Research Article Open Access
Ghrelin Plasma Levels and Gastric Tissues Expression in Patients Submitted to Laparoscopic Sleeve Gastrectomy as Primary or Revisional Weight Loss Procedure
| Claudio Di Cristofano1, Caterina Chiappetta1, Francesco De Angelis2, Natale Porta1, Jessica Cacciotti1, Eugenio Lendaro1, Vincenzo Petrozza1, Carlo Della Rocca1* and Gianfranco Silecchia2 |
|
| 1 UOC of Pathology, Department of Medical-Surgical Sciences and Bio-Technologies, Sapienza University of Rome, Polo Pontino, I.C.O.T, Latina, Italy | |
| 2 UOC of Surgery, Department of Medical-Surgical Sciences and Bio-Technologies, Sapienza University of Rome, Polo Pontino, I.C.O.T, Latina, Italy | |
| Corresponding Author : | Carlo Della Rocca, MD UOC of Pathology Department of Medical-Surgical Sciences and Bio-Technologies Sapienza University of Rome Polo Pontino, I.C.O.T, Latina Corso della Repubblica 79, Italy Tel: 0773-6513411 E-mail: carlo.dellarocca@uniroma1.it |
| Received April 29, 2014; Accepted May 21, 2014; Published May 26, 2014 | |
| Citation: Cristofano CD, Chiappetta C, Angelis FD, Porta N, Cacciotti J, et al. (2014) Ghrelin Plasma Levels and Gastric Tissues Expression in Patients Submitted to Laparoscopic Sleeve Gastrectomy as Primary or Revisional WeightLoss Procedure. J Obes Weight Loss Ther 4:215. doi:10.4172/2165-7904.1000215 | |
| Copyright: © 2014 Cristofano CD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Introduction: Ghrelin (Ghr) plays a role in the regulation of food intake and Laparoscopic Sleeve gastrectomy (LSG) is used for treatment of morbid obesity (MO) and after this the expression of ghrelin could be modulate. The aim of present study was to analyze the expression of ghr in three areas of resected stomach specimens from MO patients and co rrelate these data with plasmatic ghrelin levels before andafter surgery. Materials and Methods: 36 morbidly obese patients (17%, 6/36 with Type 2 Diabetes) were subjected to LSG and tissue samples were obtained from the fundus, body, prepyloric of the resected stomach. For mRNA and protein expression analysis. Blood samples were collected before and 1 month after surgery to evaluate the plasmatic ghrelin levels. Results: Ghrelin protein expression was higher in the fundus than in the other areas. T2DM patients showed a lower basal ghrelin plasma level compared with non-diabetic patients but they showed a high percentage of positive cells in the stomach. Was not observed a statistically difference in plasmatic, mRNA and protein expression of ghrelin between primary LSG patients and in revisional LSG group. Conclusion: Ghrelin fundal mucosal expression was comparable in primary and revisional LSG. Diabetic patients showed a compensatory higher protein mucosal expression probably to balance lower plasma Ghrelin level. Further studies will elucidate the clinical relevance of those preliminary data.
