Research Article Open Access
Gestational Weight Gain and Changes in Body Composition in Pregnant Women with BMI 25 Kg/m2 Undergoing a Healthy Lifestyle Program Starting Early in Pregnancy
| Marcella Malavolti1*, Elisabetta Petrella2, Valentina Bertarini1, Dietitian2, Cristina Cicchetti1, Isabella Neri2 and Fabio Facchinetti2 | |
| 1Technical and Applied Science Dietary Chair, Clinical Diagnostic Medicine and Public Health Department, University of Modena and Reggio Emilia, Italy | |
| 2Mother-Infant Department, University of Modena and Reggio Emilia, Modena, Italy | |
| *Corresponding Author : | Marcella Malavolti Technical and Applied Science Dietary Chair Clinical Diagnostic Medicine and Public Health Department, via: del Pozzo 71 41124 Modena, University of Modena and Reggio Emilia, Italy Tel: 39- 059-2055350 Fax: 39-059-2055483 E-mail: marcella.malavolti@unimore.it |
| Received January 30, 2016; Accepted Februray 18, 2016; Published February 21, 2016 | |
| Citation: Malavolti M, Petrella E, Bertarini V, Dietitian, Cicchetti C, et al. (2016) Gestational Weight Gain and Changes in Body Composition in Pregnant Women with BMI ≥25 Kg/m2 Undergoing a Healthy Lifestyle Program Starting Early in Pregnancy. J Obes Weight Loss Ther 6:300. doi:10.4172/2165-7904.1000300 | |
| Copyright: © 2016 Malavolti M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Introduction: we evaluated the utility of bioimpedance analysis and the SenseWear armband for monitoring gestational weight gain (GWG) in overweight/obese pregnant women. Materials and methods: Sixty overweight/obese pregnant women were recruited and advised to follow a physical activity (PA) program (sessions gradually increasing to 30 minutes of mild physical activity intensity per day at least 4-5 days a week) with caloric restriction (1500 kcal/day + 200 kcal/day for obese and 300 kcal/day for overweight women). Weight, GWG and body composition were measured at enrollment (within the 12th week of gestation), at the 16th, 20th, 32nd, and 36th week, at delivery and 12 weeks after delivery using BIA. The patients were also instructed to wear the SWA, for 5 consecutive days, at enrollment, and at the 20th and 36th weeks of gestation. Results: GWG at delivery was 12.6 ± 5.4 kg for overweight, 9.0 ± 8.0 kg for obese women and 3.3 ± 5.3 kg for morbidly obese women. Nine overweight (60.0%), 22 obese (66.7%) and 10 morbidly obese (90.9%) women remained within the Institute of Medicine recommended ranges at delivery. Overall percentual body composition changed significantly throughout pregnancy, (fat-free mass: 53.4 ± 6.1 to 56.9 ± 6.5; FM = 40.9 ± 12.8 to 44.1 ± 11.8, p < 0.05). Logistic regression analysis showed that women who adhered to the prescribed diet achieved a lower GWG (CI 95% = -1.65-0.54; r = -1.10, p = 0.001). Conclusion: The efficacy of the intervention depends directly on the adherence to the prescribed diet and PA program
| Keywords |
| Pregnancy; Lifestyle; Overweight; BIA; SenseWear armband; Physical activity |
| Abbreviations |
| BIA: Bioelectrical Impedence Analysis; BMI: Body Mass Index; FFQ: Food Frequency Questionnaire; FM: Fat Mass; FFM: Fat Free Mass; GDM: Gestational Diabetes Mellitus; GWG: Gestational Weight Gain; PA: Physical Activity; SWA: SenseWear Mini Armband |
| Key Message Box |
| A low glycemic diet with a caloric restriction associated with moderate physical activity can reduce weight gain during pregnancy in overweight/obese women. The efficacy of the intervention depends directly on the adherence to the prescribed diet and physical activity. |
| Materials and Methods |
| The Institute of Medicine (IOM) in 2009 updated the guidelines for GWG for different BMI categories [1] while emphasizing the importance of PA and diet for weight control. Several studies suggest that appropriate GWG is associated with an optimal delivery outcome both for mother and child [2,3]. On the other hand, an excessive GWG is associated with an increased risk of complications during the prenatal period [3] and at delivery. Moreover, excessive GWG is a risk factor for the development of maternal diseases later in life (i.e. diabetes, hypertension, breast cancer) as well as cardio-vascular diseases of the newborns during their adulthood [4]. |
