Research Article Open Access
Gestational Hypoglycaemia Restricts Foetal Growth and Skeletal Ossification in the Rat
Jensen Vivi FH1*, Mølck Anne-Marie1, Berthelsen Line O1, Andersen Lene2, Demozay Damien3, Renaut Ruth4 and
Bøgh Ingrid1
1Toxicology, Safety Pharm and Pathology, Novo Nordisk A/S, Novo Nordisk Park, Denmark
2Development Bioanalysis 4, Novo Nordisk A/S, Novo Nordisk Park, Denmark
3Insulin Metabolism and Safety Biology, Novo Nordisk A/S, Novo Nordisk Park, Denmark
4Toxicology Operations, Envigo, Eye, Suffolk, UK
- *Corresponding Author:
- Jensen Vivi FH
Toxicology, Safety Pharm and Pathology
Novo Nordisk A/S, Novo Nordisk Park
Denmark
Tel: 4531507319
E-mail: vfhj@novonordisk.com
Received date: April 27, 2017; Accepted date: May 16, 2017; Published date: May 20, 2017
Citation: Vivi FHJ, Anne-Marie M, Line OB, Ingrid B, Lene A, et al. (2017) Gestational Hypoglycaemia Restricts Foetal Growth and Skeletal Ossification in the Rat. J Preg Child Health 4:321. doi:10.4172/2376-127X.1000321
Copyright: © 2017 Vivi FHJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Maternal blood glucose level during gestation is crucial for normal foetal development. In the non- clinical studies in non-diabetic rats required by authorities, long-acting insulin analogous being developed to decrease blood glucose levels persistently generate safety data on embryo-foetal effects, which may be challenging to interpret. Separating potential toxicological effects of the insulin analogue from those caused by the hypoglycaemia becomes problematic, when the effects of the latter are unknown. Therefore, the aim of the present study was to investigate foetal effects of persistent maternal hypoglycaemia in rats after continuous maternal insulin-infusion throughout gestation. Effects on foetal size were measured and foetal skeletons were examined after alizarin red and alcian blue staining. Foetal hepatic glycogen content and foetal exposure to exogenous, i.e. human insulin, as well as endogenous plasma insulin levels were measured. Persistently 50% lowering maternal blood glucose throughout gestation caused a 10% decrease of foetal blood glucose levels on GD 20 and decreased foetal growth and development. Generalised decreased ossification and skeletal malformations of ribs, cranial bones, and long bones were seen with high incidences in foetuses. Furthermore, foetal but not maternal hepatic glycogen stores were decreased up to 50%. Foetuses were hypoinsulinaemic, and had no detectable exposure to exogenous insulin. The results indicate the existence of mechanisms which down-regulate placental glucose transfer to the foetus and consequently affect the foetal glucose metabolism and development reflected by counter-regulatory mobilisation of hepatic glycogen, decreased growth and delayed ossification of the skeleton as well skeleton malformations. As the foetuses were hypoinsulinaemic, these changes were most likely secondary to the maternal hypoglycaemia leading to foetal hypoglycaemia, and delayed development. This study provides novel data, which will aid the interpretation of findings in safety studies with longer-acting insulin analogues allowing differentiation between foetal effects of toxicological origin and those due to the persistent hypoglycaemia.
Keywords
Non-clinical safety studies; Long-acting insulin analogues; Insulin-induced hypoglycaemia; Non-diabetic rats; Foetal growth restriction; Decreased ossification; Human insulin; Embryofoetal development; Maternal hypoglycaemia
Abbreviations
ANOVA: Analysis of Variance; CTRL: Control; GD: Gestation Day; HE: Haematoxylin and Eosin; HI: Human insulin; HIL: Human Insulin Low dose; HI-H: Human Insulin High dose; HI-E: Human Insulin Escalated dose; LLOQ: Lower Limit of Quantification
Introduction
Maintaining normal maternal glucose homeostasis during pregnancy is pivotal, as both maternal hyperglycaemia and hypoglycaemia may affect foetal development [1-3]. Embryo-foetal development studies assessing the effects of maternal dosing during gestation are an important part as well as a regulatory requirement for non-clinical safety evaluation of blood glucose lowering drugs in development [4]. During the development of new insulin analogues, where the intended effect is to lower blood glucose levels, it is of importance to differentiate between the potential “off- target” toxicities versus effects due to hypoglycaemia on embryo-foetal development in non-diabetic animals. The use of non-diabetic animals is a regulatory requirement [5,6]. In humans, effects of hypoglycaemia per se can be avoided as target is to dose to normoglycaemia.
