Glossary of Terms Used in Evidence Based Medicine, Epidemiology and Biostatistics
Received: 24-Nov-2015 / Accepted Date: 18-Feb-2016 / Published Date: 25-Feb-2016 DOI: 10.4172/2471-9919.1000102
41689Introduction
The areas of epidemiology, biostatistics and evidence-based medicine (EBM) have their own jargon that must be understood by health professionals to better understanding and application of this knowledge. Therefore, it is necessary to publish this glossary of terms commonly used in daily life (Table 1) [1-8].
| Terms | Definition |
|---|---|
| Absolute risk reduction (ARR) | The difference in the event rate between control group (CER) and treated group (EER): ARR = CER – EER. |
| Absolute risk increase(ARI) | The absolute difference in rates of bad events, when the experimental treatment harms more patients than the control treatment; calculated as for ABI. |
| Absolute Benefit Increase (ABI) | Is the arithmetic difference between the rates of events in the experimental and control group? An Absolute Benefit Increase (ABI) refers to the increase of a good event as a result of the intervention. ABI =EER - CER. |
| Bias | Any tendency to influence the results of a trial (or their interpretation) other than the experimental intervention. |
| Blinding | A technique used in research to eliminate bias by hiding the intervention from the patient, clinician, and/or other researchers who are interpreting results. |
| Case-control study | The observational epidemiologic study of persons with the disease (or other outcome variable) of interest and a suitable control (comparison, reference) group of persons without the disease. The relationship of an attribute to the disease is examined by comparing the diseased and nondiseased with regard to how frequently the attribute is present or, if quantitative, the levels of the attribute, in each of the groups. |
| Case-series | A group or series of case reports involving patients who were given similar treatment. Reports of case series usually contain detailed information about the individual patients. This includes demographic information (age, gender, ethnic origin) and information on diagnosis, treatment, response to treatment, and follow-up after treatment. |
| Clinical practice guideline | A systematically developed statement designed to assist health care professionals and patients make decisions about appropriate health care for specific clinical circumstances. |
| Cohort study | The analytic method of epidemiologic study in which subsets of a defined population can be identified who are, have been, or in the future may be exposed or not exposed, or exposed in different degrees, to a factor or factors hypothesized to influence the probability of occurrence of a given disease or other outcome. The main feature of cohort study is observation of large numbers over a long period (commonly years) with comparison of incidence rates in groups that differ in exposure levels. |
| Confidence interval (CI). | Quantifies the uncertainty in a measure. It is usually performed as 95% confidence interval, which is the range of values within which we can be 95% certain that fall real value for the entire population (To a decrement of the risk of death 10 with an IC 5-15, we would have 95% sure that the true value would fall between 5 and 15). The greater the distance between the ranges limits, the lower the homogeneity of the results and thus a higher risk of error. |
| Confounding variable | A variable which is not the one you are interested in but which may affect the results of trial |
| Cost-benefit analysis | Assesses whether the cost of an intervention is equivalent to the benefit, by measuring both in the same units. |
| Cost-effectiveness analysis | It measures the net cost to be providing a service as well as the outcomes obtained. Outcomes or events are reported in a single unit of measure. |
| Cost-utility analysis | Converts the effects of an intervention on personal preferences - or utilities - and describes what it costs for some additional quality gain (eg cost per additional quality-adjusted life year). |
| Clinical decision analysis | It is the application of specific quantitative methods to quantify prognosis, treatment effects and values of patients in order to analyze a decision under uncertainty conditions. |
| Cost-minimisation analysis | Knowing that the effects of more than one intervention on health are the same, only the costs are analyzed and lower cost alternative is chosen. |
