Review Article Open Access
Gingival Overgrowth: Drug-induced versus Hereditary and Idiopathic
Alyaa Ragaei1* and Rehab A Abdel Moneim21Department of Supplementary General Science, Future University, Cairo, Egypt
2Department of Oral Biology, Cairo University, Cairo, Egypt
- *Corresponding Author:
- Alyaa Ragaei
Department of Supplementary General Science
Future University, Cairo, Egypt
Tel: +201004684290
E-mail: alyaa_fue@yahoo.com
Received date: October 28, 2016; Accepted date: March 09, 2017; Published date: March 13, 2017
Citation: Ragaei A (2017) Gingival Overgrowth: Drug-induced versus Hereditary and Idiopathic. Cosmetol & Oro Facial Surg 3:113.
Copyright: © 2017 Ragaei, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Cosmetology & Oro Facial Surgery
Abstract
Gingival overgrowth (GO) is the abnormal enlargement of maxillary and mandibular gingiva. It can be caused due to different etiological factors inherited (hereditary) gingival fibromatosis (HGF), accompanied with diseases characterizing syndrome; idiopathic gingival fibromatosis (IGF) or as a side effect of an adverse drug reaction (ADR) known as drug-induced gingival fibromatosis (DIGF). The hypertrophic gingiva is also accompanied with variable growth factors expression at cellular and molecular levels. It is well observed in fibroblasts activity and production of collagenous fibers in connective tissues as well as their degradation. Thus, it would be useful to identify and explore different factors related to gingival growth changes to help in treatment plans. This review article will throw the light on systemic, pathological, histological and immuniohistochemical aspects associated with both HGF and DIGF.
Keywords
Gingival overgrowth; Hereditary gingival fibromatosis; Drug-induced gingival fibromatosis; Pathogensis; Histopathology; Immuniohistochemistry; Treatment
Introduction
Gingival fibromatosis, hypertrophic gingivitis, gingival hyperplasia or hypertrophy is the overgrowth of gingival (GO). Predisposing factors include inflammation, leukemia hormonal disturbances, uncontrolled diabetes and drug use [1]. It develops as slowly progressive, local or diffuse enlargements within marginal and attached gingiva or interdental papilla which might cover the crowns of the teeth in severe cases. Thus functional, esthetic, and periodontal problems, such as bone loss and bleeding might result due to the presence of pseudo-pockets and plaque accumulation. The most common forms of GO are those induced by systemic drugs (DIGO), (Table 1) [2]; followed by the inherited HGF or idiopathic IGF conditions [3]. Although many studies were devoted to studying the clinical aspects of GO, histological and molecular basis of both HGF and DIGO were not in their scope of interest. This review will focus on the pathogenesis, histological, molecular and regulatory mechanism that have been associated with both DIGO and HGF aiming at controlling the disease and amend about the approaches of its treatment.
| Category | Pharmacologic agent |
| Anticonvulsants | Phenytoin |
| Sodium valproate | |
| Phenobarbitone | |
| Vigabatrin | |
| Primidone | |
| Mephenytoin | |
| Ethotoin | |
| Ethosuximide | |
| Methosuxinimide | |
| Immunosuppressants | Cyclosporin |
| Tacrolimus | |
| Sirolimus | |
| Calciumchannel blockers | Nifedipine |
| Nitrendipine | |
| Felodipine | |
| Nicardipine | |
| Manidipine | |
| Amlodipine |
Table 1: Drugs causing gingival overgrowth
Etiology
Enlargement associated with non-genetic diseases can be directly or indirectly linked to poor nutrition (vitamin C deficiency) [4], systemic hormonal stimulation (pregnancy or puberty) [5], leukemia [6,7], Wegener‘s granulomatosis [8], orofacial granulomatosis [9], pyogenic granuloma [10] and sarcoidosis [11]. It may also be associated with pseudotumors [12,13], benign neoplasms, e.g. giant cell fibroma [14], gingival and oral myofibroma [15,16], papilloma [17], giant cell granuloma [18], malignant neoplasms [19,20], salivary gland tumors [21,22], melanoma [23,24], adenoma and mucoepidermoid carcinoma [25].
Gingival enlargement may develop during the course of inflammatory diseases of the oral cavity, e.g. localized and generalized aggressive periodontitis, primary gingival tuberculosis [26,27], inflammatory pseudotumors [13] and inflammatory fibrous hyperplasia due to local irritants [28]. Plaque accumulation and bacterial infection resulting from poor oral hygiene are significant predisposing factors [29,30].
Gingival enlargement might be associated with hereditary factors or co-existing with genetic diseases and syndromes [28,31,32]. Despite the pharmacological heterogeneity of the three major drugs causing GO, immunosuppressants, calcium channel blockers and anticonvulsants (Table 1) still they possess similar mechanism of action at the cellular and molecular levels; leading to DIGF [33,34]. This occurs by inhibiting the intracellular calcium influx, hence having a common side effect upon gingival connective tissue. Immunosuppressive drugs as cyclosporine and tacrolimus have immunosuppressive properties and as consequence they may protect the patients against periodontal breakdown [35]. However, gingival enlargement might be of unknown etiology [36,37].
Pathogenesis
It was observed that children and adolescents are more subjected to DIGF than adults. It was postulated that drug-induce an influence on androgen and testosterone metabolism which could be a significant factor in the pathogenesis of drug induce gingival overgrowth.. Likewise, excised tissue from nifedipine and cyclosporine-induced GO demonstrates a similar increase in androgen metabolism [35].
The active androgen metabolites could target gingival fibroblasts and cause either an increase in collagen synthesis or a decrease in collagenase activity.