Little data is available on the effects of chronic (throughout gestation) persistent (24 h/day) maternal insulin-induced hypoglycaemia in the rat, which is the typical rodent species used for the embryo-foetal development studies with insulin analogues. Several in vivo studies have evaluated the effects of short-term (<1 days) maternal insulin-induced hypoglycaemia during gestation, which results in foetal skeletal changes in both mice and rats [2,3,7]. One study in rats evaluated foetal effects of chronic recurrent maternal insulin-induced hypoglycaemia throughout gestation [8]; but, studies investigating the effects of chronic persistent gestational insulin induced hypoglycaemia are to our knowledge lacking. However, with the new generation of longer-acting insulin analogues intended for once daily or even once weekly dosing, this gap in understanding the link between persistent maternal hypoglycaemia and foetal effects makes the interpretation of foetal findings in toxicity studies of these analogues problematic; especially as the regulation of materno-foetal glucose transfer during low availability is poorly understood. It has been debated whether the foetus is prioritized at expense of the mother or reversely, the mother is prioritised at the expense of the foetus [9].
Therefore, the primary aim of the present study was to generate information regarding the foetal effects of persistent maternal hypoglycaemia throughout gestation in the non-diabetic rat. This should, furthermore, increase the understanding of the regulation of materno-foetal glucose levels during prolonged periods of maternal low glucose availab ility.
Materials and Methods
Animals and study design
Forty-four female Sprague-Dawley (Crl:CD [SD]) rats were supplied by Charles River Limited (UK) in two cohorts (12-13 weeks old, cohort 1: n=33, cohort 2: n=11). Animals were acclimatized to local environmental conditions (19-23ºC, 40-70% humidity, 12 h light-dark cycle, lights on at 6 am for at least 12 days and housed up to five animals/cage before surgery. They were offered a commercial maintenance diet (VRF-1 diet, Special Diet Services, Essex, UK) and water ad libitum. At least three days after surgery (implantation of infusion cannula, see below), animals in the first cohort were randomised into three groups (n=11/group), whereas the second cohort comprised one group (n=11) (Table 1A).
| Group | n | Human insulin doses Day 1a to GD20 (nmol/kg/day) |
|---|---|---|
| CTRL | 11 | 0 |
| HI-L | 11 | 48 |
| HI-H | 11 | 66/60b |
| HI-E | 11 | 60/66/72c |
Table 1A: Study design; Doses (a) Infusion-start; b) Dosed reduced on Day 13 to GD3 due to clinical signs of potential hypoglycaemia in two animals; c)Dose escalations on GD8 and GD15).
Post-surgery, animals were single-housed except during pairing for mating, and received at least seven days recovery from surgery before start of infusion. Start of infusion was defined as Day 1 and infusion was continued until sacrifice. Doses in HI-L (low-dose human insulin [HI]) and HI-H (high-dose human insulin) groups were based on previous studies with human insulin infusion in non-pregnant rats [10-12] (Table 1B). Doses in HI-E (escalated dose of human insulin) group were increased during pregnancy to maintain the maternal counter-regulatory effect on food consumption based on the outcome of group HI-L and HI-H. From Day 5, the oestrous cycle was monitored by examination of vaginal wet smears (using pipette lavage). After a minimum of seven days of treatment, i.e., from Day 8, females in pro-oestrus were paired for mating with untreated stock males (male/female ratio 1:1).
Morning after pairing was designated as Gestation Day (GD) 0 when positive evidence of mating was detected; mating was confirmed by presence of ejected copulation plugs in the cage tray and sperm in the vaginal smear. If an animal failed to mate during pairing, an additional pairing was performed at the next prooestrus. Because of differences in oestrous cycle stage (animals were not synchronised), the duration of the pre-mating treatment period differed between animals.
