification, and Azzopardi’s effect. All data accumulation was in accordance with Canadian and Province of Quebec legislation and the tenets of the Declaration of Helsinki.
Cell line: The RB cell line Y79 (ATCC catalog number HTB-18) was purchased from ATCC (Manassas, VA, USA). Cells were incubated at 37°C in a humidified 5% CO2-enriched atmosphere. The cells were cultured in RPMI-1640 medium (Invitrogen, Burlington, ON, Canada) supplemented with 10% heat-inactivated fetal bovine serum, 1% fungizone, and 1% penicillin-streptomycin (Invitrogen). Cells were cultured in 25cm2 flasks (Fisher, Whitby, ON, Canada) and observed twice weekly at every media change for normal growth by phase contrast microscopy. Cells were centrifuged at 120 g for 10 minutes to form a pellet, and were then re-suspended in 1 mL of medium and counted using the Automated TC-20 cell counter (Bio-Rad, Mississagua, ON, Canada). Next, 1 × 106 cells were formalin fixed and paraffin embedded (FFPE) in order to use the same fixation and mounting media as human samples using the cell block technique.
Immunohistochemistry: FFPE 5µm sections of the optopupilary section of each case were used. Automated immunohistochemistry was performed using the Ventana benchmark machine according to the manufacturer´s recommended protocol (Ventana Medical Systems, Inc., Tucson, AZ, USA). The fully automated processing of bar code-labeled slides included baking of the slides, solvent-free deparaffinization, and CC1 (Tris-EDTA buffer [pH 8.0])-based antigen retrieval. Slides were incubated with a monoclonal anti-GnRHR antibody (clone A9E4;Thermo Science, Kalamazoo, MI, USA) at a dilution of 1:10 for 30 minutes at 37°C followed by application of a biotinylated secondary antibody for 8 minutes at 37°C and then an avidin/streptavidin enzyme conjugate complex was added. Finally, Fast Red was used as chromogenic substrate and slides were counterstained with hematoxylin. As a positive control, sections of adenohypophysis were used and for negative controls the primary antibody was omitted.
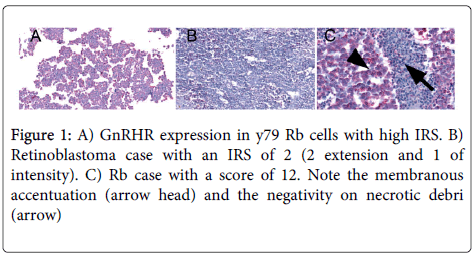
Immunohistochemical GnRHR expression was assessed by a scoring system based on the percentage of stained cells and the relative staining intensity, as previously described [17,18]. Two ocular pathologists evaluated all cases independently and the histopathological evaluation involved characterization of intensity and percentage of immunostaining. The slides were converted into whole slide images (WSI) using the Ventana icoreo gold (Ventana,. Tucson, AZ, USA) and images were analyzed using the Virtuoso image analyzer (Ventana). The extent of immunoreactive cells was evaluated based on the percentage of cells showing positive staining as follows: absence of staining was scored as 0; 1% to 10% of positive cells was scored as 1; 11% to 50% as 2; 51% to 80% as 3; and 81% to 100% as 4. Staining intensity was rated on a semi-quantitative scale using the staining of the positive control as a reference and scored from 0 to 3 as follows: 0=negative; 1=weak; 2=moderate; and 3=strong nuclear staining. Data were then converted to the numeric German Immuno-Reactive Score (IRS) by multiplying the staining extent and intensity scores [17,18] resulting in a possible range of 0 to 12. A score of 0 was considered negative; 1 to 6 was considered low and higher than 6, classified as high. Discrepancies were re-evaluated and consensus was reached in all cases.
Data management and statistical analysis: Data are presented as means ± standard error of the mean (SEM), and n represents the number of cases. Mean GnRHR IRS for tumor and retina were compared using the Student’s t-test. The Fisher’s exact test was used to compare GnRHR IRS with presence or absence of each morphological high-risk feature. Differences were considered significant at P<0.05. Statistics were performed using Microsoft Excel (Microsoft, Redmond, WA, USA) and the GraphPad Prism 5.0 software (Graphpad software, la Jolla, CA, USA).