It was reported that a certain threshold concentration of the drug or its metabolites is necessary to activate gingival fibroblasts. Moreover, it was obvious that there is a direct relation between salivary cyclosporine or phenytoin and gingival overgrowth [35]. Recent attention has focused on local drug concentration in gingiva.
Phenytoin (anticonvulsant) was first introduced in 1938 as an antiepileptic drug but due to its adverse side effects leading to a significant gingival overgrowth; other anticonvulsant agents were introduced as shown in Table 1.
These drugs have been linked to clinically significant forms of GO [38]. Though, cyclosporine is widely used in tissue rejection prevention after transplantation, it might have damaging side effects such as nephrotoxicity, hepatotoxicity and gingival fibromatosis [39].
Recently, tacrolimus or FK506 is considered as an alternative to cyclosporine, since its side effects are less severe with frequent association of GO with its use [40].
Extracellular matrix (ECM) plays an important role in the regulation of cell functions, storage for various growth factors and participation in the regulation of their activation. Thus, altered abundance or composition of ECM may play an active part in the pathogenesis of GO [41]. Collagen type-1 which is considered as the major component of EMC, is evaluated by observing the balance between its synthesis and degradation intercede by matrix metalloproteinases (MMPs) and tissue matrix metalloproteinase inhibitors (TMPIs) [42-44].
The connective tissue turnover is largely controlled by chemokines and cytokines. It was observed that high levels of specific cytokines as transforming growth factor TGF-B, platelet drive growth factor B (PDGF-B), fibroblast growth factor-2 (FGF-2) and connective tissue growth factor (CTGF) are increased in patients treated with immunosuppressive, anticonvulsants and calcium channel blocker drugs.
It has been confirmed that exposure of gingival fibroblasts to different drugs as cyclosporine increases the level of transferable collagen RNA causing an overproduction of collagen and hence inducing gingival enlargement. At the same time gingival fibroblasts are heterogeneous in respect of their ability to synthesize collagenase and TMP which may be affected by drug induction.
Regarding (HGF), the pathologic manifestation of GF is correlated to the excessive accumulation of ECM and proteins, including collagen type I [45,46]. During collagen biosynthesis, single procollagen polypeptides undergo posttranslational modification in the endoplasmic reticulum (ER), forming triple-helical chains, which are then secreted into the extracellular space. This process involves heat shock protein 47 (HSP47), a 47 kDa glycoprotein localized in the ER.
It binds to type I procollagen peptides to prevent premature folding and aggregation of procollagen chains, and participates in the translocation and secretion of procollagen I into the extracellular space result in subnormal gingival growth [47].
Fibroblast cultures from patients with HGF exhibited high levels of type I collagen and HSP47, mRNA and protein [48]. Moreover, transforming growth factor (TGF)-β 1 and interleukin (IL)-6 induce the expression of type I collagen and HSP47 and downregulate matrix metalloproteinase MMP-1 and MMP-2 in fibroblast cultures from HGF patients. On the other hand, interferon-γ (IFN-γ) reduced collagen I and HSP47 expression, and slightly affected MMP-1 and MMP-2 [48]. This observation suggests that HSP47 might be a crucial molecule in the post-translational processing of the overproduced type I procollagen chains, while enhanced TGF-β 1 and IL-6 production in patients with GF may favor the accumulation of collagen fibrils in the gingiva.
MMPs are key enzymes regulating the composition of the ECM; thus the alteration in their expression has been implicated in the pathogenesis of GF. Several studies showed a significant decrease in the expression and activity of MMP-1 and MMP-2 in fibroblasts from HGF patients [48,49].
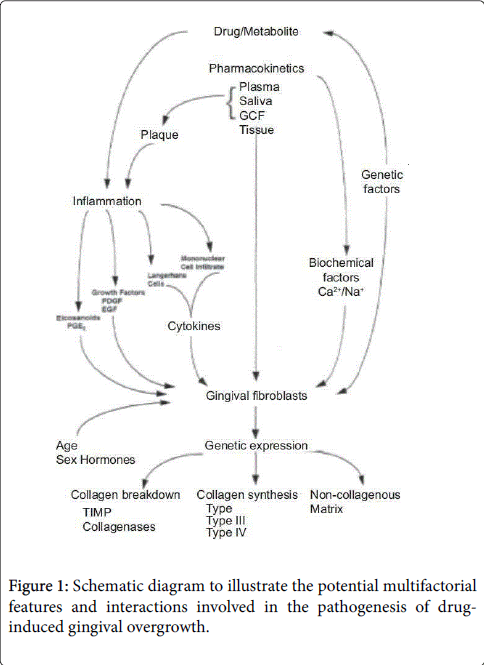
MMP-1 is a collagenase that degrades interstitial collagen, while MMP-2 acts predominantly on type IV collagen, but it has also been shown to degrade type I collagen [50]. Similarly, the inhibition of MMP-1, MMP-2, and MMP-3 has been reported, a condition also associated with enhanced TGF-β1 production [51,52]. The catalytic activity of the MMPs is regulated at the transcriptional level as well as by TMPIs. Coletta et al. [49] concluded that the addition of anti-TGF-β1 antibodies resulted in a slight increase in MMP-1 and a decrease in MMP-2 expression, whereas TIMP-1 and TIMP-2 expression were unaffected. These results confirm previous observations that enhanced TGF-β1 production may lead to the accumulation of ECM by altering the proteolytic activities of fibroblasts (Figure 1).
Genetic Predisposition
Although, the hereditary condition of HGF exhibits autosomal dominant mode of transmission, an autosomal recessive inheritance has also been reported [53]. Gingival fibromatosis may be familial or idiopathic [2].