| Despite both ACOG and ACSM [5,6] recommend a certain amount of PA during pregnancy, several studies on GWG both in the US and Europe indicate that only a small sample of women remain within the IOM recommendations [7,8]. Moreover, according to the most recent Cochrane review [9], interventions to prevent and/or limit extra GWG have not been adequately evaluated. Indeed, only a few studies have included both diet and PA in their intervention program. In a previous paper, we demonstrated that a multilevel intervention (including caloric restriction, changes in eating behavior, and regular PA from the beginning of pregnancy) in obese women was able to reduce both GWG and related pregnancy complications [10]. |
| In addition to measuring GWG, it is important to evaluate changes in body composition, in particular, differentiating between FM and FFM. Octapolar bioelectrical impedance analysis (BIA) allows a simple and reproducible method of body composition assessment in a variety of conditions including obesity and diabetes [11,12]. Moreover, a few studies have used the metabolic Holter monitors to record PA levels and walking steps in pregnant women [13] while others have evaluated the validity of the SWA to estimate energy expenditure during pregnancy [14]. The purpose of the present study was to evaluate the usefulness of tools such as the SWA and BIA in monitoring body composition changes of pregnant women with BMI ≥ 25 kg/m2 enrolled in a healthy lifestyle program designed to help them to reach an optimal GWG. |
| Materials and Methods |
| Study design |
| This observational study was approved by the local Ethics Committee. All volunteers gave written informed consent prior to the start of the study. |
| Pregnant women with a pre-pregnancy BMI ≥ 25 kg/m2, age >18 years and a single pregnancy were included. At enrollment, an accurate obstetric and lifestyle history was collected and FFQ for investigate eating habits before starting a proper nutrition was filled-in. None among the patients followed a diet prior to enrollment or a physical activity program. Moreover, it was the first experience with a dietitian for each of them. |
| They were enrolled within the 12th week of gestation at the Obstetric Unit of Policlinico Hospital - University of Modena, and were scheduled for specific follow-up appointments at the 16th, 20th, 32nd and 36th weeks of gestation to evaluate the adherence to the program. Each visit was carried out with a gynecologist and dietitian simultaneously present. |
| Exclusion criteria were: multiple pregnancy, chronic disorders (i.e., diabetes mellitus, hypertension, and untreated thyroid diseases), GDM in previous pregnancies, a smoking habit, intake of dietary supplements or herbal products known to affect body weight, contraindications for exercise [5] and intent to deliver outside our Birth Center. |
| Every subject received care in the Antenatal Clinics of Modena National Health System. Two ultrasound examinations and at least 5 antenatal obstetric evaluations until term were routinely provided. |
| Blood pressure, weight (Wt) and height (Ht) were measured and BMI was calculated. At enrollment, at each follow-up visit and 12 weeks after the delivery, Wt and specific body composition changes were measured using the BIA. Body Wt was also measured at delivery. Postpartum Wt retention was calculated as the difference between Wt before pregnancy and 12 weeks after delivery. At enrollment, each subject completed a food frequency questionnaire (FFQ) and the dietitian and gynecologist provided caloric restriction instructions and explained the PA program. FFQ was completed again at the 36th week of gestation to evaluate any change in eating habits. The patients were instructed to wear the SWA, continuously for five whole consecutive days (from Thursday morning to Monday evening), at enrollment, at the 20th and at the 36th week of gestation. |
| All participants were advised to participate in sessions of at least 15 minutes gradually increasing to 30 minutes of mild intensity PA per day at least 4-5 days a week according to ACOG and ACSM [14]. |
| Food frequency questionnaire (FFQ) |
| The FFQ included questions about specific food items, such as the frequency of sauce consumption, the fat content of several food items, and seasonal consumption of fruit and vegetables. Questions about general patterns of consumption of the main food groups (bread, luncheon meat and cheese on bread, meat, fruit, and vegetables) at the end of the FFQ were used to adjust the consumption patterns that were reported when several single food items from these food groups were requested. The development, reproducibility, and validity of the FFQ were described in previous studies [15,16]. |