| Keywords |
| Ghrelin; Laparoscopic sleeve gastrectomy; Morbid obesity |
| Introduction |
| Ghrelin (Ghr), a ligand for the growth hormone secretagogue receptor, is an orexigenic hormone produced primarily by cells in the oxyntic glands of the stomach [1-4]. It is known to increase appetite and regulate weight and body composition [5]; it plays a physiological role in the regulation of food intake (meal initiator) with plasma ghr levels rising shortly before meals and declining rapidly after food intake in humans and rodents [3,6,7]. Although produced throughout the length of the intestine, ghr is mainly secreted by the stomach, followed by the small intestine but it is also expressed by peripheral tissues, such as pancreas and brain [1,8,9]. |
| Interestingly, fasting plasma ghr concentration is negatively correlated with Body Mass Jndex (BMI), so the ghr concentration is lower in obese patients than in lean subject [7,10]. Plasma ghr levels increased in patients with eating disorder, like anorexia nervosa and cachexia [11-13]. The regulatory mechanism of ghr expression is not completely understood and moreover the plasmatic ghr levels are affected by several conditions like pre-analytic factors in plasmatic ghr analysis, stress, diet and its circadian rhythm [5]. |
| Laparoscopic Sleeve Gastrectomy (LSG) is rapidly gaining acceptance both as a first stage of biliopancreatic diversion with duodenal switch (BPD-DS) or bridge procedure to gastric bypass in high risk patients or definitive procedure for treatment of morbid obesity (MO) [14,15]. Recently, LSG has been proposed as an alternative revisional procedure for failed or complicated gastric banding [16]. Most bariatric surgeons perform the revisional procedure in one stage and the outcomes in terms of weight loss seem to be comparable with the primary LSG [17-22]. In order to reduce perioperative complications rate, some authors have proposed to perform the LSG in “two stage” after the band removal [23-25]. The main steps of the procedure includes a vertical resection of most of the stomach volume calibrated on an orogastric bougie, removing part of the antrum, most of the body, and all of the fundus [14,26]. The proposed mechanism of action of this procedure is a combination of volume restriction, creation of a high pressure system and the induction of a favourable hormonal change [27,28]. Therefore crucial points of the technique are the amount of antrum resected (distance of the stapler line from the pylorus), orogastric bougie size and completed fundus dissection to allow a proper total fundectomy. The left crus of the diaphragm should be systematically exposed and the posterior attachment of the fundus released [27]. Since LSG removes the area of stomach producing ghr, low plasma ghr levels were monitored up to 5 years [15]. |
| MO individuals report a decline in appetite after LSG, presumably due, in part, to ghr cell removal [1]. Moreover, there are strong evidence that surgery can “heal” most of the MO patients with type 2 diabetes mellitus (T2DM) diagnosed less 10 years but the mechanism is not completely understood [29-32]. |
| Recently, some authors have studied the localization of ghr expression to try to clarify the clinical significance of ghr in obese patient’s submitted to LSG [5,33] |
| Aim of this prospective study was to evaluate ghr protein tissues distribution in different stomach areas by immunohistochemical analysis and mRNA gene expression by Real-Time Polymerase chain reaction (RT-PCR) on fresh stomach specimens tissue of LSG carried out as primary or revisional intent. Another important end point was to analyze the circulating plasma ghr levels before and 1 month after surgery and to correlate these values with mRNA and protein ghr expression. |
| Materials and Methods |
| Population study |
| Thirty-six MO patients, 6 men and 30 women with median age of 39 years old (range 22 to 61 years) consecutively undergone LSG, has been enrolled prospectively. Exclusion criteria were: not compensated chronic liver or renal disease; BMI >60 Kg/m2, procedure intent as first stage of BPD-DS, conversion to open surgery, age <18 and >65 years old. Median pre-sleeve BMI value was 44.5 Kg/m2 (range 36-57) and 9 (25%) patients underwent LSG as revisional procedure after gastric banding failure (mean interval band removal-sleeve was 5 months). Six patients (17%), all women with median age of 52 years old, presented T2DM diagnosed following ADA criteria [34]; all T2DM patients underwent to primary LGS and the median pre-sleeve BMI value was 43.5 Kg/m2. |