The familial variation may occur with a number of other inherited syndromes; e.g. Zimmerman Laband syndrome, [54,55] Murray Puretic drescher (juvenile hyaline fibromatosis) [55].
Rutherfurd, Cross, Cowden syndrome, multiple hamartomas, tuberous sclerosis [56,57] and, HGF may be associated with other clinical manifestations such as hypertrichosis, [58] growth retardation, [59] hypopigmentation, mental deficiency, [60] epilepsy, [61] splenomegaly, [54] optic and auditory defects, cartilage and nail defects and dentigerous cysts [62].
The most common syndrome of HGF includes hypertrichosis, epilepsy and mental retardation, however the two latter features were not present in all cases [63]. Autosomal dominant forms of gingival fibromatosis, which are usually non-syndromic, have been genetically linked to the chromosome 2p21-p22 and 5q13-q22 [57].
A mutation in the Son of Sevenless 1 (SOS-1) gene has been suggested as a possible cause of non-syndromic gingival fibromatosis, but no definite linkage has been established yet [57]. Two genetically distinct loci seemed to be responsible for this type of HGF [64], though a locus for autosomal dominant HGF has been mapped to a region on chromosome 2 [2,65].
Hiura et al, [66]; Landstrom and Ackerman, [67] reported that most common diseases are recognized to have an inherited genetic susceptibility, which leads to disease onset when combined with environmental factors. Traits for several uncommon dental diseases such as amelogenesis imperfecta or pre-pubertal periodontitis are inherited in an autosomal dominant or recessive pattern [68-70].
In these cases, a single gene defect is responsible for disease occurrence, being potentially targetable by specific management strategies once the genetic mutation is identified. Three genetic defects on chromosome 2 and 5 have so far been identified as responsible for HGF [71-73]. Thus, a whole-exome or whole-genome sequencing will be needed to identify the mutation causing HGF in a certain family.
This technology will also address the remote possibility of an Xlinked recessive inheritance because all genes on all chromosomes are sequenced and can therefore be assessed for variants on the Xchromosome.
On the other hand, evidence from previous researches postulated that individual susceptibility to drug may be related to genetic predisposition variation in drug responsive and tolerance, as well to different drug concentration which in turn play an important role in the decrease or increase of fibroblasts proliferation and hence lead to (GO) [74,75].
Gingival overgrowth individual susceptibility (GOIS) may be related to genetic predisposition where gingival fibroblasts exhibit functional heterogeneity in response to various drug stimulation. Several drugs as cyclosporine lead to increase synthetic activity of the fibroblasts and decrease collagenolytic activity as expressed by collagenase and TMPI production [76].
This may be influenced by drug receptor binding, drug metabolism, collagen synthesis and many other factors. Human Lymphocyte Antigen (HLA-DR1) and (DR2) expressions may prove to be a useful genetic markers for the identification of patients with high risk of developing drug-induced gingival overgrowth.
Clinical Description
Clinically, the onset coincides with the eruption of primary or permanent dentition, and rarely presents at birth [77]. GF may also occur as a local, nodular-like lesion.
In severe cases, the excess gingival tissue can cover part of or the entire crown, and can result in diastemas, teeth displacement, or retention of primary or impacted teeth, and may also cause masticatory, phonetic, psychological, and esthetic problems [78].
Unlike DIGO which usually occurs as a generalized diffuse enlargement, HGF is characterized by a slow, progressive growth of the gingival tissue (Figures 2 and 3).
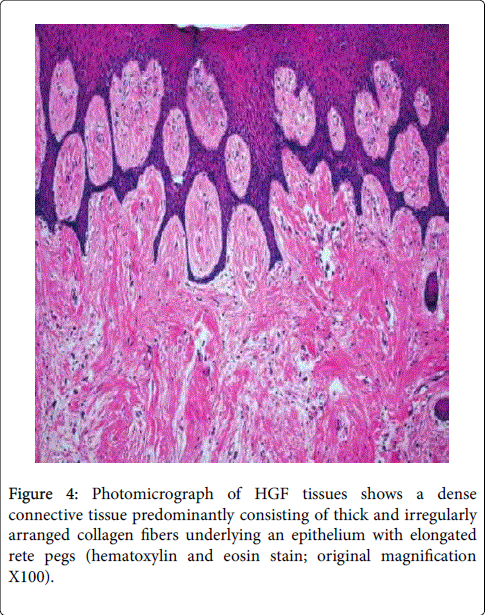
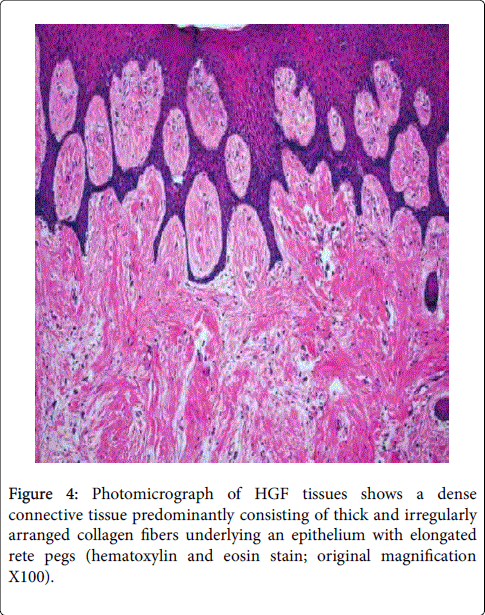
Figure 3: Photomicrograph of gingival section of (a, b) HGF Gp showing mild hyperplastic stratified squamous epithelium with elongated rete-ridges showing a tubular pattern. c) CsA treated group showing pronounced increase in epithelial thickness (hyperplasia) (E), marked elongation of papillae (P) and hypertrophy of epithelial lining mucosa. d) Mild hyperplasia (E), notable elongation of papillae (P), and mild degenerative areas (The figures showed difference in Epithelial and connective tissue structure in both DIG and HGF).