| Dietary intervention |
| The lifestyle program started with a one-hour multidisciplinary counseling session (carried out by both the dietitian and gynecologist) about the importance of reaching an appropriate GWG according to the Institute of Medicine – IOM-guidelines [1] and on the benefits of a healthy lifestyle. The proposed nutritional intervention was a hypocaloric, low-glycemic (low-GI) diet consisting an average intake of 1500 kcal/day, which corresponded approximately to the baseline metabolism of a pregnant woman. In view of the PA program, the dietitian added 200 kcal/day for obese and 300 kcal/day for overweight women [17]. The target macronutrient composition was arranged as follow: 55% carbohydrate (80% complex with low glycemic index and 20% simplex), 20% protein (50% animal and 50% vegetable) and 25% fat (12% monounsaturated, 7% polyunsaturated and 6% saturated) with moderately low saturated fat levels. The daily calories were divided into small frequent meals to avoid ketonuria and acidosis, which often occur following prolonged fasting. The daily intake of carbohydrates was at least 225 g/day (the minimum daily intake to prevent ketosis is 180 g/ day) [17,18]. The dietitian encouraged a wide consumption of fresh vegetables and fruit, cereals, legumes, and fish, with olive oil as the main source of fat and moderate consumption of red wine, in harmony with the Mediterranean eating habits. |
| Mediterranean style is characterized by the wide consumption of plant foods, cereals, legumes, fish and olive oil as the main source of fat and moderate red wine consumption; for each meal or snack pregnant women have several alternatives all suitably calibrated. However, the dietitian customized the caloric restriction at light of the different ethnicities or of the intensity of the PA exerted by each patient. |
| The dietary intervention, previously used by our research group [10], was introduced to the pregnant women at the first visit in the presence of both a gynecologist and a dietitian and further detailed through a one hour counseling session about the appropriate GWG at term for each BMI category as recommended by IOM [1]. The primary focus of the dietary intervention was decreasing consumption of foods with a high glycemic index and substituting them with healthier alternatives (fresh fruit and vegetables with low glycemic index). The second goal was to redistribute the number of meals throughout the day and to include the last snack two hours after dinner to avoid hypoglycemia during the night. |
| Diet adherence score |
| The degree of adherence to the official guidelines of the Health Ministry for Physiological Pregnancy Healthy Eating recommended by the Italian National Institute of Health (ISS) (www.snlg-iss.it/cms/ fails/lg_gravidanza.pdf 2011) [16,18], was measured by means of a diet adherence index. The adherence to the diet was assessed using FFQ as a reference. Each question of the FFQ was assigned, by the dietitian, an answer and a numerical value from 0 to 2. The patients responses to the FFQ allowed us to quantify how they followed the diet because the questionnaire takes into account most of the dietary components: total fruit, total vegetable, total grain (pasta and bread), dairy products (all milk products includes yogurt,), animal protein (meat, fish, processed meat, eggs, cheese), vegetable protein (legumes), salty-snacks (crackers, breadsticks), drinks, added sugar (cake, ice-cream, dessert, cookies), and oils and saturated fats (butter, margarine, cream). For most dietary components an increasing score indicated increasing consumption, whereas, for a few of them, an increasing score indicated decreasing consumption, as shown in Table 1. Scores ranged between 0 and 144. We divided the patients into three numeric groups. Scores between 96- 144 confirmed that the women were following the diet, an intermediate score (45-95) indicated that the diet was being followed but with a few mistakes in choosing suggested nutrients, especially low consumption of fruits and vegetables and large consumption of sugar foods, those that have a low score (0-47) did not follow the diet. |
| Anthropometry |
| Wt was measured to the nearest 100 g and Ht to the nearest 0.1 cm using BIA (TANITA, BC418 Tokyo, Japan) and a stadiometer, respectively. BMI was calculated as Wt(Kg)/Ht(m2). |
| Bioelectrical impedence analysis (BIA) |