Body weight was recorded on arrival, day of surgery, for at least three days during the post-surgery recovery period, every other day from Day 1 until positive evidence of mating, day of mating (GD0), and daily on GD3-20. Animals were inspected visually for clinical signs of hypoglycaemia and function of the infusing system at least four times daily during the infusion phase.
| = 7 days prior to Day 1 | Day 1 to Day 7 | From Day 8 | GD0 to GD20/termination |
|---|---|---|---|
| Surgery and recovery from surgery | Premating | Paired for matinga) | Gestation |
| Not infused | Infused | ||
Table 1B: Infusion period.
Two types of in life sampling for maternal blood glucose monitoring were included: plasma for glucose profiling (sublingual vein with isoflurane anaesthesia) and whole blood for continuous single timepoint assessment (tail vein, no anaesthesia). The plasma glucose profiles were taken to monitor the decrease in blood glucose levels following start of infusion Day 1, on GD6 and GD17 (3-5 animals/ group at each time-point), as well as a single sample at termination immediately prior to completion of infusion (all dams). These samples were taken at 0 and 12 h after commencement of infusion on Day 1 and at the same time of day on GD6 and GD17. Whole blood glucose sampling was performed from Day 1 (pre-dose) and hereafter every other day until termination on GD20 (including GD0 and GD20). Samples were taken at approximately the same time of day on each occasion. If a value was ≤ 2.5 mM or >10 mM an immediate resample was taken to confirm the reading. Values >10 mM in the insulininfused groups were considered outliers, and a further blood glucose measurement was performed towards the end of the working day. Additionally, where possible blood was sampled from each foetus at termination following transverse sectioning of the umbilical cord for whole blood glucose measuring.
Blood samples for measurement of human insulin levels in maternal plasma were sampled at the same time-points and from the same animals as for plasma glucose sampling. At termination, umbilical blood was taken from litters from approximately half of the dams (pooled for each litter) for measurement of human insulin concentration in foetal plasma. Spare plasma from these samples were analysed for endogenous insulin level. Litters in the remaining dams were sampled for measurement glucose concentration of foetal plasma.
Dams were killed by carbon dioxide asphyxiation on GD20 (infusion was on going until ≤ 30 min before termination, corresponding to approximately two half-lives [13]), with subsequent exsanguination. All viable foetuses were dissected from the uterus, killed by chilling on a cool plate (approximately 0°C), individually weighed and identified within the litter using a coding system based on their position in the uterus. They were examined externally with abnormalities recorded. For dams surviving to termination at GD20 gravid uterine weight (including cervix and ovaries) was recorded. For all dams (including those prematurely sacrificed), the following parameters were recorded for each ovary/uterine horn: number of corpora lutea, implantation sites, and number of live foetuses. For each foetus, weight and crown-rump length were recorded. Maternal liver as well as foetal livers and placentas were isolated. Maternal liver weight and foetal livers and placentas (pooled per litter) weight was recorded and each tissue frozen in liquid nitrogen and stored at -80°C until analysis for glucose and lipid concentrations. Foetuses were then eviscerated and fixed in Industrial Methylated Spirit for skeletal investigations, as described below.
All procedures involving live animals were performed under the Project Licence authorized by the United Kingdom Secretary of State and according to EC Commission Directive 2004/10, OECD Principles and Good Laboratory Practice, and The Good Laboratory Practice (Codification Amendments Etc.) Regulations 2004 [14-16], as well as Envigo and Novo Nordisk A/S company policies on the care and use of laboratory animals.
Insulin formulations and infusion system
Infusion was achieved by external infusion pumps (Harvard Apparatus Pump 11 Plus Syringe Pump, Harvard Apparatus, Holliston, MA, USA) through a surgically implanted vascular cannula inserted into the caudal vena cava through the femoral vein as previously described [10]. The test substance used was recombinant human insulin formulated in a phosphate buffered vehicle (HI-L, HI-H and HI-E groups) and buffered vehicle (control [CTRL] group). Recombinant human insulin stock solution (600 nmol/ml) as well as vehicle was supplied by Novo Nordisk A/S (Maaloev, Denmark). Recombinant human insulin stock solution and vehicle were diluted with dilution medium to obtain the dose formulations for infusion. The composition of human insulin stock formulation, vehicle, and dilution medium was as previously described [10].
Quantification of glucose in plasma and whole blood
Plasma glucose levels were quantified as described previously [10]. Whole blood was sampled for snap-measuring of glucose levels with an Accu-Check device (Aviva, Cat/Type 05911974002, Roche Diagnostics, Burgess Hill, West Sussex, UK).