This enlargement may project into the vestibule and floor of the mouth; interfere with normal mastication and even lip closure that makes the speech difficult. The enlarged gingival tissue appears firm and pink with exaggerated stippling [2,70].
The gingival hyperplasia may be generalized (symmetric) or localized (nodular) [79]. Local involvement mainly affects the maxillary tuberosity’s and lingual surfaces of lower molars and is typically characterized by the presence of multiple large masses [2,80].
On the other hand, the symmetric form, which is the most common type of disorder, results in uniform enlargement of the gingiva that is firm, dense, tough resilient, insensitive fibrous tissue that covers the alveolar ridges and extends over the teeth resulting in extensive pseudo pockets [81].
Histopathological Description
Hyperplastic epithelium with elongated rete ridges extending into the underlying connective tissue is the typical histopathological feature of the lesion [69,82]. The connective tissue consists of excess collagen, but it has relatively few fibroblasts and blood vessels [83,84]. Enlarged fibroblasts appear scattered among thin and thick collagen fibrils.
It appears that the human gingival fibroblasts from hereditary fibromatosis tissue exhibit characteristics of permanently activated fibroblasts as they grow faster and produce more collagen and fibronectin than fibroblasts from normal human gingiva [84]. Elastic and oxytalan fibers are also present. Unlike in normal gingiva, coarse and fine dense collagen fiber bundles are oriented in all directions [36, 85-87].
Small osseous calcifications and abundant neurovascular bundles might also be present. Overgrowth of the gingival tissue might provide a chance for the growth of microorganisms, plaque accumulation and pseudo pockets formation resulting in inflammatory infiltration of the gingival connective tissue [88-89].
The histopathological appearances of the various DIGO are somehow similar regardless of few differences as appeared in (Figure 2c and 2d). The potential drug related difference has been described only when different staining techniques have been employed. The epithelial acanthuses observed in DIGO may be due to an entranced keratinocyte life spam by the action of drugs. Epithelial hyperplasia and elongated papillae observed in drug induced gingiva may suggest that the increase in epithelial tissue observed, is a result of direct effect of drugs on epithelial cells [35].
However an indirect result of drug interactions with other cells in the gingival tissues as fibroblast cells in the underlying connective tissue may play role in the GO. The close relation between the epithelial and its adjacent connective tissue in their development has been the subject of strong examination. The interaction between both was mediated by two growth factors keratinocyte factor (KGF) and Scatter Factor (SF) in mesenchymal cells in close vicinity to epithelial structure [41-44].
Gingival fibroblasts are capable of synthesizing both FGF and SF. Recent studies show the increase of KGF and its receptor (KGF-R) in pathological over grown gingiva compared with normal gingival tissue. Thus it was suggested that epithelial changes in gingiva accompanied with drug induction is considered as secondary alteration due to abnormalities drug induced in the underlying lamia propria and mainly the adverse effect on fibroblast and growth factors.
A recent study using immunohistochemical stain for different growth factors, showed variability in concentration of these growth factors (TGF-ß1, PDGF-ß, TMPI and MMP) in relation to different drugs induced gingival over growth [62]. It was observed that not all drugs induce an adverse effect on gingiva, causing a significant overgrowth. The different results of both immunosuppressive drugs cyclosporine and tacrolimus showed that tacrolimus must be used in favor of cyclosporine [35].
Treatment
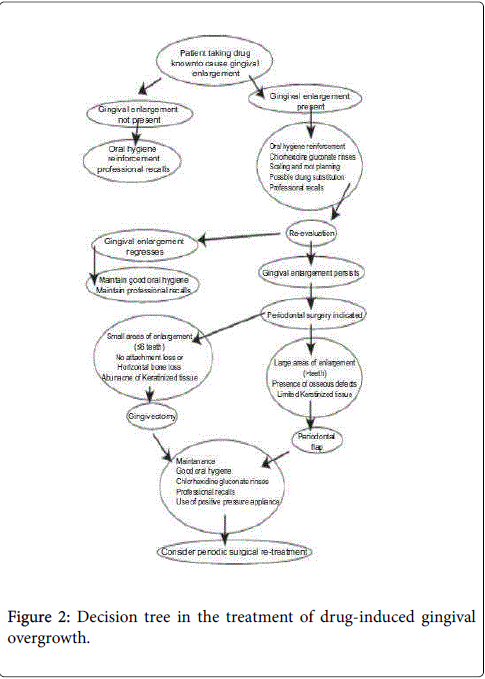
The patient’s management depends on: a) The medical history (e.g. patient’s age and the presence of other diseases) b) The clinical examination c) The size of the gingival overgrowth. Accordingly, when the enlargement is minimum, good scaling of the teeth, oral hygiene instructions and administration of antibiotics, usually amoxicillin and metronidazole, along with anti-inflammatory (ibuprofen) and analgesic (paracetamol) drugs and the use of chlorhexidine mouth rinses may be essential. The use of azithromycin in the management of GO was recently reported by researchers. The use of nonsteroidal antiinflammatory agent could be used to control interlukin-1 thus mediating inflammation as well low dose of androgen receptors antagonists to block the receptors of androgen and hence decrease abnormal collagen production (Figure 4). Furthermore, the discontinuing of using certain drug or using alternative medication replacing the used ones is sometimes required. Phenytoin was replaced by sulthiame and topiramate and cyclosporine-induced gingival fibromatosis was substituted by tacrolimus. Also, azathioprine was used as a protective drug against gingival hyperplasia through its antiproliferative and anti- inflammatory effect [89].