| Whole and regional body impedance was measured using an eightpolar tactile-electrode BIA (BIA, TANITA, BC418 Tokyo, Japan). This instrument makes use of 8 tactile electrodes: two are in contact with the palm and thumb of each hand and two with the anterior and posterior aspects of the sole of each foot. The subject stands with his soles in contact with the foot electrodes and grabs the hand electrodes. No precaution was taken to standardize the subject’s posture before BIA, as suggested by the manufacturer. This BIA measured the resistance, at 5, 50, 250 and 500 kHz frequencies, of arms, legs and trunk. Whole-body resistance was calculated as the sum of each segmental resistance (right arm + left arm + trunk + right leg + left leg). We obtained the equivalent FM and FFM (which included body water) in different regions (arms and legs) and in the whole body. The BIA analyzer computed resistance and reactance, which can be used to calculate the fluid changed in body. Previous study showed that BIA is useful in estimating body fluid during pregnancy [19] and commonly used in studies on energy balance in pregnancy [20]. Measurements were performed in the morning at room temperature (21°C), after at least 12 hours of rest, and following an overnight fast. |
| Sense Wear Armband (SWA) and physical activity assessment |
| According to the manufacturer’s instructions, the SWA was placed over the triceps muscle of the right arm, at the midpoint between the acromion and olecranon processes, of all participants during monitoring. We have used the SWA in previous studies [21,22]. The SWA is a portable sensing device, 8.8 × 5.6 × 2.1 cm in size and 82 g in weight that can provide information regarding the total energy expenditure and circadian rhythm. The sensors in the SWA measure skin temperature, galvanic skin response, heat flux from the body, and movement. Subsequently, these data are processed by advanced algorithms to calculate and report total energy expenditure, metabolic PA, and sleep duration in a free-living environment. SWA’s sensors provide an ideal combination of physiologic data collectors with respect to sleep parameters [23]. Participants wore SWA for five consecutive days (from Thursday morning to Monday evening, 24 hours/day). Data from the SWA were analyzed by algorithms using Body Media® InnerView® Research Software (version 6.1) provided by BodyMedia, Inc. |
| Exercise intervention focused on increasing walking and developing a more active lifestyle (i.e., walking rather than driving for short distances). Recommendations set forth by the U.S. Department of Health and Human Services (DHHS) in 2008 PA Giudelines for Americans state that pregnant women should engage in a minimum of 150 minutes of moderate-intensity aerobic activity a week, even if not physically active prior to pregnancy [24]. The American College of Sports Medicine (ACSM) currently recommends a minimum of 3 exercise sessions completed in at least 15 minute sessions, gradually increasing to 30 minutes per day, preferably all days of the week [14]. |
| All participants were advised to participate minimum in 15 minute sessions gradually increasing to 30 minutes of mild intensity PA per day at least 4-5 days a week. Because of heart rate variability during pregnancy, women were encouraged to use the “talk test” (being able to maintain a conversation during activity) to monitor exercise intensity defined by physicians using the Metabolic Equivalent of Task (METs) [25]. |
| Participants received a “PA diary” to record the frequency and duration of the activity. The SWA allowed us to verify the PA performance of every women and the number of steps they made. |
| Statistical analysis |
| A p-value of <0.05 was considered statistically significant. For demographic variables and body composition parameters we used frequencies and Student’s t-test for paired data. |
| Graphic analysis of linear regression was used to assess the adherence to the diet and the weight gained during pregnancy. Data are reported as the mean ± SD. Statistical analysis was conducted using Stata 12.0 (Stata- Corp, College Station, TX 2012) |
| Results |