Quantification of human insulin in plasma
Levels of HI in plasma were quantified as previously described [10]. The lower limit of quantification (LLOQ) of the assay was 25 pmol/l. Results are reported as the mean of two replicates.
Quantification of endogenous insulin in foetal plasma
The measurements were performed using a commercially available ELISA kit (Cat. no. 80-INSRT-E01, E10, Alpco, New Hampshire, USA) according to manufacturer’s instructions. The LLOQ was 0.124 ng/ml. Results are reported as the mean of two replicates.
Fetal skeletal investigations
All viable foetuses went through the following processing after sacrifice: The skin was removed followed by evisceration and fixation in Industrial Methylated Spirit for one day and then stored in acetone for one day for degreasing. Hereafter, the foetuses were stored in 1% potassium hydroxide for maceration for one day, processed and double stained with Alizarin Red and Alcian Blue for calcified bone and cartilage, respectively. After staining foetuses were stored in 20% glycerol+1% potassium hydroxide for two days, 20% glycerol for one day, 50% glycerol for one day, and then 100% glycerol for long term storage. Foetuses were then evaluated for the degree of calcification and skeletal malformations.
Quantification of tissue glucose and lipid content
Approximately 20-40 mg of frozen tissue for each measurement was homogenized in a sodium acetate (0.15 M) buffer supplemented with Triton X-100 (0.75%) allowing the hydrophobic lipids to be extracted together with the hydrophilic glucose molecules (from glycogen). Samples of tissue homogenate for enzyme incubation were then denatured by incubation at 100ºC for 2 min and centrifuged at 9000 g. The supernatant was collected for direct measurements of lipids (triglyceride, free fatty acids, and cholesterol) and free glucose concentrations using the Cobas 6000 Analyser (Roche). For total glucose concentration measurement, supernatants were incubated with amyloglucosidase, an enzyme that converts glycogen to D-glucose, prior to analysis with the Cobas Analyser. The glycogen concentration in the tissue samples was then calculated by subtracting the free glucose from the total glucose concentrations. The results are given as a mean value of two duplicate measurements in μmol/g wet tissue.
Pancreatic histopathology
At termination, pancreas from the dams was sampled and fixed in 10% neutral buffered formalin, processed to tissue blocks, and sections of a nominal thickness of 4–5 μm were stained with H&E. All of the sections were evaluated microscopically for histopathologic changes by two experienced pathologists and the result represents a consensus hereof.
Statistical analysis
The dam or litter was considered the experimental unit except for reported incidences of foetal skeletal changes where the foetus was considered the experimental unit. Incidences of histopathologic changes in pancreas as well as skeletal changes were compared between groups using Fisher’s exact test. An overall effect of maternal insulininfusion on foetal blood glucose levels as well as endogenous insulin levels was evaluated using a paired t-test followed by a Tukey’s multiple comparison test in case of a significant effect. A two-way analysis of variance (ANOVA) was performed for the following data: Whole blood glucose measurements (analysis performed on data set including outliers), food consumption, and body weight measurements testing for effect of group and time-point, and for evaluating the effect of uterine horn position (effect of group or position [mid-position or adjacent to ovary or cervix]) on foetal size (effect of group and uterine location) within each group. The maternal plasma human insulin and glucose levels measured at two consecutive time-points 12 h apart on GD6 and GD17 were analysed using a two-way ANOVA for an effect of time-point and group. If there was no effect of time-point, but an effect of group, the two time-points were pooled within each group and a post hoc Tukey’s multiple comparison tests for an overall effect of group was performed. The remaining results were analysed using a one-way ANOVA for an effect of group. The ANOVAs were followed with the post hoc Tukey’s multiple comparison test in case of significant effects. A p-value <0.05 was considered statistically significant.
Results
Dosing, clinical signs and mortality
Pre-mating treatment period spanned from 8 to 15 days except for one animal (HI-L group) which never showed signs of pro-oestrus and consequently was sacrificed on Day 21. A total of three animals showed clinical signs of hypoglycaemia during the study: one HI-L group animal exhibited fast breathing on Day 1, but recovered later on the same day without glucose treatment and two animals from the HI-H group displayed under-activity, irregular breathing, piloerection, reduced body temperature and pale skin colour on GD1 and Day 11, respectively. Both of these animals had a whole blood glucose level of 2.2 mM during clinical signs, and despite of glucose treatment and decreasing insulin dose by 50% following recognition of clinical symptoms.