When the gingival mass increases; the need for surgical intervention is required owing to the functional and esthetic compromise. The treatment consists of surgical excision of the enlarged tissue; often in a series of gingivectomies, that should be accompanied by an effective program of oral hygiene. Few studies have documented the use of carbon dioxide laser, [48] however; the most widely used method of removing large quantities of tissue is the conventional external bevel gingivectomy with gingivoplasty particularly when there are pseudo pockets and no attachment loss [88,89]. A periodontal flap procedure may be preferred if fibromatosis is accompanied by attachment loss and osseous defects [80]. While non-specific gingival surgical excisions are the standard treatment for HGF, interferon-gamma is an example of a potential therapeutic adjunct for HGF cases, because of its activity on fibroblast myofibroblast differentiation [89]. Whenever possible the treatment should be performed after the complete eruption of permanent dentition. Regenerative techniques include the use of bone grafts, barrier membranes, wound healing agents and enamel matrix protein.
It has been reported that recurrence might be a common feature over varying periods. One report indicated that there is less chance of recurrence if the gingivectomy is delayed until permanent dentition is in place [58]. However slight recurrence was seen after 20 months [89]. In several reported cases, there was no recurrence in a period of 2 years, [81] 3 years, [82] or even 14 years follow up [83].
Conclusion
GF is a rare and slowly progressive condition that is also characterized by etiological heterogeneity. Moreover, it constitutes a typical symptom of several genetic syndromes and may occur sporadically in several other syndromes and diseases. By contrast, DIGO may occur as soon as several months from the onset of systemic therapy in susceptible individuals treated with certain immunosuppressant’s or calcium channel blockers. DIGO and HGF are disorders characterized by varying degrees of attached gingival overgrowth which might cause masticatory, phonetic, psychological, and esthetic problems. In general, the histological features of GF are similar, but phenytoin-induced GO is reported to be most fibrotic and to express higher levels of CTGF than nifedipine and CsA-induced GO. Excessive accumulation of ECM components, particularly collagen type I, seems to contribute to the pathologic manifestation of all etiological types of GF. Understanding of the molecular mechanisms leading to this disease may give better options for possible novel management strategies and allow the implementation of less invasive therapeutic methods than surgery into routine dental practice. In the current review, we tried to verify the various conditions of GF and focus mainly on their pathogenesis, histopathological, and regulatory mechanism as an overview and in an attempt for controlling the disease.
References
- DongariBagtzoglou A (2004) Drug-associated gingival enlargement. J Periodontol 75: 1424-1431.
- VipinBharti, ChhayaBansal (2013) Drug-induced gingival over growth: The nemesis of gingiva unraveled. J Indian SocPeriodontol 17: 182-187.
- Bhowmick SK, Gidvani VK, Retting KR (2001) Hereditary gingival fibromatosis and growth retardation. EndocrPract 7: 383-387
- Li R, Byers K, Walvekar RR (2008) Gingival hypertrophy: a solitary manifestation of scurvy. Am J Otolaryngol 29: 426-428.
- McIntosh CL, Kolhatkar S, Winkler JR, Ojha J, Bhola M (2010) An unusual case of generalized severe gingival enlargement during pregnancy. Gen Dent 58: e272-e278.
- Howard MR, Hamilton PJ (2008) Haematology. 3rd ed. Philadelphia: Elsevier.
- Dalirsani Z, Ghazi A (2015) T-cell lymphoblastic lymphoma in the maxilla and mandible of a child: a rare case report. J ClinDiagn Res 9: ZD22–ZD24.
- Stewart C, Cohen D, Bhattacharyya I, Scheitler L, Riley S, et al. (2007) Oral manifestations of wegenersgranulomatosis: a report of three cases and a literature review. J Am Dent Assoc 138: 338–348.
- Rangdhol RV, Madhulika N, Dany A, Jeelani S, Asokan GS (2014) Idiopathic orofacialgranulomatosis – a diagnostic and treatment challenge. J ClinDiagn Res 8: ZD07–ZD10
- Jané-Salas E, Albuquerque R, Font-Muñoz A, González-Navarro B, EstrugoDevesa A, et al. (2015) Pyogenic granuloma/peripheral giant-cell granuloma associated with implants. Int J Dent : 839032.
- Tripathi P, Aggarwal J, Chopra D, Bagga S, Sethi K (2014) Sarcoidosis presenting as isolated gingival enlargement: a rare case entity. J ClinDiagn Res 8: ZD25–ZD26.
- Oota S, Shibuya H, Hamagaki M, Yoshimura R, Iwaki H, et al. (2003) Oral pseudotumor: benign polypoid masses following radiation therapy. Cancer 97: 1353–1357.
- Liston SL, Dehner LP, Jarvis CW, Pitzele C, Huseby TL (1981) Inflammatory pseudotumors in the buccal tissues of children. Oral Surg Oral Med Oral Pathol 51: 287–291.
- Magnusson BC, Rasmusson LG (1995) The giant cell fibroma. A review of 103 cases with immunohistochemical findings. ActaOdontolScand 53: 293–296.
- Abdul Jalil A, Lau SH (2007) Gingival myofibroma in children: report of 4 cases with immunohistochemical findings. Malays J Pathol 29: 53–56.