| Institutional review board approval was obtained prior to study initiation and all subjects signed an informed consent form. |
| Laparoscopic sleeve gastrectomy |
| The LSG was performed according to the technique described by Gagner [23]. The division of the gastric greater curvature vascular supply started 6 cm from the pylorus (antrum preserving procedure) and proceeded upwards to the angle of His; ultrasound dissection or radiofrequency were used. The gastro-esophageal junction was always assessed, and a hiatal hernia, if found, was repaired. The LSG was created using a linear stapler. The stapler was applied next to a 42 Fr bougie placed next to the lesser curve. The stomach was transected and a gastric pouch of 80-100 ml created. In the case of a primary LSG, two sequential green cartridges were used for the transection of antrum, followed by two or three sequential yellow cartridges for the gastric corpus and fundus. For LSG revision, only green reinforced (Seamguard®) cartridges were used. An intraoperative methylene blue test was performed and a 19 Fr drain was placed alongside the transected stomach. The stomach was removed throughout a 12 mm port incision enlarged to avoid specimen damage. A stitch was put on the distal resection margin (pre-pyloric area). |
| Plasma ghrelin determination |
| The plasmatic levels of ghr were measured by a commercial radioimmunoassay kit (LINCO Cat# GHRT-89HK). In all fasting overnight patients a draw of peripheral blood was obtained the day of surgery and 1 month after surgery. Radioactivity was determined by the WALLAC gamma counter (1261). Two samples were excluded due to technical error in sample handling. |
| Immunohistochemical analysis |
| Stomach specimens resected during SG were fixed in buffered formalin 10% for 24 hours. After fixation, three tissue samples were obtained from 3 areas of each stomach: the fundus, body and prepyloric area. |
| The avidin–biotin complex (ABC) method was performed on 4 μm thick tissue sections for immunohistochemical analysis. Sections were deparaffinized with xylene for 15 min and were treated in a microwave oven using 0.01 M citrate buffer (pH 6.0) for 30 min. The mouse IgG monoclonal antibody directed against ghrelin (Neomarker, Fremont, CA, USA) was used. The reaction was performed in an automated system (Bond Max, Menarini, Italy). Results were expressed according to a semiquantitative analysis: two pathologists evaluated the percentage of cells positive in 3 different areas (20X, fundus, body, pre-pyloric) of each sample; the mean value of the three different areas was used to define the value of positive cells in each sample. For each patient, the ghr expression was calculated using the mean value of positive cells of three areas. |
| Real-Time Polymerase Chain reaction |
| RNA from frozen tissue was extracted from fundus of the stomach using SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the supplier’s instructions, which provided an elution in a final volume of 100 μL. The extracted RNA was quantitated by OD260/280 measurement; next total RNA was reverse-transcribed in a final volume of 20uL using High Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, CA, USA). The cDNA was storage -20°C until it was used. |
| Then, 30 ng cDNA was added to 2X TaqMan Fast Universal PCR Master Mix (Life Technologies, Foster City, CA, USA) in a final volume of the 10 μl. Real-Time quantitative PCR for ghr mRNA was performed on an ABI PRISM 7500 Fast Real Time PCR System (Life Technologies, Foster City, CA, USA). Amplification of cDNA was performed under the following conditions: 20s at 95°C, 3s at 95°C and 30s at 60°C for 40 cycles. PCR products for ghr were detected using gene-specific primers and probes labeled with reporter dye FAM (Ghr gene ID: 51738, Life Technologies, Foster City, CA, USA). GAPDH was selected as endogenous control and the expression of ghrelin was compared to this housekeeping gene; GAPDH was detected using gene-specific primers and probes labeled with reporter dye FAM (gene ID: 2597, Life Technologies, Foster City, CA, USA). Amplification products length of ghr and GAPDH was 62 and 58 base pairs respectively. PCR reaction was carried out in triplicate on 96-well plate, at the end of the reaction; the results were evaluated using the ABI PRISM 7500 software. The Ct (Cycle threshold) values for each set of three reactions were averaged for all subsequent calculations. |