| The cohort of women with BMI ≥ 25 kg/m2 was composed by 60 subjects. Their socio-demographic characteristics are summarized in Table 2. Women were divided according to their pre-pregnancy BMI: 15 were overweight (25 ≤ BMI ≤ 29.9 kg/m2), 33 were obese (30≤BMI≤39.9 kg/m2) and 12, classified as morbidly obese, had a BMI ≥ 40 kg/m2. Weight increased significantly for all women during pregnancy (p < 0.05). Overweight women increased from a pre-pregnancy weight of 72.9 ± 4.9 kg to 85.5 ± 5.2 kg at delivery, obese women increased from 91.6 ± 9.3 kg to 100.7 ± 10.5 kg, and morbidly obese subjects increased from 119.8 ± 18.7 kg to 124.1 ± 22.1 kg. |
| GWG at delivery was 12.6 ± 5.4 kg for overweight, 9.0 ± 8.0 kg for obese women and 3.3 ± 5.3 kg for morbidly obese women. Nine overweight (60.0%), 22 obese (66.7%) and 10 morbidly obese (90.9%) women remained within IOM recommended ranges [1]. |
| Overall, percentual body composition changed significantly throughout pregnancy (FFM% = 53.4 ± 6.1 to 56.9 ± 6.5; FM% = 40.9 ± 12.8 to 44.1 ± 11.8, p = 0.0001). Changes based on BMI categories are reported in Table 3. When measured at enrollment and at the 36th week of gestation, morbidly obese women showed the lowest increase in FM and FFM (2.1% and 5.6%, respectively) compared to overweight women (FM: 18% and FFM: 9.1%). Using BIA, we also measured and compared FFM and FM in every single region (trunk, arms and legs) and the results confirmed those obtained in the whole body analysis (data not shown). |
| In morbidly obese women, in contrast to overweight and obese women, percentual FM increase in the legs was not significant (23.7 ± 4.9 at the first visit; 25.2 ± 7.1 at the last visit) although it did increase significantly in the arms (9.02 ± 2.1 at the first visit; 11.4 ± 3.5 at the last visit; p = 0.03). |
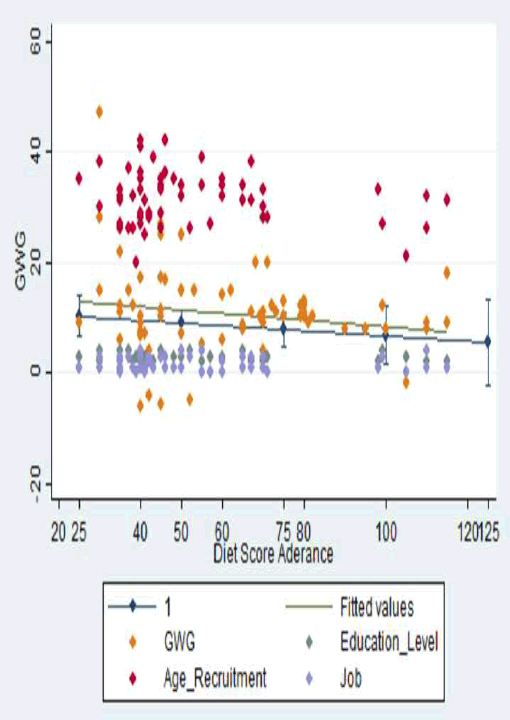
| Dietary habits during the first trimester, before the intervention took place, deviated from the Italian recommendations. Mean daily intakes of fat and saturated fat were above the range of 25% and 9%, respectively, that is recommended; similarly, the intake of simplex carbohydrates was, for most of the women, above the range of 20% of energy intake. Significant changes in eating habits occurred in our study population as supported by the dietary adherence score (Table 3). An increase in the consumption of fruit, vegetable, vegetable protein, olive oil, and fish with a decreased intake of high caloric food, sweet food and saturated fats was reported. Logistic regression, adjusted for confounding factors (age recruitment, education level and job) showed that women who closely adhered to the prescribed diet achieved a lower GWG (CI 95% = -0.15 -0.06; r = -0.046; p = 0.378) (Figure 1). |
| None contraindication to stop PA in our population during pregnancy was recorded. The number of steps, measured by SWA, did not change significantly during pregnancy even after considering the three BMI subgroups (Table 4). The time spent for PA remained constant with the exception of women with 30 ≤ BMI ≤ 40 kg/m2 who showed a trend towards decreased PA time (p = 0.07). |
| The intensity of PA, measured in METS, remained constant during pregnancy for each groups. The time that pregnant women spent lying down decreased while the time spent sleeping did not change during pregnancy for all groups (Table 4). Overall 41/60 women increased their Wt according to IOM recommendations. As reported in Table 3, this corresponds to those women who showed a greater adherence to the diet prescribed. Moreover, they also showed a smaller increase in FM (Δ = 1.5 kg) compared to those with an excessive GWG (Δ = 6.9 kg). Both groups, however, showed comparable increases in FFM. |
| At the planned follow up three months after delivery, only 31/60 women came back to the planned visit, then evaluated (52.5%). Overweight women showed a significant increase in Wt (74.3 ± 4.7 vs 78 ± 6.2, p = 0.042) while women with BMI ≥ 30 did not (97.8 ± 13.4 to 98.2 ± 13.4, p = NS). |
| None women studied presented a detectable ketonuria during pregnancy. |
| Discussion |
| We developed a behavioral intervention to improve eating habits in women with pre-pregnancy BMI ≥ 25 kg/m2. Intervention included education and behavioral strategies to promote a healthy lifestyle through proper nutrient intake and a PA program. We found that greater adherence to our prescribed intervention was associated with a lower GWG that remained within IOM recommendations [1]. |
| A critical issue with interventions that target behavioral changes is adherence to the program. In many studies, follow-up is not rigorous and only consists of phone calls, self-reported monitoring, and group sessions, etc. [26-28]. In our program, monitoring through well-defined follow-up visits planned at the first meeting, as well as early pregnancy counseling with a dietitian, helped the participants to better adhere to the program. |