- Brasileiro BF, Martins-Filho PR, Piva MR, da Silva LC, Nonaka CF, et al. (2010) Myofibroma of the oral cavity. A rare spindle cell neoplasm. Med Oral Patol Oral Cir Bucal 15: e596–e600.
- Scrieciu M, Mercuţ V, Mercuţ R, Am�?r�?scu MO, Popescu SM, et al. (2015) Immunohistochemical aspects of apoptosis in gingival mucosa with papilloma and condyloma acuminate. Rom J MorpholEmbryol 56: 425–431.
- Tandon PN, Gupta SK, Gupta DS, Jurel SK, Saraswat A (2012) Peripheral giant cell granuloma. ContempClin Dent 3: S118–S121.
- Qaisi M, Vorrasi J, Lubek J, Ord R (2014) Multiple primary squamous cell carcinomas of the oral cavity. J Oral MaxillofacSurg 72: 1511–1516.
- Tsubochi H, Suzuki T, Suzuki S (2000) Immunohistochemical study of basaloid squamous cell carcinoma, adenoid cystic and mucoepidermoid carcinoma in the upper aerodigestive tract. Anticancer Res 20: 1205–1211.
- Namboodiripad PC (2014) A review: immunological markers for malignant salivary gland tumors. J Oral BiolCraniofac Res 4: 127–134.
- Alos L, Lujan B, Castillo M, Nadal A (2005) Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J SurgPathol 29: 806–813.
- Ardekian L, Rosen DJ, Peled M, Rachmiel A, Machtei EE, et al. (2000) Primary gingival malignant melanoma. Report of 3 cases. J Periodontol 71: 117–120.
- Thomas PS, Babu GS, Anusha RL, Shetty S (2012) Oral malignant melanoma-an unusual presentation. Gerodontology 29 :e1241–e1243.
- Rasheed FS, MajeedAhlam H (2011) Immunohistochemical expression of actin and S100 in pleomorphic adenoma and mucoepidermoid carcinoma. Oral Diag 23: 51–54.
- Meng H, Xu L, Li Q, Han J, Zhao Y (2007) Determinants of host susceptibility in aggressive periodontitis. Periodontol 2000 43: 133–159.
- Gupta G, Khattak BP, Agrawal V (2011) Primary gingival tuberculosis: a rare clinical entity. ContempClin Dent 2: 31–33.
- Shukla P, Dahiya V, Kataria P, Sabharwal S (2014) Inflammatory hyperplasia: From diagnosis to treatment. J Indian SocPeriodontol 18: 92–94.
- Gawron K, Lazarz-Bartyzel K, Lazarz M, Steplewska K, Pyrc K, et al. (2014) Invitro testing the potential of a novel chimeric IgG variant for inhibiting collagen fibrils formation in recurrent hereditary gingival fibromatosis: chimeric antibody in a gingival model. J PhysiolPharmacol 65: 585–591.
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366: 1809–2000
- Breen GH, Addante R, Black CC (2009) Early onset of hereditary gingival fibromatosis in a 28-month-old. Pediatr Dent 31: 286–88
- Hart TC, Pallos D, Bozzo L, Almeida OP, Marazita ML, et al. (2000) Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res 79: 1758–1764.
- Seymour RA, Heasman PA (1988) Drugs and the periodontium. J ClinPeriodontol 15: 1–16.
- Dongari-Bagtzoglou A (2004) Research, science and therapy committee,american academy of periodontology. Drug-associated gingival enlargement. J Periodontol 75: 1424–1431.
- Hala El-Menoufy, Lobna Abdel Aziz Aly, AlyaaRagaei (2016). Collagen turnover induced by cellular connective tissue cytokines of drug induced gingival overgrowth and hereditary gingival fibromatosis (Histological and immunohistochemical comparative study), Future Dental Journal.
- Gawron K, Łazarz-Bartyzel K, Chomyszyn-Gajewska M (2014) Clinical presentation and management of a rare case of unilateral idiopathic gingival fibromatosis. Dent Med Probl 51: 546–552.
- Shetty AK, Shah HJ, Patil MA, Jhota KN (2010) Idiopathic gingival enlargement and its management. J Indian SocPeriodontol 14: 263–265.
- Dongari-Baqtzoglou A (2004) Research, Science and therapy committee, american academy of periodontology. Drug-associated gingival enlargement. J Periodontol 75: 1424-1431.
- Wentz LA, Oliveira SC, Moreira CH, Rösing CK (2012) Low prevalence of gingival overgrowth associated to new immunosuppressive protocols with cyclosporin. Braz Oral Res 26: 64-70.
- Sekiguchi RT, Paixão CG, Saraiva L, Romito GA, Pannuti CM, et al. (2007) Incidence of tacrolimus-induced gingival overgrowth in the absence of calcium channel blockers: A short-term study. J ClinPeriodontol 34: 545-550.
- Li J, Zhang YP, Kirsner RS (2003) Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 60: 107-114.
- Domei JH, Modeer T, Yecel Lindberg T (2004) Matrix metallo proteinase-1 and tissue inhibitors of metallo proteinase-1 production in human ginvial fibroblasts: the role of protein kinase C. J Periodontol Res 39: 308-314.
- Gangliano N, Moscheni C, Dellavia C, Masiero S, Torri C, et al. (2005) Morphological and molecular analysis of idiopathic gingival fibromatosis: a case report. J ClinPeriodontol 32: 1116-1121.
- Mark Bartold P, Sampath Narayanan A (2006) Molecular and cell biology of healthy and diseased periodontal tissues. Periodontology 40: 29-49.