| Control event rate (CER) | It is often the outcome or event of interest occurs in the study group that is not receiving the experimental intervention. |
| Critically appraised | A short summary of an article from the literature, created to answer a specific clinical question. |
| Crossover study design | It consists of the administration of two or more experimental interventions (or against placebo), one after another, in a specific or random order to the same group of patients. |
| Decision analysis | The application of explicit, quantitative methods to analyses decisions under conditions of uncertainty. |
| Diagnosis | The process of determining health status and the factors responsible for producing it; may be applied to an individual, family, group or community. The term applied both to the process of determination and to its findings. |
| Diagnostic Test | Any test performed to confirm, or determine the presence of disease in an individual suspected of having the disease, usually following the report of symptoms, or based on the results of other medical tests. |
| Ecological survey | A survey based on aggregate data of a given population the way it exists at any given time or in evolutionary points, to investigate the relationship of exposure to a known or suspected risk factor for a particular event. |
| Effectiveness | A measure of the benefit resulting from an intervention for a given health problem under usual conditions of clinical care for a particular group. |
| Efficacy | A measure of the benefit resulting from an intervention for a given health problem under the ideal conditions of an investigation. |
| Event rate | The proportion of patients in a group in whom an event is observed. Considering 100 patients, if the event is observed at 27, the event rate is 0.27. Control event rate (TEC) refers to the proportion of patients in the control group who experienced the event and the experimental event rate (TEE) is the proportion of patients in the intervention group under who experienced the event of interest |
| Experimental event rate (EER) | The proportion of patients in the group under experimental intervention presenting the event or outcome of interest |
| Forrest plot. | A diagrammatic representation of the results of individual trials in a meta-analysis |
| Funnel plot | A method of graphing the results of trials in a meta-analysis to show if the results have been affected by publication bias. |
| Heterogeneity | In systematic reviews, the amount of incompatibility between trials included in the review, whether clinical (i.e., the studies are clinically different) or statistical (i.e., the results are different from one another). |
| Historically Controlled Study | A control study recruiting control subject(s) for whom data were collected at a time preceding that at which the data are gathered on the group being studied. |
| Inception cohort study | A group of individuals identified for subsequent study at an early, uniform point in the course of the specified health condition, or before the condition develops. |
| Incidence | The number of new cases of illness commencing, or of persons falling ill, during a specified time period in a given population. |
| Intention-to-treat | Characteristic of a study where patients are analyzed in the groups to which they were originally assigned, even though they may have switched treatment arms during the study for clinical reason |
| Likelihood ratio | The likelihood that a given test results would be expected in a patient with the target disorder compared to the likelihood that the same result would be expected in a patient without that disorder. A. for a positive test result = LR+ = sensitivity/(1-specificity) B. for a negative test result = LR- = (1-sensitivity)/specificity |
| Mechanism-based reasoning | Involves an inference from mechanisms to claims that an intervention produces a patient-relevant outcome. Such reasoning will involve an inferential chain linking the intervention (such as antiarrhythmic drugs) with a clinical outcome (such as mortality) |
| MeSH | Medical Subject Headings: a thesaurus of medical terms used by many databases and libraries to index and classify medical information. |
| Monitoring Test | Any test performed to confirm, or determine the presence of disease in an individual suspected of having the disease, usually following the report of symptoms, or based on the results of other medical tests. |