Therefore, it was decided to sacrifice these animals for welfare reasons. This happened <24 h apart, consequently, the dose of 66 nmol/kg/day was considered too high and was immediately reduced to 60 nmol/kg/day in the remaining animals in the HI-H group (spanning from Day 13 to GD3 due to different mating days). In the two prematurely sacrificed HI-H animals with clinical signs, necropsy later revealed oedamatous and thickened perirenal adipose tissue around vena Cava, a full stomach and/or a dark enlarged left kidney and oedematous mesovarian adipose tissue. Based on these findings in combination to the lack of response to glucose treatment and dose reduction, it was concluded that the clinical signs in these two animals were secondary to complications of the presence of the infusion cannula in Vena Cava causing a general poor clinical condition.
Additionally, a total of five animals were sacrificed prematurely during the study due to loss of cannula patency; one CTRL (GD1) and four HI-E group animals (GD1, GD6, GD7, and GD13). All dams surviving up to scheduled sacrifice on GD20 were confirmed as pregnant.
Human insulin plasma levels
Maternal: In all CTRL group animals plasma levels were below LLOQ (25 pmol/l) and all samples from insulin-infused dams had detectable levels of human insulin, with high inter-individual variation as seen previously with this infusion model (Table 2) [10]. On GD6 and GD17, where steady state should be present (half-life of human insulin in the rat is approximately 0.25 h [13]) there was no effect of time-point (0 h vs. 12 h) or group within the insulin-infused groups (2-way ANOVA). Similarly, on GD20 (terminal) there was no difference between insulin-infused groups (one way ANOVA).
| CTRL | HI-L | HI-H | HI-E | ||
|---|---|---|---|---|---|
| GD6 | |||||
| n/time-point | 5 | 5 | 04-May | 04-May | |
| 0 ha | <LLOQc) | 806.8 (720.2) | 738.0 (590.5) | 1181.5 (703.7) | |
| 12 hb | <LLOQ | 674.4 (567.7) | 454.8 (404.5) | 636.3 (642.2) | |
| GD17 | |||||
| n/time-point | 5 | 5 | 04-May | 03-Apr | |
| 0 ha | <LLOQ | 1000.6 (486.4) | 1592.6 (1511.3) | 3268.3 (3269.9) | |
| 12 hb | <LLOQ | 906.2 (682.5) | 862.3 (381.6) | 466.3 (366.0) | |
| Termination | |||||
| n | 10 | 10 | 9 | 7 | |
| <LLOQ | 111.7 (68.7) | 425.3 (1071.7) | 168.7 (106.9) | ||
Table 2: Maternal human insulin plasma levels (Means (± SD); a) Relates back to time of infusion-start on Day 1; b) Relates back to 12 h after infusion-start on Day 1; c) 25 pmol/l).
Foetal: Human insulin was below LLOQ in samples from litters, irrespective of group.