- Bonnaure-Mallet M, Tricot-Doleux S, Godeau GJ (1995) Changes in extracellular matrix macromolecules in human gingiva after treatment with drugs inducing gingival overgrowth. Arch Oral Biol 40: 393–400.
- Bolzani G, Della Coletta R, MartelliJúnior H, MartelliJúnior H, Graner E (2000) Cyclosporin A inhibits production and activity of matrix metalloproteinases by gingival fibroblasts. J Periodontal Res 35: 51–58.
- Satoh M, Hirayoshi K, Yokota S, Hosokawa N, Nagata K (1996) Intracellular interaction of collagen-specific stress protein HSP47 with newly synthesized procollagen. J Cell Biol 133: 469–483.
- Martelli-Junior H, Cotrim P, Graner E, Sauk JJ, Coletta RD (2003) Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingival and hereditary gingival fibromatosis. J Periodontol 74: 296–306.
- Coletta RD, Almeida OP, Reynolds MA, Sauk JJ (1999) Alteration in expression of MMP-1 and MMP-2 but not TIMP-1 and TIMP-2 in hereditary gingival fibromatosis is mediated by TGF-beta 1 autocrine stimulation. J Periodontal Res 34: 457–463.
- Aimes RT, Quigley JP (1995) Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J BiolChem 270: 5872–5876.
- Shin GT, Khanna A, Ding R, Sharma VK, Lagman M, et al. (1998) In vivo expression of transforming growth factor beta-1 in humans: stimulation by cyclosporine. Transplantation 65: 313–318.
- Thomason JM, Sloan P, Seymour RA (1998) Immunolocalization of collagenase (MMP-1) and stromelysin (MMP-3) in the gingival tissues of organ transplant patients medicated with cyclosporin. J ClinPeriodontol 25: 554–560.
- Emerson TG (1965) Hereditary gingival fibromatosis: A family pedigree of four generations. Oral Surg Oral Med Oral Pathol 19: 1-9.
- Laband P, Habib G, Humphrey G (1964) Hereditary gingival fibromatosis: Report of an affected family with associated splenomegaly and skeletal and soft tissue abnormalities. Oral Surg Oral Med Oral Pathol 17: 339-351.
- Oikawa K, Cavaglia AM, Lu D (1979) Laband syndrome: Report of case. J Oral Surg 37: 120-122.
- Sciubba JJ, Niebloom T (1986) Juvenile hyaline fibromatosis (Murray-PureticDrescher Syndrome): Oral and systemic findings in siblings. Oral Surg Oral Med Oral Pathol 62: 397-409.
- Bansal A, Narang S, Sowmya K, Sehgal N (2011) Treatment and two year follow up of a patient with hereditary gingival fibromatosis. J Indian SocPeriodontol 15: 406-409.
- James PL, Prasad SV (1971) Gingival fibromatosis: Report of case. J Oral Surg 29: 55-59.
- Horning G, Fischer J, Barker B, Killoy WJ, Lowe JW (1985) Gingival fibromatosis with hypertrichosis. A case report. J Periodontol 56: 344-347.
- Bhowmick SK, Gidvani VK, Retting KR (2001) Hereditary gingival fibromatosis and growth retardation. EndocrPract 7: 383-387.
- Araiche M, Brode H (1959) A case of fibromatosis gingivae. Oral Surg Oral Med Oral Pathol 12: 1307-1310.
- Ramon Y, Berman W, Bubis JS (1967) Gingival fibromatosis combined with cherubism. Oral Surg Oral Med Oral Pathol 24: 435-448.
- Doufexi A, Mina M, Ioannidou E (2005) Gingival overgrowth in children: Epidemiology, pathogenesis and complications. A literature review. J Periodontol 76: 3-10.
- Gorlin RJ, Cohen MM, LevisLS (1990) Syndromes of head and neck. 3rd editionx. New York: Oxford University Press 847-855.
- Hart TC, Pallos D, Bozzo L, Almeida OP, Marazita ML, et al. (2000) Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res 79: 1758-1764.
- Hart TC, Pallos D, Bowden DW, Bolyard J, Pettanati MJ, et al. (1998) Genetic linkage of hereditary gingival fibromatosis to chromosome 2p21. Am J Hum Genet 62: 876-883.
- Hiura Y, Shen CS, Kokubo Y et al (2009) Identification of genetic markers associated with high-density lipoproteincholesterol by genome-wide screening in a Japanese population-the Suita study. Circul J 73: 1119–1126.
- Landstrom AP, Ackerman MJ (2009) GWAS or Gee Whiz, PSAS or Pshaw: elucidating the biologic and clinical significance of genetic variation in cardiovascular disease. Heart Rhythm 6: 1751–1753.
- Lau EC, Mohandas TK, Shapiro LJ, Slavkin HC, Snead ML (1989) Human and mouse amelogenin gene loci are on the sex chromosomes. Genomics 4: 162–168.
- Hart TC, Pallos D, Bozzo L (2000) Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res 79: 1758–1764.
- Hart TC, Hart PS (2009) Genetic studies of craniofacial anomalies: clinical implications and applications. OrthodCraniofac Res 12: 212–220.
- Xiao S, Bu L, Zhu L (2001) A new locus for hereditary gingival fibromatosis (GINGF2) maps to 5q13-q22. Genomics 74: 180–185.
- Ye X, Shi L, Cheng Y (2005) A novel locus for autosomal dominant hereditary gingival fibromatosis, GINGF3, maps to chromosome 2p22.3-p23.3. Clin Genet 68: 239-244
- Hassell TM, Hefti AF (1991) Drug-induced gingival over –growth:Oldproblem,new problem. Crit Rev Oral Biol Med 2: 103-137
- Boland J , Atkinson K, Britton K, Darveniza P, Johnson S, (1984) Tissue distribution and toxicity of cyclosporin A in the mouse. Pathology 1984; 16: 117-123.