| Statistical analysis |
| All data obtained from the various analyses will be collected in a customizable database built with Microsoft Access (Microsoft Corporation). Relevant data will be extracted from the database with appropriate queries and exported in Microsoft Excel (Microsoft Corporation) and SSPS v.08 for further manipulations and statistical analyses. |
| Results |
| Plasmatic ghrelin levels |
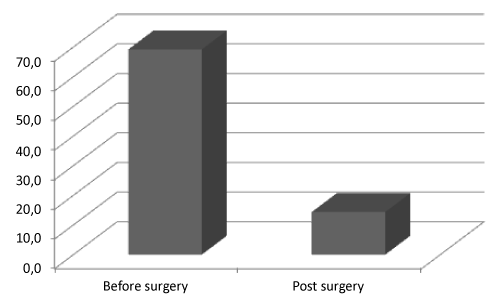
| Total ghr plasma levels decreased from 69.2 ± 99.4 pg/mL before surgery to 14.4 ± 31.6 pg/mL after surgery (Figure 1). In T2DM patients the ghr level before surgery was 40.7 ± 44.5 pg/mL while in non-T2DM patients was 75.1 ± 127.2 pg/mL; one month after surgery, ghr levels in T2DM and non-T2DM patients were 21.5 ± 31.6 pg/mL and 12.7 ± 27.2 pg/mL respectively. |
| Was not observed a statistically significant difference in the values of plasma ghr before surgery between patients undergone LSG as primary procedure versus those subjected to revisional (two stages approach) LSG, which showed 73.9 ± 127.2 pg/mL and 55.5 ± 99.4 pg/mL respectively; a statistically difference was not observed in the plasma ghr values one month after surgery too (16.1 ± 31.6 pg/mL and 5.7 ± 17.2 pg/mL). |
| Mean BMI was 44.5 ± 10.5 kg/m2; a negative correlation between the basal plasma ghr levels and BMI was observed (p=0.01). T2DM patients showed basal plasmatic ghr value lower than non-T2DM patients (40.7 pg/mL vs. 75.1 pg/mL, p=0.05, Table 1). |
| Immunohistochemical results |
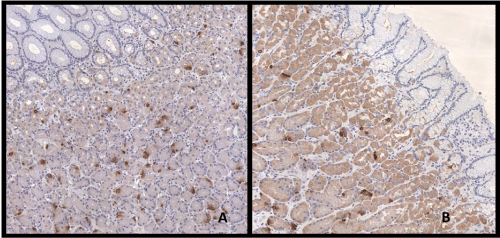
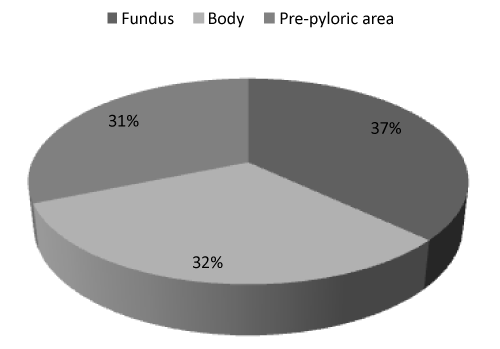
| Immunohistochemical expression of ghr was detected in 25.0% of gastric cells; furthermore, we observed that ghr-producing cells were distributed differently in investigated areas. Indeed, the prevalence of ghr-producing cells was higher in the fundus than in the body and even less in the pre-pyloric area near the distal resection surgical margin. The average ghr cells counts were 27.7 ± 45.6, 23.9 ± 29.3, 23.6 ± 28.0 cells/ field in the fundus, body and pre-pyloric area respectively (Figures 2 and 3) but this trend was not statistically significant. In T2DM patients the immunohistochemical expression of ghr was 30.7% while in non- T2DM patients was 23.9% (p=0.02). The ghr immunohistochemical expression was similar in primary and revisional sleeve (25.0% and 25.1%). We didn’t find correlation between protein expression and ghr plasma level. |
| Quantitative mRNA ghrelin expression |
| Level of ghr mRNA expression was normalized to the expression of GAPDH; the mean value of RQ (Relative Quantification) of ghr mRNA expression was 2.6 ± 12.5. The T2DM patients showed a value of ghr mRNA expression of 2.1 ± 2.2 while non-T2DM patients showed a mean value of 2.6 ± 12.3. Ghr expression was significantly higher (3.0 ± 12.2) in primary LSG group than in revisional 1.2 ± 2.0 (p<0.05). |
| We find a strong correlation between the mRNA expression and the protein expression of ghr in the fundus (p=0.0001), while we found a weak correlation between the mean value of mRNA expression and the mean value of ghr protein expression obtained from the three areas (fundus, body, pre-pyloric, p=0.04). Also in this case, we didn’t find correlation between mRNA expression and plasmatic levels of ghr. |
| Discussion |
| The decrease of plasma ghr after LSG is advocated as one of the hormonal mediator of the weight loss as well as glucose homeostasis in the early phase in absence of a significant weight loss. Several experimental and clinical studies suggest a change of incretin levels related to the marked post-LSG ghr decrease [35-38]. |
| In this prospective study, ghr expression and tissue distribution of cells producing this protein were evaluated to better understand the ghr distribution in different areas of stomach and correlate those findings with ghr plasmatic levels in different groups of patients undergone LSG (primary sleeve, revisional sleeve, diabetic). |