Blood glucose levels
Maternal: Whole blood glucose levels
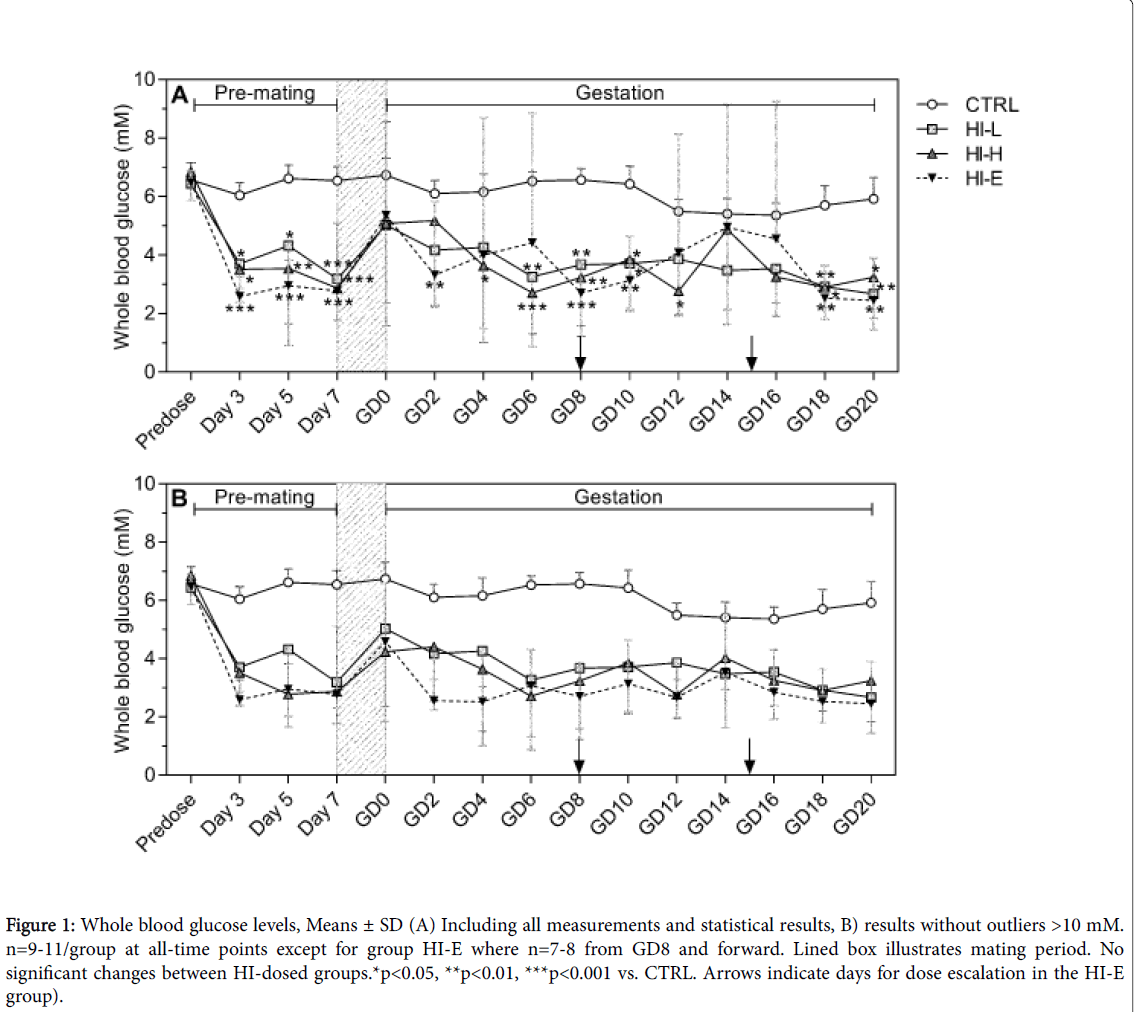
Pre-dose glucose values were similar in all groups (Figure 1). After infusion-start, mean glucose levels declined in insulin-infused groups and were generally within the range of 2 to 5 mM throughout the study in all insulin-infused groups with no dose-response versus 6 to 7 mM in the CTRL group.
There were individual animals in the insulin-infused groups with values transiently >10 mM as previously seen with this infusion model [10], therefore, the means without these outliers are also depicted in Figure 1, for illustration purposes. For the animals with values >10 mM, the values at the repeated afternoon sampling were <4 mM, thus confirming the transient nature of these high values.
Figure 1: Whole blood glucose levels, Means ± SD (A) Including all measurements and statistical results, B) results without outliers >10 mM. n=9-11/group at all-time points except for group HI-E where n=7-8 from GD8 and forward. Lined box illustrates mating period. No significant changes between HI-dosed groups.*p<0.05, **p<0.01, ***p<0.001 vs. CTRL. Arrows indicate days for dose escalation in the HI-E group).