- Gawron K, Łazarz-Bartyzel K, Jan Potempa, Maria Chomyszyn-Gajewska (2016) Gingival fibromatosis: clinical, molecular and therapeutic issues. Orphanet Journal of Rare Diseases 11: 9
- Breen GH, Addante R, Black CC (2009) Early onset of hereditary gingival fibromatosis in a 28-month-old. Pediatr Dent 31: 286–288.
- Millet C, Rodier P, Farges JC, Labert N, Duprez JP (2012) Surgical and prosthetic treatment in an elderly patient affected by unilateral idiopathic gingival fibromatosis: a case report. Gerodontology. 29: e1185–e1189.
- Lobao DS, Silva LC, Soares RV, Cruz RA (2007) Idiopathic gingival fibromatosis: A case report. Quintessence Int 38: 699-704.
- Bansal A, Narang S, Sowmya K, Sehgal N (2011) Treatment and two year follow up of a patient with hereditary gingival fibromatosis. J Indian SocPeriodontol 15: 406-409.
- Shafer WG, Hine MK, Levy BM (1983) A textbook of oral pathology. 4th edition. philadelphia: WB Saunders 785-786.
- Hart TC, Pallos D, Bozzo L, Almeida OP, Marazita ML, et al. (2000) Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res 79: 1758–1764.
- Baptista IP (2002) Hereditary gingival fibromatosis: A case report. J ClinPeriodontol 29: 871–874.
- Kataoka M, Kido J, Shinohara Y, Nagata T (2005) Drug-induced gingival overgrowth-a review. Biol Pharm Bull 28:1817–1821.
- Kelekis-Cholakis A, Wiltshire WA, Birek C (2002) Treatment and long-term follow-up of a patient with hereditary gingival fibromatosis: a case report. J Can Dent Assoc 68: 290–294.
- Sakamoto R, Nitta T, Kamikawa Y, Kono S, Kamikawa Y, et al. (2002) Histochemical, immunohistochemical, and ultrastructural studies of gingival fibromatosis: a case report. Med Electron Microsc 35: 248-254.
- Gawron K, Łazarz-Bartyzel K, Chomyszyn-Gajewska M (2014) Clinical presentation and management of a rare case of unilateral idiopathic gingival fibromatosis. Dent Med Probl 51: 546-552.
- Pasupuleti MK, Musalaiah SV, Nagasree M, Kumar PA (2013) Combination of inflammatory and amlodipine induced gingival overgrowth in a patient with cardiovascular disease. Avicenna J Med 3: 68–72.
- Casavecchia P, Uzel MI, Kantarci A, HaticeHasturk H, Dibart S, et al. (2004) Hereditary gingival fibromatosis associated with generalized aggressive periodontitis: a case report. J Periodontol 75: 770-778.
- Gawron K, Lazarz-Bartyzel K, Lazarz M, Steplewska K, Pyrc K, et al (2014) In vitro testing the potential of a novel chimeric IgG variant for inhibiting collagen fibrils formation in recurrent hereditary gingival fibromatosis: chimeric antibody in a gingival model. J PhysiolPharmacol 65: 585–591.
- Camargo PM, Melnick PR, Pirih FQ, Lagos R, Takei HH (2001) Treatment of drug-induced gingival enlargement: Aesthetic and functional considerations. Periodontol 2000 27: 131-138.
- Miller M, Truhe T (1993) Lasers in dentistry: An overview. J Am Dent Assoc 124: 32-35.
- Shafer WG, Hine MK, Levy BM. A textbook of oral pathology. 4th edition. philadelphia: W.B. Saunders; 1993.p. 785-6.
- Millet C, Rodier P, Farges JC, Labert N, Duprez JP (2012) Surgical and prosthetic treatment in an elderly patient affected by unilateral idiopathic gingival fibromatosis: a case report. Gerodontology 29: e1185-e1189
- Martelli-Junior H, Cotrim P, Graner E, Sauk JJ, Coletta RD (2003) Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. J Periodontol 74: 296-306.
- Bittencourt LP, Campos V, Moliterno LF, Ribeiro DP, Sampaio RK (2000) Hereditary gingival fibromatosis: Review of literature and a case report. Quintessence Int 31: 415-418.
- Gunhan O, Gardener DG, Bostanci H, Gunhan M (1995) Familial gingival fibromatosis withunusual histologic findings. J Periodontol 66: 1008-1011 .
Relevant Topics
- Blepharoplasty
- Bone Anchored Hearing Aids
- Chemical peel
- Cleft Surgery
- Congenital Craniofacial Malformations
- Cosmetic Facial Surgery
- Craniofacial Surgery
- Dental Orofacial Surgery
- Dentoalveolar Surgery
- Head and Neck Reconstruction
- Injectable Cosmetic Treatments
- Lip Reconstruction
- Mandibular Nerve Surgery
- Maxfax Surgery
- Maxillofacial Surgery
- Neck Liposuction
- Oral and Maxillofacial Surgery
- Oral Surgery Surgeon
- Orofacial Surgery Braces
- Pediatric Maxillofacial Surgery
- Rhytidectomy
- Sleep Apnea Orofacial Surgery
- Temporomandibular Joint Disorders
- Upper Jaw Surgery
Recommended Journals
Article Tools
Article Usage
- Total views: 7238
- [From(publication date):
June-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 6002
- PDF downloads : 1236