| By using SWA, it was possible to assess for consistent participation in a regular PA program. Every woman regularly spent time practicing PA (except for women with 30 ≤ BMI ≤ 40 kg/m2) as shown by the constant number of steps, time spent lying, sleep time from first trimester to term. This underlines that these pregnant women had not had a sedentary lifestyle during pregnancy and possibly could justify the limited GWG at term. Indeed more than 60% of women in our study met the IOM recommendations for GWG, in particular, morbidly obese patients. Body composition changes indicated that the FM did not increase in morbidly obese women. The success in this particular group seems to be related not only to greater adherence to the diet but also to the PA that they regularly practiced, as demonstrated by the unchanged FM in their legs. |
| The efficacy of our intervention is supported by the significant changes in eating behavior as measured by FFQ analysis and the diet adherence score. At enrollment, women had a diet that was inclined towards the consumption of food not suitable for their gestation. Many of them did not have breakfast or lunch, and the consumption of fruits, vegetables, and legumes was rare and often replaced with unhealthy foods (high-caloric snacks, sweets, sodas, processed meat). Near term, pregnant women increased consumption of vegetables, at every meal, and fruits, while they decreased the intake of sugar especially candy and cold cuts, thus increasing their adherence to the prescribed diet. |
| Such changes in dietary habits are of paramount importance because it is directly correlated with the main outcome, i.e., meeting the recommended GWG. Moreover, those women who maintained a GWG that was within IOM recommendations showed a reduced increase of FM. The limitations of this study include the small sample size, which cannot take into account the heterogeneity of our population (ethic group and sociocultural status). There are several factors involved in our decision about the sample size. First, it was clear the inadequate eating habits and the sedentary life-style of such at risk population, thus we were conscious that it would not be easy recruit and maintain at follow-up a large sample of women. In fact, try to change so widely the wrong eating habits in an adult population are a goal not so easy to reach. Another important factor to take into account is the difficulties to explain the importance of a certain amount of PA in women wont to spend the most of the time sleeping or watching television. |
| Further, the several follow-up visits at Policlinico Hospital, added to the scheduled obstetric visits of the National Health System, are object of losses at follow-up and poor compliance, namely in this kind of sedentary population that underestimate the risks related to their high body weight and to the excessive GWG. However, neither BMI, weight nor GWG reflect the subtle changes in body composition during pregnancy [20], namely the FM and FFM, because the GWG itself has to be considered as a composite parameter, since it accounts for the growing products of conception and enlarging uterus beside maternal weight increase. The evaluation of these components and their modifications could be of the utmost importance during pregnancy, since they can vary on an individual basis, thereby affecting GWG to a variable degree. Nowadays, there aren’t studies evaluating the BIA during the different trimesters of pregnancy in obese women with the purpose of monitoring the FM and FFM increase, since the most of them namely consider the total body water [29-32]. Moreover, there are not so many studies evaluating both the adherence to a proper nutrition and the PA in obese pregnant women, namely through respectively FFQ and SWA. |
| Thus, we considered to enroll a small sample of women assuming that it was enough to evaluate the usefulness of emerging tools such as the SWA and BIA in monitoring body composition changes, since not employed in clinical practice. |
