Case Report Open Access
Glucose and the Gut: A Case Report of Acute Gastric Dilatation in Type I Diabetes Mellitus
Al-Ani AH1*, Hebbard GS2 and Ajani AE3
1Basic Physician Trainee Year One, The Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3051, Australia
2Director, Gastroenterology and Hepatology, The Royal Melbourne Hospital, 300 Grattan Street, Parkville, Victoria 3051, Australia
3Cardiologist, The Melbourne Private Hospital, Melbourne and Monash University, Australia
- *Corresponding Author:
- Dr Aysha Al-Ani
Basic Physician Trainee Year One
The Royal Melbourne Hospital
300 Grattan Street, Parkville
Victoria 3051, Australia
Tel: +61429837640
E-mail: aysha.alani@mh.org.au
Received date: November 23, 2015 Accepted date: December 23, 2015 Published date: December 29, 2015
Citation: Al-Ani AH, Hebbard GS, Ajani AE (2015) Glucose and the Gut: A Case Report of Acute Gastric Dilatation in Type I Diabetes Mellitus. J Gastrointest Dig Syst 5:367. doi:10.4172/2161-069X.1000367
Copyright: © 2015 Al-Ani AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
This case report details the complex management of gastroparesis in a 23 year old female with long standing type I diabetes mellitus (T1DM). Despite substantial efforts to control her diabetes and gastrointestinal symptomology, the disease course prevailed and a vicious cycle ensued of unstable blood glucose levels and gut instability. Her gastroparesis deteriorated despite meticulous evidence-based medical and surgical management. The patient’s demise eventuated from acute colonic dilatation requiring pancolectomy followed by acute gastric dilatation that ultimately resulted in her death.
Keywords
Type I Diabetes mellitus; Gastroparesis, gastric dilatation; Autonomic neuropathy; Autonomic enteropathy; Diabetic gut
Introduction
Gastroparesis is complex syndrome of delayed gastric emptying, occurring more commonly in diabetic patients, particularly T1DM. It is relatively common with an estimated 40% of outpatients having delayed gastric emptying. Recurrent exacerbations of gastroparesis can lead to malnutrition, worsening diabetic control, and reduced quality of life. Management therefore targets glycaemic as well as symptomatic control. The case provides a profound insight into the devastating outcome of gastroparesis in T1DM refractory to conservative, medical and surgical intervention.
Case Report
We describe a rare case of a 23 year old female with longstanding type 1 diabetes mellitus (T1DM) and severe autonomic enteropathy leading to acute colonic dilatation and eventually acute gastric dilatation with fatal sequelae. The patient was diagnosed with T1DM at the age of 11 years, commencing on an insulin pump six years later. Despite close endocrinology input, with weekly clinic visits and inpatient endocrinology involvement, her HbA1C fluctuated between 11-14% (96.7 – 129.5 mmol/mol) and numerous intensive care unit (ICU) admissions were needed to manage recurrent diabetic ketoacidosis (DKA) and acute illness. Notably, there was no underlying evidence of psychological problems nor a history of sexual abuse in this case, with the patient having a supportive family throughout her illness and a sound psychological assessment early in her diagnosis. Autonomic neuropathy was a hallmark complication of T1DM in this case, prominently enteropathy, however it also manifested in postural hypotension, neuropathic bladder requiring self-catheterisation, and autonomic cardiac neuropathy. Moreover, she had nephropathy and severe retinopathy requiring bilateral photocoagulation. Other medical past history included secondary hypogonadism with premature ovarian failure; osteoporosis due to hypogonadism and vitamin D deficiency; recurrent urinary tract infections; and non-alcoholic fatty liver disease. She had no known allergies.
The patient experienced a complex pattern of symptoms, consistent with gastroparesis. Incapacitating cramping abdominal pain was followed by urgency and passage of liquid bowel motions up to eight times daily, without blood nor mucus, usually postprandial. Episodes were associated with nocturnal faecal incontinence, borborygmi, nausea and vomiting. This culminated in a 25-30 kg weight loss in three years, weighing 38 kg at a height of 1.65 cm.
Investigations
Gastroparesis was confirmed on transit studies with markedly delayed gastric emptying. Small bowel transit was satisfactory and series of investigations performed over many years did not identify other underlying aetiology. Gastroscopy demonstrated reflux oesophagitis, colonoscopy was unremarkable, and capsule endoscopy revealed duodenitis. Duodenal biopsies and serology for Coeliac disease were negative. Magnetic resonance imaging of the abdomen excluded stricture, ulceration, inflammatory masses or obstruction. Repeated endoscopic visualisations showed no further changes. Other relevant studies included elevated breath hydrogen tests and an abnormally elevated 72 hour faecal fat collection test.