| The total plasma ghr level in all study patients before surgery was correlate to BMI as previously reported [10,39]. The ghr plasma level significantly decreased after surgery in all study groups as a results of proper total fundectomy [11,15]. On the other hand, several studies don’t find a modification of ghr plasma levels and other authors report controversial results including an increment of ghr plasma value after surgery [40,41]. The contradictory results could be due to different study designs, follow-up periods, measure methods, surgical intervention and circadian rhythm [5,11]. |
| In agreement with Goiten et al. [33], our immunohistochemical results showed that ghr protein expression was higher in the fundus than in the body and pre-pyloric area; although this difference was not statistically significant. Miyazaki et al. hypothesize that greater is the number of positive cells present on the stomach, better will be the surgery outcome and, if the number of positive cells present in the stomach correlates with the mRNA ghr expression, this value could be considered a favourable predictor of LSG outcome [5]. We found a distinct correlation between ghr mRNA expression and ghr protein expression in the fundus and a weak correlation between ghr mRNA expression and the mean value of ghr protein expression obtained from the three areas (fundus, body, pre-pyloric). Nevertheless, as previously reported [33], levels of ghr mRNA didn’t correlate with plasmatic protein levels, this could be due to compensatory productions from extra gastric organs. |
| Moreover, T2DM patients showed a lower basal (pre-operative) ghr plasma level compared with non-diabetic patients. Several studies have found that low ghr plasma levels are associated with elevated fasting insulin concentration and the prevalence of T2DM and insulin resistance and levels of plasmatic ghr is found to be lower in obese T2DM patients than to equally obese insulin-sensitive controls [42,43]. However, we observed that the percentage of ghr positive cells in T2DM patients was significantly higher compared to no-T2DM; this could suggest that T2DM patients developed a hyperplasia to compensate the lack of circulating ghr. |
| Recently, there is a debate on the extension of the antral resection during SG: some authors recommend to preserve the antrum because this site is important as a pumping mechanism for gastric emptying, because the partial antrum resection doesn’t affect significantly the long term pouch volume [44,45] and because the removal of antral tissue allows a more extensive reduction of ghr producing cells [33]. On the contrary, other authors argue the cells population present on antral tissue could be not the real population of cells producing ghr because is very different from other areas and because is numerically poor [46,47]. According to this, we believe that is not necessary to remove antral tissue since the majority of positive cells were present on fundus tissue, as previously described [46,47] but this should be confirmed on a larger population. |
| We didn’t find differences between patients who underwent to primary SG and revisional SG, both in protein expression and mRNA levels and basal plasmatic levels of ghr. These findings support the positive short and mid-terms results of LSG as revisional procedure compared to primary. |
| The limits of the present study are: small numbers of diabetic patients and non-comparable size group (primary vs. revisional), short term plasma level measurements, no long term follow up. Further studies are needed to confirm the preliminary results in a large population. |
| In conclusion, the preliminary results of the on-going prospective study confirm that ghr protein expression was higher in the fundus than in the body and pre-pyloric areas. Moreover, T2DM patients showed a lower pre-operative ghr plasma level and more ghr positive cells in stomach compared with non-diabetic patients. We didn’t found difference in ghr plasma level between patients who underwent to primary or revisional SG supporting the clinical evidence of similar excess weight loss. Further evaluation on a large population will confirm or not those preliminary findings. |
| Acknowledgements |
| The authors thank the “Serena Talarico Association”, “Fondazione Roma” and “AISOS” for the precious support to this research. |
| Conflict of Interest |
| No potential conflict of interest relevant to this article was reported. |
References
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, et al. (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J ClinEndocrinolMetab 86: 4753-4758.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656-660.
- Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, et al. (2001) Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 24: RC19-21.
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, et al. (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120-1128.
- Miyazaki Y, Takiguchi S, Seki Y, Kasama K, Takahashi T, et al. (2013) Clinical significance of ghrelin expression in the gastric mucosa of morbidly obese patients. World J Surg 37: 2883-2890.
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, et al. (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714-1719.
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, et al. (2002) Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J ClinEndocrinolMetab 87: 240-244.
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, et al. (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255-4261.
- Simonsson M, Eriksson S, Håkanson R, Lind T, Lönroth H, et al. (1988) Endocrine cells in the human oxyntic mucosa. A histochemical study. Scand J Gastroenterol 23: 1089-1099.
- Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, et al. (2001) Circulating ghrelin levels are decreased in human obesity. Diabetes 50: 707-709.
- Gelisgen R, Zengin K, Kocael A, Baysal B, Kocael P, et al. (2012) Effects of laparoscopic gastric band applications on plasma and fundicacylated ghrelin levels in morbidly obese patients. ObesSurg 22: 299-305.
- Kojima M, Hosoda H, Kangawa K (2004) Clinical endocrinology and metabolism. Ghrelin, a novel growth-hormone-releasing and appetite-stimulating peptide from stomach. Best Pract Res ClinEndocrinolMetab 18: 517-530.
- Dadan J, Hady HR, Zbucki RL, Iwacewicz P, Bossowski A, et al. (2009) The activity of gastric ghrelin positive cells in obese patients treated surgically. Folia HistochemCytobiol 47: 307-313.
- Roa PE, Kaidar-Person O, Pinto D, Cho M, Szomstein S, et al. (2006) Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. ObesSurg 16: 1323-1326.
- Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, et al. (2010) Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. ObesSurg 20: 535-540.
- Eisenberg D, Bellatorre A, Bellatorre N (2013) Sleeve gastrectomy as a stand-alone bariatric operation for severe, morbid, and super obesity. JSLS 17: 63-67.
- Goitein D, Feigin A, Segal-Lieberman G, Goitein O, Papa MZ, et al. (2011) Laparoscopic sleeve gastrectomy as a revisional option after gastric band failure. SurgEndosc 25: 2626-2630.
- Foletto M, Prevedello L, Bernante P, Luca B, Vettor R, et al. (2010) Sleeve gastrectomy as revisional procedure for failed gastric banding or gastroplasty. SurgObesRelat Dis 6: 146-151.
- Elnahas A, Graybiel K, Farrokhyar F, Gmora S, Anvari M, et al. (2013) Revisional surgery after failed laparoscopic adjustable gastric banding: a systematic review. SurgEndosc 27: 740-745.
- Gagnière J, Slim K, Launay-Savary MV, Raspado O, Flamein R, et al. (2011) Previous gastric banding increases morbidity and gastric leaks after laparoscopic sleeve gastrectomy for obesity. J ViscSurg 148: e205-209.
- Rebibo L, Mensah E, Verhaeghe P, Dhahri A, Cosse C, et al. (2012) Simultaneous gastric band removal and sleeve gastrectomy: a comparison with front-line sleeve gastrectomy. ObesSurg 22: 1420-1426.
- Acholonu E, McBean E, Court I, Bellorin O, Szomstein S, et al. (2009) Safety and short-term outcomes of laparoscopic sleeve gastrectomy as a revisional approach for failed laparoscopic adjustable gastric banding in the treatment of morbid obesity. ObesSurg 19: 1612-1616.
- Iannelli A, Schneck AS, Ragot E, Liagre A, Anduze Y, et al. (2009) Laparoscopic sleeve gastrectomy as revisional procedure for failed gastric banding and vertical banded gastroplasty. ObesSurg 19: 1216-1220.
- Bernante P, Foletto M, Busetto L, Pomerri F, Pesenti FF, et al. (2006) Feasibility of laparoscopic sleeve gastrectomy as a revision procedure for prior laparoscopic gastric banding. ObesSurg 16: 1327-1330.