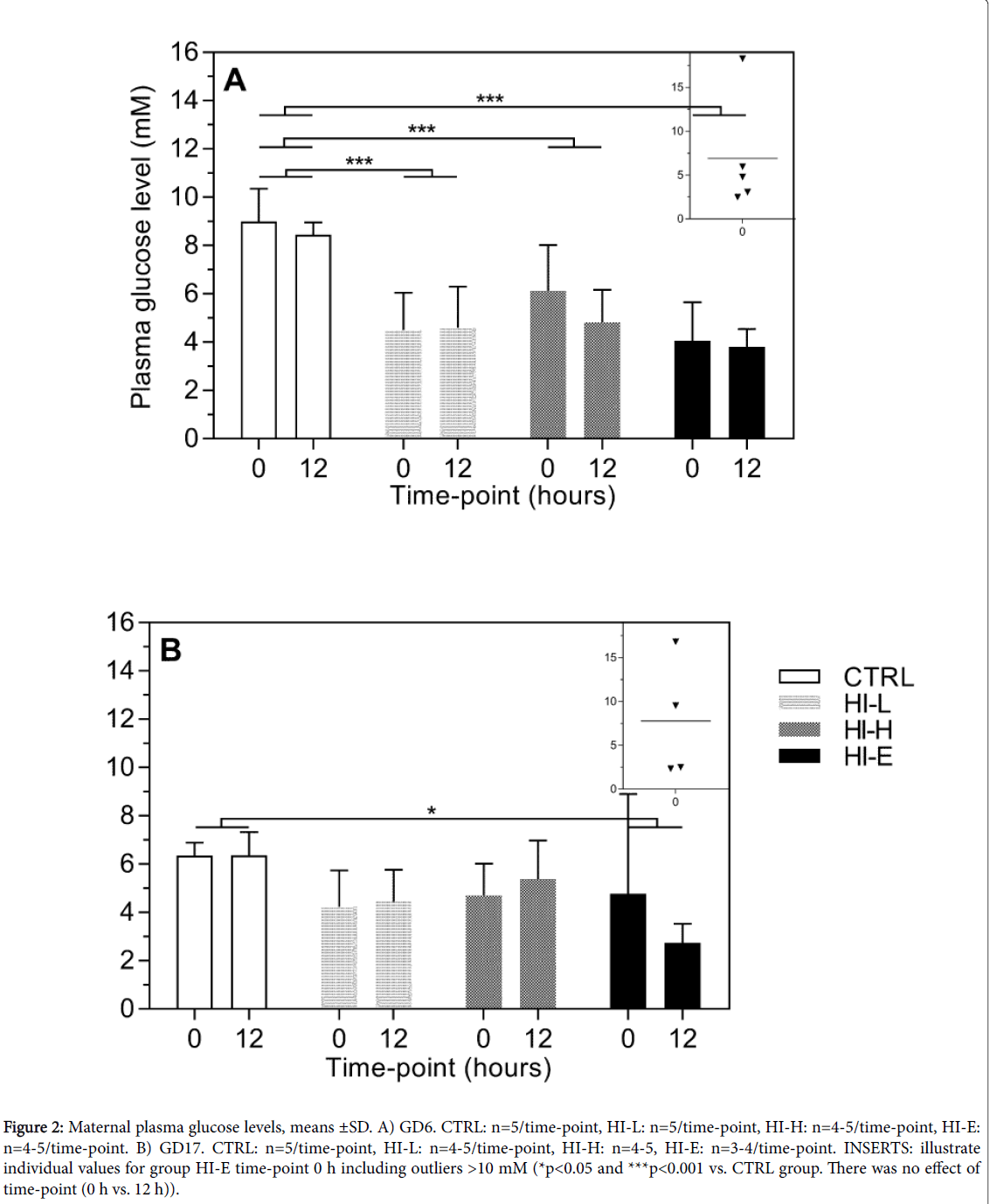
Figure 2: Maternal plasma glucose levels, means ±SD. A) GD6. CTRL: n=5/time-point, HI-L: n=5/time-point, HI-H: n=4-5/time-point, HI-E: n=4-5/time-point. B) GD17. CTRL: n=5/time-point, HI-L: n=4-5/time-point, HI-H: n=4-5, HI-E: n=3-4/time-point. INSERTS: illustrate individual values for group HI-E time-point 0 h including outliers >10 mM (*p<0.05 and ***p<0.001 vs. CTRL group. There was no effect of time-point (0 h vs. 12 h)).