Management and Progress
The management aims were to optimise her diabetic control, whilst also improving her symptoms and quality of life. Therapy was targeted to treat any potential underlying pathogenesis but symptomatic relief and weight restoration, if achieved, were transient. A spectrum of anti-diarrhoeals, antispasmodics, probiotics, prokinetics, tricyclic antidepressants and antiemetics were unsuccessful in controlling her symptoms. Several cycles of antibiotics to extinguish bacterial overgrowth were successful in reducing breath hydrogen levels, however, proved ineffective in relieving symptoms. Similarly, there was no improvement with Creon. Pancreatic islet cell transplantation was considered in an attempt to stabilise the diabetes, however, she was deemed unsuitable given grave concern about malabsorption of immunosuppressant agents. Sustained trials of gluten-free and FODMAP diets, enteral and parenteral nutrition and jejunostomy feeding were futile. Although she was adherent to her medication regimen, she was unwilling to comply with a gastroparesis diet, which she found to be too socially restrictive. Enteral feeding was discussed but was also rejected.
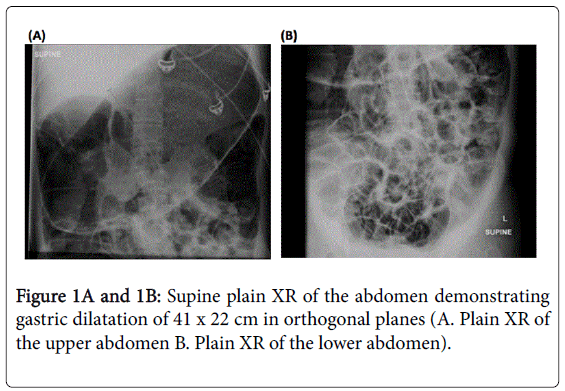
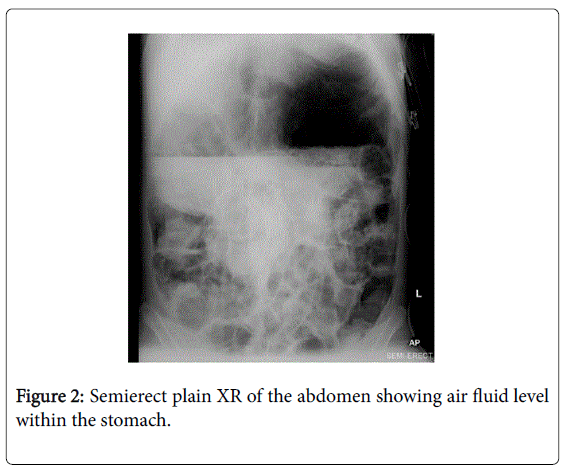
Despite extensive efforts to optimise medical and surgical therapies, her condition deteriorated. Palliative and end-of-life care was not discussed as although her condition was severe, the terminal events were very rapid. Five months prior to her death, she presented with acute gastric and colonic dilatation with ischaemic colitis (Figures 1,2).
She had septic shock and acute respiratory distress syndrome (ARDS) requiring intravenous (IV) antimicrobials and a tracheostomy. Colonoscopic decompression was temporarily successful before a pancolectomy with creation of ileostomy was necessitated to manage bowel obstruction. High stoma output post-operatively exacerbated malnutrition and a VF arrest prolonged her ICU stay.
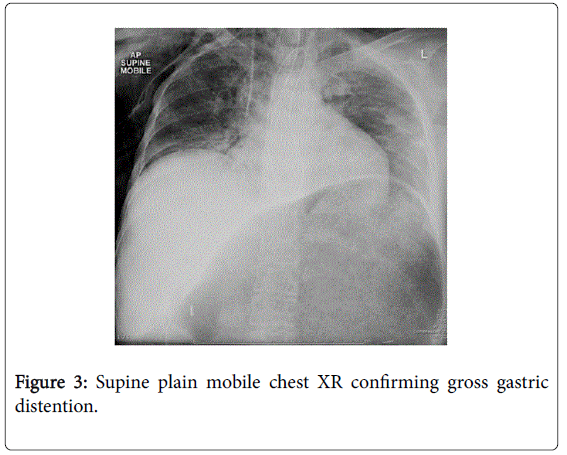
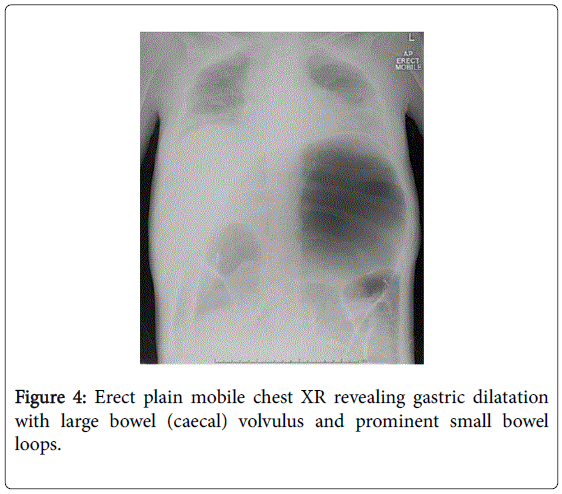
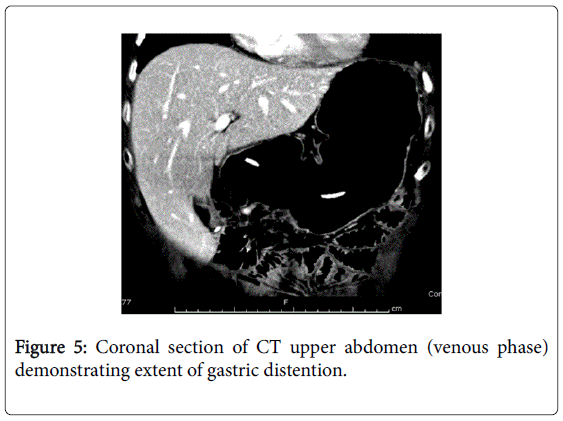
She recovered and was discharged only to represent within weeks with acute abdominal pain. Abdominal x-rays revealed massive gastric dilatation leading to intraabdominal compartment syndrome with secondary complication of ischaemic lower limbs (Figures 3-5). Full measures were undertaken to resolve this emergency including IV fluid hydration, analgesia, antiemetics, insulin infusion, and an exploratory laparotomy, however on return to ICU, she suffered VF asystolic arrest.