| Nested Case-control study | A case control study in which cases and controls are drawn from the population in a cohort study. As some data are already available about both cases and controls, the effects of some potential confounding variables are reduced or eliminated. In this type of case control study, a set of controls is selected from subjects, i.e. non-cases, at risk at the time of occurrence of each case that arises in a cohort, thus allowing for the confounding effect of time in the analysis. |
| Network meta-analysis | A method used to assess the comparative effectiveness of experimental treatment among similar patient populations that have not been compared directly in a randomized clinical trial. Unlike traditional meta-analysis, which summarizes the results of trials that have evaluated the same treatment/placebo combination, Network Meta-Analyses compare the results from two or more studies that have one treatment in common. |
| n-of-1 trial | A variation of a randomized controlled trial in which a sequence of alternative treatment regimens is randomly allocated to a patient. The outcomes of regimens are compared, with the aim of deciding on the optimum regimen for the patient. |
| Negative predictive value (-PV) | The proportion of people with a negative test who are free of disease. |
| Number needed to treat (NNT) | The number of patients who need to be treated to prevent one bad outcome. It is the inverse of the ARR: NNT=1/ARR. |
| Number needed to harm( NNH) | The number of patients who, if they received the experimental treatment, would lead to 1 additional person being harmed compared with patients who receive the control treatment; calculated as 1/ARI. |
| Observational study | A family of studies in which investigators compare people who take an intervention with those who do not. The investigators neither allocate patients to receive the intervention not administer the intervention. Instead, they compare records of patients who had taken an intervention and been treated in routine practice with similar patients who had not taken the intervention. The most common observational designs are case-studies, case-series, case-control studies, cohort studies, and historically controlled studies. |
| Odds | A ratio of events to non-events. If the event rate for a disease is 0.2 (20%), its non-event rate is 0.8 and therefore its odds are 2/8. |
| p value: | The probability that a particular result would have happened by chance. |
| Positive predictive value (+PV) | The proportion of people with a positive test who have disease. |
| Post-test probability | The probability that a patient has the disorder of interest after the test result is known. |
| Pre-test probability | The probability that a patient has the disorder of interest prior to administering a test. |
| Post-marketing surveillance | A procedure implemented after a drug has been licensed for public use, designed to provide information on the actual use of the drug for a given indication and on the occurrence of side effects, adverse reactions, etc. A method for epidemiologic study of adverse drug reactions. |
| Prevalence | The baseline risk of a disorder in the population of interest. The proportion of patients who have the target disorder, as determined before the test is carried out ([a + c]/[a + b + c + d]) (Table 2). |
| Prevention | Prevention refers to measures taken by an individual or a society to prevent disease happening or its consequences. In general, prevention includes a wide range of interventions, aimed at reducing risks to health. |
| Pretest odds | The odds that the patient has the target disorder before the test is carried out (pretest probability/[1 - pretest probability]) |
| Post-test odds | The odds that the patient has the target disorder after the test is carried out (pretest odds ´ LR). |
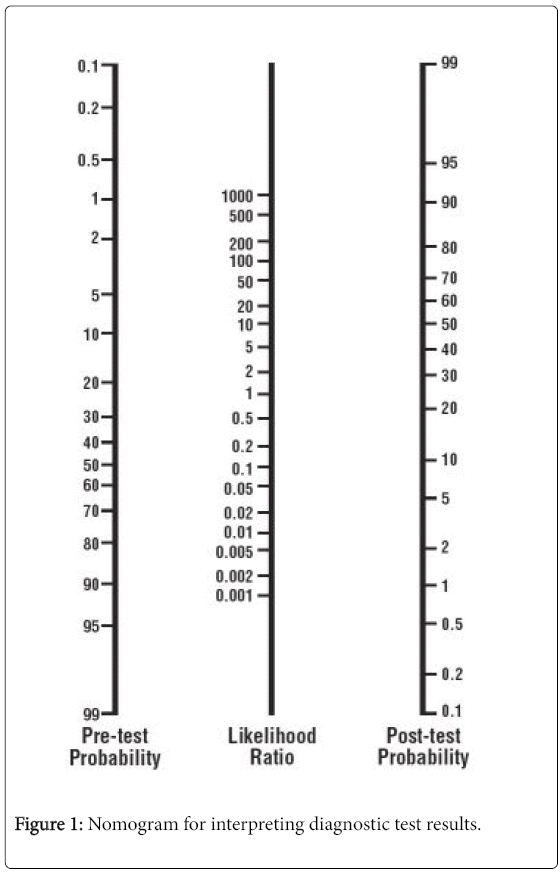
| Post-test probability | The proportion of patients with that particular test result who have the target disorder (post-test odds/[1 + post-test odds]). Use of a nomogram (Figure 1) avoids the need for these calculations. |
| Prognosis | The prospect of survival and recovery from a disease as anticipated from the usual course of that disease or indicated by special features of the case. |
| Publication bias | A bias in a systematic review caused by incompleteness of the search, such as omitting non-English language sources, or unpublished trials (inconclusive trials are less likely to be published than conclusive ones, but are not necessarily less valid). |