Results
GnRHR was expressed in all retinoblastoma cases and in the Y79 cell line .The expression was located in the cytoplasm and has a membranous accentuation that was seen in high expression cases. Low expression was found in 62.5% of the cases and high expression was evident in 37.5% (Figure 1).
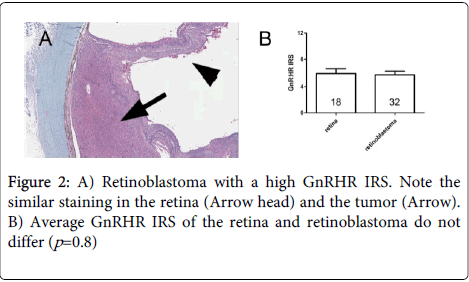
Retinal GnRHR expression was also noted in all cases in which it was evaluated (n=18, 56.25%). The IRS of the retina and the tumor did not differ significantly (Figure 2, P=0.8), but in 14 cases (77.78%) the IRS of the tumor was higher or equal than the retina.
GnRHR was not identified as a prognostic factor. No correlation between low and high GnRHR expression with respect to choroidal invasion, extension to the sclera, optic nerve invasion in the lamina cribosa or beyond, or extraocular extension was observed (Table 1). All cases with choroidal invasion observed in this series were massive.
| Low | High | P value | Odds Ratio (95% CI) | N (%) | |
|---|---|---|---|---|---|
| Choroidal invasion | p=1.0 | 1.077 (0.23-4.88) | |||
| Yes | 7 | 4 | 11 (34.4) | ||
| No | 13 | 8 | 21 (65.6) | ||
| Scleral invasion | p=1.0 | 0.88 (0.13-6.22) | |||
| Yes | 3 | 2 | 5 (15.6) | ||
| No | 17 | 10 | 27 (84.4) | ||
| ON invasion in lamina cribosa or beyond | p=0.63 | 2.75 (0.26-28.05) | |||
| Yes | 4 | 1 | 5 (15.6) | ||
| No | 16 | 11 | 27 (84.4) | ||
| ON margin positive | p=1.0 | 1.22 (0.10-15.12) | |||
| Yes | 2 | 1 | 3 (9.4) | ||
| No | 18 | 11 | 29 (90.6) | ||
| Extraocular extension | p=0.54 | 0.26 (0.02-3.27) | |||
| Present | 1 | 2 | 3 (9.4) | ||
| Absent | 19 | 10 | 29 (90.6) | ||
| Any risk factor | p=0.71 | 1.6 (0.36-7.26) | |||
| Yes | 9 | 4 | 13 (40.6) | ||
| No | 11 | 8 | 19 (59.4) |
Table 1: Univariate analysis examining association between high risk of malignancy characteristics and GnRHR expression
Other classic histopathological findings not correlated with prognosis are compared with low and high GnRHR IRS (Table 2).
| Low | High | P Value | Odds Ratio (95% CI) | N (%) | |
|---|---|---|---|---|---|
| Growth type | p=0.33 | 0.33(0.05-2.37) | |||
| Endophytic | 2 | 3 | 5(15.6) | ||
| Mixed | 18 | 9 | 27(84.4) | ||
| Rossette formation | p=1.0 | 0.75(0.11-5.33) | |||
| Yes | 3 | 2 | 5(15.6) | ||
| No | 17 | 10 | 27(84.4) | ||
| ON invasion up to head | p=1.0 | 1.92 (0.07-51.07) | |||
| Yes | 1 | 0 | 1(3.13) | ||
| No | 19 | 12 | 31(96.87) |
Table 2: Univariate analysis examining association between classic morphological features of RB and GnRHR expression
Discussion
Using immunohistochemistry, GnRHR expression was found in all RB cases and in the y79 cell line. This suggests that RB might undergo autocrine/paracrine stimulation of GnRH, with the consequent growth stimulation, as was demonstrated in melanoma [13]. Moreover, recently, a novel group of cytotoxic drugs have shown anticancer effects in cells expressing GnRHR. To the best of our knowledge, this is the first case series of RB assessing the expression of GnRHR.