| Another limitation is the lack of evaluation of total body water, which has been reported in other studies although only in non-obese people [29,30]. Moreover we can’t exactly quantify the fetus contribution to changes in body composition, even if other authors previously confirmed the progressive expansion of all body compartments during pregnancy and the positive correlation of total body water with birth weight [19,20,29,30]. The strength of this study is the focus on the issue of adherence to a prescribed program; both PA and diet, with a strict follow up schedule. |
| In conclusion this study demonstrates that early pregnancy promotion of healthy diet and PA provided by a team of gynecologists and dietitians helps women with a pre-pregnancy BMI ≥ 25 kg/m2 to achieve the recommended GWG, particularly in morbidly obese patients. The efficacy of the intervention was directly dependent upon adherence to the prescribed diet and PA program. We found highquality evidence that diet or exercise interventions, or both, help to reduce excessive weight gain in pregnancy. They may also reduce caesarean deliveries, especially with combined diet and exercise interventions, and maternal hypertension. |
| Moderate-intensity exercise appears to be an important part of weight-control strategies in pregnancy; however, more research is needed on side-effects to inform safe guidelines [31,32]. |
| Funding Statement |
| This study was supported by the Policlinico University Hospital of Modena funds. The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the article. |
| Conflict of Interest Statement |
| All the authors declare no conflict of interest. |
| Ethics Statement |
| The study has been approved by a suitably constituted Ethics committee of the Institution within which work was undertaken and that it conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). |
| Acknowledgements |
| We would like to extend our appreciation to the women who participated in the trial and the residents and midwives who collaborated with the research group. |
| The study was conducted in accordance with ethical principles derived from the Declaration of Helsinki, Good Clinical Practice, and International Conference of Harmonization Guidelines. All patients provided written informed consent. Ethical approval was provided by the institutional review board of the Academic Medical Centre in Amsterdam (MEC 05/286, January 2006), and all boards of the participating hospital. |
References
- Rasmussen KM, Yaktine AL (2009) Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines
- DeVader SR, Neeley HL, Myles TD, Leet TL (2007) Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. See comment in PubMed Commons below ObstetGynecol 110: 745-751.
- Thorsdottir I, Torfadottir JE, Birgisdottir BE, Geirsson RT (2002) Weight gain in women of normal weight before pregnancy: complications in pregnancy or delivery and birth outcome. See comment in PubMed Commons below ObstetGynecol 99: 799-806.
- Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, et al. (2014) Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J ObstetGynecol 211: 137
- (2002) American College of Obstetrics and Gynecology: Committee opinion number 267: Exercise during pregnancy and the postpartum period. ObstetGynecol99:171-3.
- (2006) American College of Sports Medicine: Impact of physical activity during pregnancy and postpartum on chronic disease risk: Roundtable consensus statement. Med Sci Sports Exerc38:989–1006.
- Cedergren M1 (2006) Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. See comment in PubMed Commons below Int J GynaecolObstet 93: 269-274.
- Abrams B, Altman SL, Pickett KE (2000) Pregnancy weight gain: still controversial. Am J ClinNutr 71:1233S-1241S
- Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. (2012) Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev 4:CD007145.
- Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, et al. (2014) Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. See comment in PubMed Commons below J Matern Fetal Neonatal Med 27: 1348-1352.
- Ozhan H, Alemdar R, Caglar O, Ordu S, Kaya A, et al. (2012) MELEN Investigators. Performance of bioelectrical impedance analysis in the diagnosis of metabolic syndrome. J Investig Med 60:587-591
- Baltadjiev AG, Baltadjiev GA (2011) Assessment of body composition of male patients with type 2 diabetes by bioelectrical impedance analysis. See comment in PubMed Commons below Folia Med (Plovdiv) 53: 52-57.
- Berntsen S, Richardsen KR, Mørkrid K, Sletner L, Birkeland KI, et al. (2012) Objectively recorded physical activity in early pregnancy: A multiethnic population-based study. Scand J Med Sci Sports 24: 594-601.