Cardiopulmonary resuscitation and compressions were initiated and continued for one hour with every attempt made to reverse correctable causes however she remained acidotic (pH 7.14, pCO2 68 mmol/L, pO2 6 mmol/L) and hyperkalaemic (K+ 10.4 mmol/L). There was no return of spontaneous circulation.
Discussion
This case demonstrates the challenges in maintaining glycaemic control in the face of worsening gastroparesis, exacerbating malnutrition, malabsorption, and making prediction of insulin dosing difficult. The effects of glucose levels on the gastrointestinal systemic in diabetic patients remains an area of interest. Gastroparesis is a complex syndrome of delayed gastric emptying that manifests in a symptom pattern of variable severity. It is commonly associated with diabetes mellitus, more pronounced in T1DM than T2DM, however can also be of idiopathic or post surgical aetiology [1,2].
As we see in this case, severe gastroparesis confers a poor prognosis due to aspiration of gastric contents and erratic glycaemic control [3]. It is thought that gastroparesis is a significant contributor to unstable glycaemic control in diabetic patients due to a discrepancy between the onset of insulin action and release of nutrients into the small intestine [4]. At a cellular level, diabetic gastroparesis leads to dysfunction of the gut’s pacemaker system through loss of interstitial cells of Cajal (ICCs) [5].
While upper gastrointestinal symptoms have been attributed to gastroparesis in diabetics before, acute gastric dilatation in diabetes is much less frequently described [6-8] and yet is life threatening in postoperative [9], post-traumatic [10], or neuromuscular disease cases [11]. Proposed mechanisms include diabetic neuropathy causing gastric hypomotility leading to acute gastric dilatation [6]. Three cases report concurrent respiratory tract infections suggesting that bacterial toxin may inhibit gastric motility and precipitate this process [9,10]. Recommended treatment begins with optimisation of glycaemic state, supportive dietary and hydration measures, then pharmacological and surgical therapies in refractory cases [3]. Emerging therapies include botulinum toxin injection and electrical stimulation of gastric smooth muscle, however data are limited.
In conclusion, we need to further explore and research into this topic to guide both therapeutic and symptomatic management. Ultimately, this case has demonstrated that gastric and/or colonic dilatation in patients with diabetic enteropathy can be a serious and potentially rapidly progressive condition. It requires prompt investigation, with diagnosis confirmed on plain abdominal x-ray, and instigation of appropriate management, likely urgent surgery.
References
- Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, et al. (2011) Similarities and differences between diabetic and idiopathic gastroparesis. ClinGastroenterolHepatol 9: 1056-1064.
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology (2013) Clinical guideline: management of gastroparesis. Am J Gastroenterol 108: 18-37.
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association (2004) American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology 127: 1589-1591.
- Ishii M, Nakamura T, Kasai F, Onuma T, Baba T, et al. (1994) Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care 17: 901-903.
- Tack J, Carbone F, Rotondo A (2015) Gastroparesis. CurrOpinGastroenterol 31: 499-505.
- Nagai T, Yokoo M, Tomizawa T, Mori M (2001) Acute gastric dilatation accompanied by diabetes mellitus. Intern Med 40: 320-323.
- Nagai T, Imamura M, Konaka S, Tomizawa T, Shimizu H, et al. (1995) A dead case of diabetes mellitus accompanied by lung tuberculosis and acute dilatation of the stomach. Journal of the Japan Diabetes Society 38: 187-193.
- Nagai T, Negishi T, Tomizawa T, Mitimata T, Mori M (1995) A case of acute gastric dilatation accompanied by diabetic neuropathy. Journal of the Japan Diabetes Society 38: 959-964.
- Dragstadt LR, Montogomery ML, Ellis JC, Matthews WB (1931) The pathogenesis of acute dilatation of the stomach. Surgery, Gynecology & Obstetrics 52: 1075-1086.
- Jambor CR, Steedman DJ (1991) Acute gastric dilation after trauma. J R CollSurgEdinb 36: 29-31.
- Bensen ES, Jaffe KM, Tarr PI (1996) Acute gastric dilatation in Duchenne muscular dystrophy: a case report and review of the literature. Arch Phys Med Rehabil 77: 512-514.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 12613
- [From(publication date):
December-2015 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 11734
- PDF downloads : 879