- Dapri G, Cadière GB, Himpens J (2009) Feasibility and technique of laparoscopic conversion of adjustable gastric banding to sleeve gastrectomy. SurgObesRelat Dis 5: 72-76.
- Cutolo PP, Nosso G, Vitolo G, Brancato V, Capaldo B, et al. (2012) Clinical efficacy of laparoscopic sleeve gastrectomyvs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. ObesSurg 22: 1535-1539.
- Yehoshua RT,Eidelman LA, Stein M, Fichman S, Mazor A, et al. (2008) Laparoscopic sleeve gastrectomy--volume and pressure assessment. ObesSurg 18: 1083-1088.
- Baltasar A, Serra C, Pérez N, Bou R, Bengochea M (2006) Re-sleeve gastrectomy. ObesSurg 16:1535-1538.
- Lee WJ, Chong K, Chen CY, Chen SC, Lee YC, et al. (2011) Diabetes remission and insulin secretion after gastric bypass in patients with body mass index <35 kg/m2. ObesSurg 21: 889-895.
- Thaler JP, Cummings DE (2009) Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150: 2518-2525.
- Rubino F, Kaplan LM, Schauer PR, Cummings DE (2010) Diabetes Surgery Summit Delegates; Diabetes Surgery Summit Delegates. The Diabetes Surgery Summit Consensus Conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 251:399-405.
- Rizzello M, De Angelis F, Campanile FC, Silecchia G (2012) Effect of gastrointestinal surgical manipulation on metabolic syndrome: a focus on metabolic surgery. Gastroenterol Res Pract 2012: 670418.
- Goitein D, Lederfein D, Tzioni R, Berkenstadt H, Venturero M, et al. (2012) Mapping of ghrelin gene expression and cell distribution in the stomach of morbidly obese patients--a possible guide for efficient sleeve gastrectomy construction. ObesSurg 22: 617-622.
- American Diabetes Association (2008) Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 31:S55-60.
- Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, et al. (2013) Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J ClinEndocrinolMetab 98: 4391-4399.
- Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK (2008) Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247: 401-407.
- Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, et al. (2009) Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250:234-241.
- Pacheco D, de Luis DA, Romero A, González Sagrado M, Conde R, et al. (2007) The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg 194: 221-224.
- Ueno H, Yamaguchi H, Kangawa K, Nakazato M (2005) Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. RegulPept 126: 11-19.
- Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, et al. (2003) Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidily obese subjects. J ClinEndocrinolMetab 88:1594-1602.
- Holdstock C, Engström BE, Ohrvall M, Lind L, Sundbom M, et al. (2003) Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J ClinEndocrinolMetab 88: 3177-3183.
- Ikezaki A, Hosoda H, Ito K, Iwama S, Miura N, et al. (2002) Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes 51: 3408-3411.
- McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE (2004) Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J ClinEndocrinolMetab 89: 1630-1635.
- Weiner RA, Weiner S, Pomhoff I, Jacobi C, Makarewicz W, et al. (2007) Laparoscopic sleeve gastrectomy--influence of sleeve size and resected gastric volume. ObesSurg 17: 1297-1305.
- Givon-Madhala O, Spector R, Wasserberg N, Beglaibter N, Lustigman H, et al. (2007) Technical aspects of laparoscopic sleeve gastrectomy in 25 morbidly obese patients. ObesSurg 17: 722-727.
- Choe YH, Song SY, Paik KH, Oh YJ, Chu SH, et al. (2005) Increased density of ghrelin-expressing cells in the gastric fundus and body in Prader-Willi syndrome. J ClinEndocrinolMetab 90: 5441-5445.
- Maksud FA, Barbosa AJ (2013) Letter to: Mapping of ghrelin gene expression and cell distribution in the stomach of morbidly obese patients – a possible guide for efficient sleeve gastrectomy construction. ObesSurg 23:115-116.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 13964
- [From(publication date):
June-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9394
- PDF downloads : 4570