- American College of Sports Medicine: Exercise prescription for healthy populations and special considerations. In Guidelines for Exercise Testing and Prescription. 8thedition. Edited by: Thompson WR. Philadelphia: Wolters Kluwer, Lippincott Williams 2010:183-187
- da Vico L, Agostini S, Brazzo S, Biffi B, Masini ML (2012) [Mediterranean diet: not only food]. See comment in PubMed Commons below Monaldi Arch Chest Dis 78: 148-154.
- Mello G, Ottanelli S, Buti G. Nutrizione e gravidanza (2010) In: NutrizioneIndividuoPopolazione. Binetti P, MarcelliMx, Baisi R, Editors, issue 1:601-620.
- Binetti P, Marcelli M, Baisi R, Buti G, Mello G (2010) NutrizioneIndividuoPopolazione; Nutrizione e gravidanza p. 601-620, Casa EditriceUniverso, Roma.
- S.I.N.U (SocietàItaliana di NutrizioneUmana) (1996) Livelli di AssunzioneRaccomandati di Energia e Nutrienti per la PopolazioneItaliana – LARN revision p-85-86.
- Van Loan MD, Kopp LE, King JC, Wong WW, Mayclin PL (1995) Fluid changes during pregnancy: use of bioimpedance spectroscopy. See comment in PubMed Commons below J ApplPhysiol (1985) 78: 1037-1042.
- Larciprete G, Valensise H, Vasapollo B, Altomare F, Sorge R, et al. (2003) Body composition during normal pregnancy: reference ranges. See comment in PubMed Commons below ActaDiabetol 40 Suppl 1: S225-232.
- Malavolti M, Pietrobelli A, Dugoni M, Poli M, Romagnoli E, et al. (2007) A new device for measuring resting energy expenditure (REE) in healthy subjects. See comment in PubMed Commons below NutrMetabCardiovasc Dis 17: 338-343.
- Predieri B, Bruzzi P, Lami F, Vellani G, Malavolti M, et al. (2013) Accuracy of SenseWear Pro2 Armband to predict resting energy expenditure in childhood obesity. See comment in PubMed Commons below Obesity (Silver Spring) 21: 2465-2470.
- Papazoglou D, Augello G, Tagliaferri M, Savia G, Marzullo P, et al. (2006) Evaluation of a multisensor armband in estimating energy expenditure in obese individuals. See comment in PubMed Commons below Obesity (Silver Spring) 14: 2217-2223.
- Physical Activity Guidelines Advisory Committee: 2008 Physical Activity Guidelines for Americans. US Dept of Health and Human Services 2008.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, et al. (2000) Compendium of physical activities: an update of activity codes and MET intensities. See comment in PubMed Commons below Med Sci Sports Exerc 32: S498-504.
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, et al.( 2011) Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J ClinNutr93:772-779.
- Polley BA, Wing RR, Sims CJ (2002) Randomized controlled trial to prevent excessive weight gain in pregnant women. See comment in PubMed Commons below Int J ObesRelatMetabDisord 26: 1494-1502.
- Guelinckx I, Devlieger R, Mullie P, Vansant G. (2010) Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr91:373-8037.
- Lukaski HC, Siders WA, Nielsen EJ, Hall CB (1994) Total body water in pregnancy: assessment by using bioelectrical impedance. See comment in PubMed Commons below Am J ClinNutr 59: 578-585.
- Ghezzi F, Franchi M, Balestreri D, Lischetti B, Mele MC, et al. (2001) Bioelectrical impedance analysis during pregnancy and neonatal birth weight. See comment in PubMed Commons below Eur J ObstetGynecolReprodBiol 98: 171-176.
- Valensise H, Andreoli A, Lello S, Magnani F, Romanini C, et al. (2000) Multifrequency bioelectrical impedance analysis in women with a normal and hypertensive pregnancy. See comment in PubMed Commons below Am J ClinNutr 72: 780-783.
- Valensise H, Larciprete G, Vasapollo B, Novelli G, Altomare F, et al. (2003) Nifedipine-induced changes in body composition in hypertensive patients at term. See comment in PubMed Commons below Eur J ObstetGynecolReprodBiol 106: 139-143.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 12378
- [From(publication date):
February-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11498
- PDF downloads : 880