One of these cytotoxic drugs is AN-152(AEZS-108), which consists of a GnRH carrier linked to doxorubicin. It has been established that drug uptake is mediated by receptor endocytosis. Phase I/II clinical trials in patients with gynecological cancers have demonstrated anticancer activity without important side effects. It is important to highlight that GnRHR are localized not only in hormone related tissue, but also in other organs and hematopoietic stem cells, including the central nervous system and the retina.
Our study was able to demonstrate the presence of GnRH receptors in all RB cases. This novel finding provides an opportunity for cytotoxic GnRH analogues as a treatment option for RB specially, in cases where conventional chemotherapy was unsuccesful. The amount of chemotherapy given should be much smaller than the conventional chemotherapy as has been demonstrated in xenograft animal models [19]. Moreover, AN-152 has been studied in vitro in glioblastoma, melanoma, renal cell carcinoma, and also in hormone related tumors, showing that its activity is not affected by the multidrug resistant 1 (MDR-1) system [20].
In our series, the retina stains similarly to previous reports [21]. However, it is important to highlight that even though the expression in the retina did not differ from tumors, the volume of the tumor is much greater than the retina. Based on this fact we presume that the drug will have more affinity for the tumor than normal cells, minimizing the side effect on the uninvolved retina. Furthermore and to the best of our knowledge, in Phase I and II studies in gynecological tumors, no ocular side effects have been reported. Perhaps the receptors present in the mature retina are not functional and due to the cytotoxic agent must enter the cell by endocytosis, the drug is not effective. This theory needs to be confirmed in further studies.
Another important value of GnRHR expression in RB is the possible link between increased risk of the disease in women that underwent infertility treatment with GnRH analogues. Some studies have shown that it is a risk factor while newer studies failed to show that association [22,23]. It is important to know the effect of GnRH agonists in cell lines, because in melanoma [13], glioblastoma [24] and other hormone-related tumors [11] it has shown an antiproliferative effect. If that is the case in RB, the agonist should be more protective than deleterious.
When compared with prognostic features, no association was found. It is important to highlight that this is a retrospective study that has a limited number of cases from a single center. This could represent selection bias that resulted in this lack of correlation. We believe that increasing the number of cases will provide more accurate conclusions regarding the prognostic value of GnRHR expression in RB.
In summary, GnRHR is expressed in varying degrees in RB. GnRHR IRS did not correlate with prognostic factors of this particular tumor. Therefore, GnRHR may be a novel therapeutic target for the treatment of RB, but further studies are needed to confirm this. Moreover, it is important to analyze the response of the Y79 cell line to agonist and antagonist drugs to confirm the functionality of this receptor.
References
- Mitra D, Shaw AK, Hutchings K (2012) Trends in incidence of childhood cancer in Canada, 1992-2006. Chronic Dis Inj Can 32: 131-139.
- Kivela T (2009) The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol 93: 1129-1131.
- Goddard AG, Kingston JE, Hungerford JL (1999) Delay in diagnosis of retinoblastoma: risk factors and treatment outcome. Br J Ophthalmol 83: 1320-1323.
- Nyamori JM, Kimani K, Njuguna MW, Dimaras H (2012) The incidence and distribution of retinoblastoma in Kenya. Br J Ophthalmol 96: 141-143.
- Benavente CA, Dyer MA (2015) Genetics and Epigenetics of Human Retinoblastoma. Annu Rev Pathol 10: 547-562.
- Abramson DH, Beaverson K, Sangani P, Vora RA, Lee TC, et al. (2003) Screening for retinoblastoma: presenting signs as prognosticators of patient and ocular survival. Pediatrics 112: 1248-1255.
- Shields CL, Shields JA (1999) Recent developments in the management of retinoblastoma. J PediatrOphthalmol Strabismus 36: 8-18.
- Kunkele A, Wilm J, Holdt M, Lohmann D, Bornfeld N, et al.(2015)Neoadjuvant/adjuvant treatment of high-risk retinoblastoma: a report from the German Retinoblastoma Referral Centre. Br J Ophthalmol.