| Randomized trial | An epidemiological experiment in which subjects in a population are randomly allocated into groups, usually called study and control groups, to receive or not receive an experimental preventive or therapeutic procedure, maneuver, or intervention. The results are assessed by rigorous comparison of rates of disease, death, recovery, or other appropriate outcome in the study and control groups. |
| Relative risk (RR) (or risk ratio) | The ratio of the risk of an event in the experimental group compared to that of the control group (RR=EER / CER). |
| Relative risk reduction (RRR) | The percentage reduction in events in the treated group event rate (EER) compared to the control group event rate (CER): RRR = (CER-EER) / CER. |
| Receiver Operator Curves (ROC) | A graph plotting the true positive rate (1-specificity) over a series of cutoffs for defining a positive test. A diagonal line indicates no ability to distinguish persons with and without the condition. The farther the curve reaches toward the upper left corner of the graph the better the test in discriminating diseased from non-diseased persons. |
| Sensitivity | The proportion of people with disease who have a positive test. |
| Specificity | The proportion of people free of a disease who have a negative test. |
| SnNout | When a sign / test / symptom has a high sensitivity, a negative test rule out the diagnosis. (Sensitivity Negative out = SnNout ) |
| SpPin | When a sign or symptom test has a high specificity, a positive test to confirm the diagnosis. (Specificity Positive in = SpPin) |
| Systematic review | The application of strategies that limit bias in the assembly, critical appraisal, and synthesis of all relevant studies on a specific topic. Systematic reviews focus on peer-reviewed publications about a specific health problem and use rigorous, standardized methods for selecting and assessing articles. A systematic review may or may not include a meta-analysis, which is a quantitative summary of the results. |
| Treatment benefits | Positive patient-relevant outcome associated with an intervention, quantifiable by epidemiological measures such as absolute risk reduction (ARR) and number needed to treat (NNT). |
| Validity | The extent to which a variable or intervention measures what it is supposed to measure or accomplishes what it is supposed to accomplish. A.The internal validity of a study refers to the integrity of the experimental design. B.The external validity of a study refers to the appropriateness by which its results can be applied to non-study patients or populations. |
| Variability | A.Intraobserver Variability: Variation in test results during repeat testing by the same observer. B.Interobserver Variability: Variation in test results by different observers. The variability is often quantified with the Kappa value. |
Table 1: Glossary of Terms Used in Evidence-Based Medicine
| Test result | Target Disorder | |
|---|---|---|
| Present | Absent | |
| Positive | a | b |
| Negative | c | d |
Table 2: Comparison of test results with a diagnostic standard.
References
- Guyatt G, Meade MO, Cook DJ, Rennie D (2014) Users' Guides to the Medical Literature: A Manual for Evidence-based Clinical Practice, Third edition. New York.
- Greenhalgh T (2001) How to Read a Paper. The basics of evidence based medicine. Second edition. BMJ Books.
- Silva E, Drummond JP (1999) MedicinaBaseadaemEvidências. Novo ParadigmaAssistencial e Pedagógico. São Paulo.
- Fletcher RH, Fletcher SW and Wagner EH (1996) EpidemiologiaclÃnica. Bases cientÃficas da condutamédica. ArtesMédicas, . Porto Alegre.
- Last JM (2001)A Dictionary of Epidemiology ҠFourth Edition.Oxford University Press, Oxford.
- Spitalnic S (2004) Test Properties 1: Sensitivity, Specificity and Predictive Values. Hospital Physician, 27-31.
- Spitalnic S (2004) Test Properties 2: Likelihood ratios, Bayes’ formula, and receiver operating characteristic curves. Hospital Physician, 53-58.
Citation: Roever L (2016) Glossary of Terms Used in Evidence Based Medicine, Epidemiology and Biostatistics. Evidence Based Medicine and Practice 1: 102. DOI: 10.4172/2471-9919.1000102
Copyright: © 2016 Roever L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 19548
- [From(publication date): 4-2016 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 18540
- PDF downloads: 1008