- Zheng W, Grafer CM, Kim J, Halvorson LM (2015) Gonadotropin-Releasing Hormone and Gonadal Steroids Regulate Transcription Factor mRNA Expression in Primary Pituitary and Immortalized Gonadotrope Cells. Reprod Sci.
- Rugo HS (2014) Hormone therapy in premenopausal women with early-stage breast cancer. N Engl J Med 371: 175-176.
- Novikova OV, Andreeva I, Novikova EG, Vostrov AN, Pronin SM, et al. (2014) [Hormone therapy alone for pre-cancerous conditions and early endometrial cancer: pros and cons]. VoprOnkol 60: 306-312.
- Rick FG, Schally AV (2014) Bench-to-bedside development of agonists and antagonists of luteinizing hormone-releasing hormone for treatment of advanced prostate cancer. UrolOncol.
- Moretti RM, MontagnaniMarelli M, Van Groeninghen JC, Limonta P (2002) Locally expressed LHRH receptors mediate the oncostatic and antimetastatic activity of LHRH agonists on melanoma cells. J ClinEndocrinolMetab 87: 3791-3797.
- Keller G, Schally AV, Gaiser T, Nagy A, Baker B, et al. (2005) Receptors for luteinizing hormone releasing hormone expressed on human renal cell carcinomas can be used for targeted chemotherapy with cytotoxic luteinizing hormone releasing hormone analogues. Clin Cancer Res 11: 5549-5557.
- Keller G, Schally AV, Gaiser T, Nagy A, Baker B, et al. (2005) Human malignant melanomas express receptors for luteinizing hormone releasing hormone allowing targeted therapy with cytotoxic luteinizing hormone releasing hormone analogue. Cancer Res 65:5857-5863.
- Sastre X, Chantada GL, Doz F, Wilson MW, de Davila MT, et al. (2009) Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Archives of pathology & laboratory medicine 133: 1199-1202.
- Rush DS, Tan J, Baergen RN, Soslow RA (2001) h-Caldesmon, a novel smooth muscle-specific antibody, distinguishes between cellular leiomyoma and endometrial stromal sarcoma. Am J SurgPathol 25: 253-258.
- Remmele W, Stegner HE (1987) [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemicalestrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe 8: 138-140.
- Miyazaki M, Nagy A, Schally AV, Lamharzi N, Halmos G, et al. (1997) Growth inhibition of human ovarian cancers by cytotoxic analogues of luteinizing hormone-releasing hormone. J Natl Cancer Inst 89: 1803-1809.
- Gunthert AR, Grundker C, Bongertz T, Schlott T, Nagy A, et al. (2004) Internalization of cytotoxic analog AN-152 of luteinizing hormone-releasing hormone induces apoptosis in human endometrial and ovarian cancer cell lines independent of multidrug resistance-1 (MDR-1) system. Am J ObstetGynecol 191: 1164-1172.
- Dukic-Stefanovic S, Walther J, Wosch S, Zimmermann G, Wiedemann P, et al. (2012) Chorionic gonadotropin and its receptor are both expressed in human retina, possible implications in normal and pathological conditions. PLoS One 7: e52567
- Moll AC, Imhof SM, Cruysberg JR, Meeteren SAY, Boers M, et al. (2003) Incidence of retinoblastoma in children born after in-vitro fertilisation. Lancet 361: 309-310.
- Foix-L'Helias L, Aerts I, Marchand L, Rouic LL, Villars GM, et al. (2012) Are children born after infertility treatment at increased risk of retinoblastoma? Hum Reprod 2012, 27: 2186-2192.
- Marelli MM, Moretti RM, Mai S, Muller O, Groeninghen VJC, et al. (2009) Novel insights into GnRH receptor activity: role in the control of human glioblastoma cell proliferation. Oncol Rep 21: 1277-1282.
Citation: Zoroquiain P, Aldrees S, Logan P, Alkharashi M, Toledo Diaz AB, et al. (2015) Gonadotropin-Releasing Hormone Receptor is expressed in Retinoblastoma. J Clin Exp Pathol 5:231. DOI: 10.4172/2161-0681.1000231
Copyright: © 2015 Zoroquiain P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15450
- [From(publication date): 6-2015 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 10826
- PDF downloads: 4624