Plasma glucose levels
On GD6 mean plasma glucose levels were generally between 8 and 9 mM in CTRL group, and 4-6 mM in insulin-dosed groups (Figure 2A). There was one single outlier >10 mM on the 0 h time-point in the HI-E group, which was excluded from statistical analysis, as it was predefined as an outlier. This is included in the individual values in an insert in Figure 2A for illustration purposes. As there was no effect of time-point (0 h vs. 12 h), but an effect of group (two-way ANOVA, p<0.0001), both time-points were pooled within each group for statistical analysis. Mean plasma glucose level in all insulin-infused groups was significantly lower than in the CTRL group with no doseresponse. On GD17 mean plasma glucose level was 6.4 mM at both time-points in the CTRL group (Figure 2B). Again an outlier >10 mM was seen at the 0 h time-point in the HI-E group. This was excluded from statistical analysis (individual values incl. outlier in insert for illustration), as it was predefined as an outlier. There was no effect of time-point, but an effect of group (two-way ANOVA, p=0.0179). Post hoc analysis, with time-points pooled within each group, showed significant lower levels in the HI-E group only versus the CTRL group and no dose-response, although levels in the HI-H versus CTRL group approached statistical difference (p=0.0538).
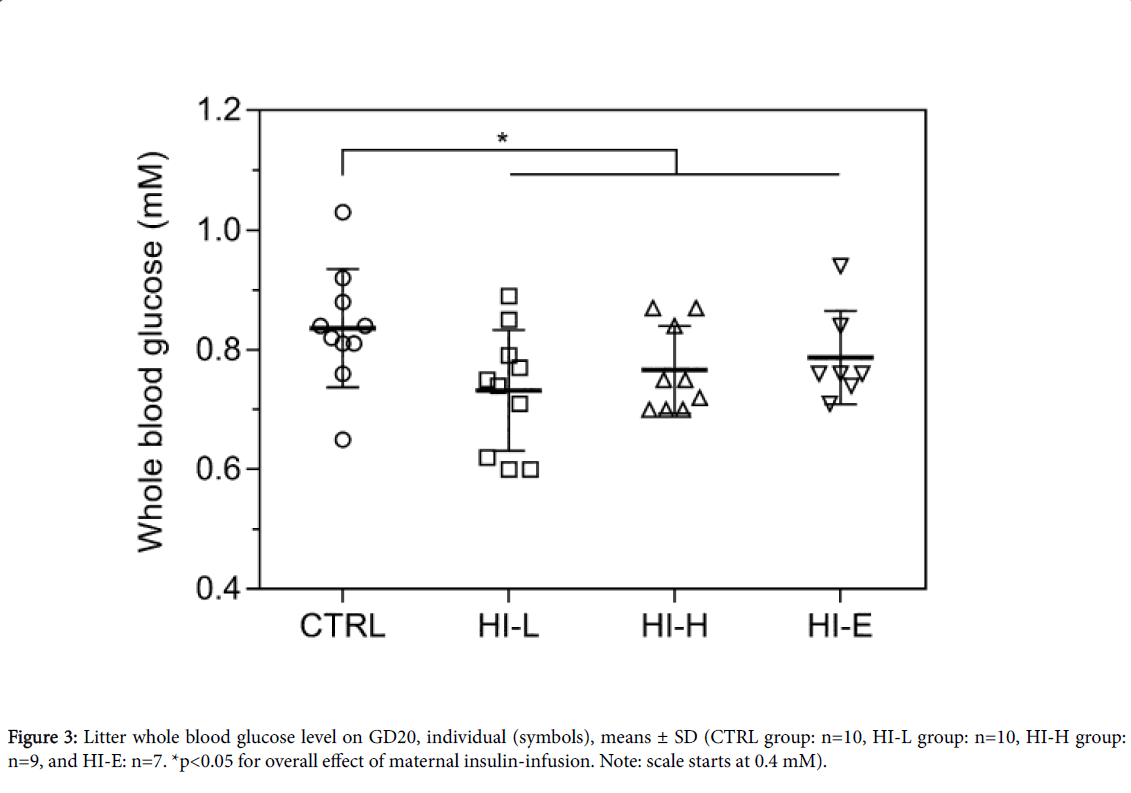
Foetal: Whole blood glucose level
Whole litter blood glucose levels on GD20 are depicted in (Figure 3). There was a statistically significant lowering of blood glucose level in litters from insulin-infused dams (p=0.0269). There were no significant differences between individual groups at the post hoc analysis.
Plasma glucose level: On GD20, litter plasma glucose levels were below the LLOQ (1 mM) for this method.
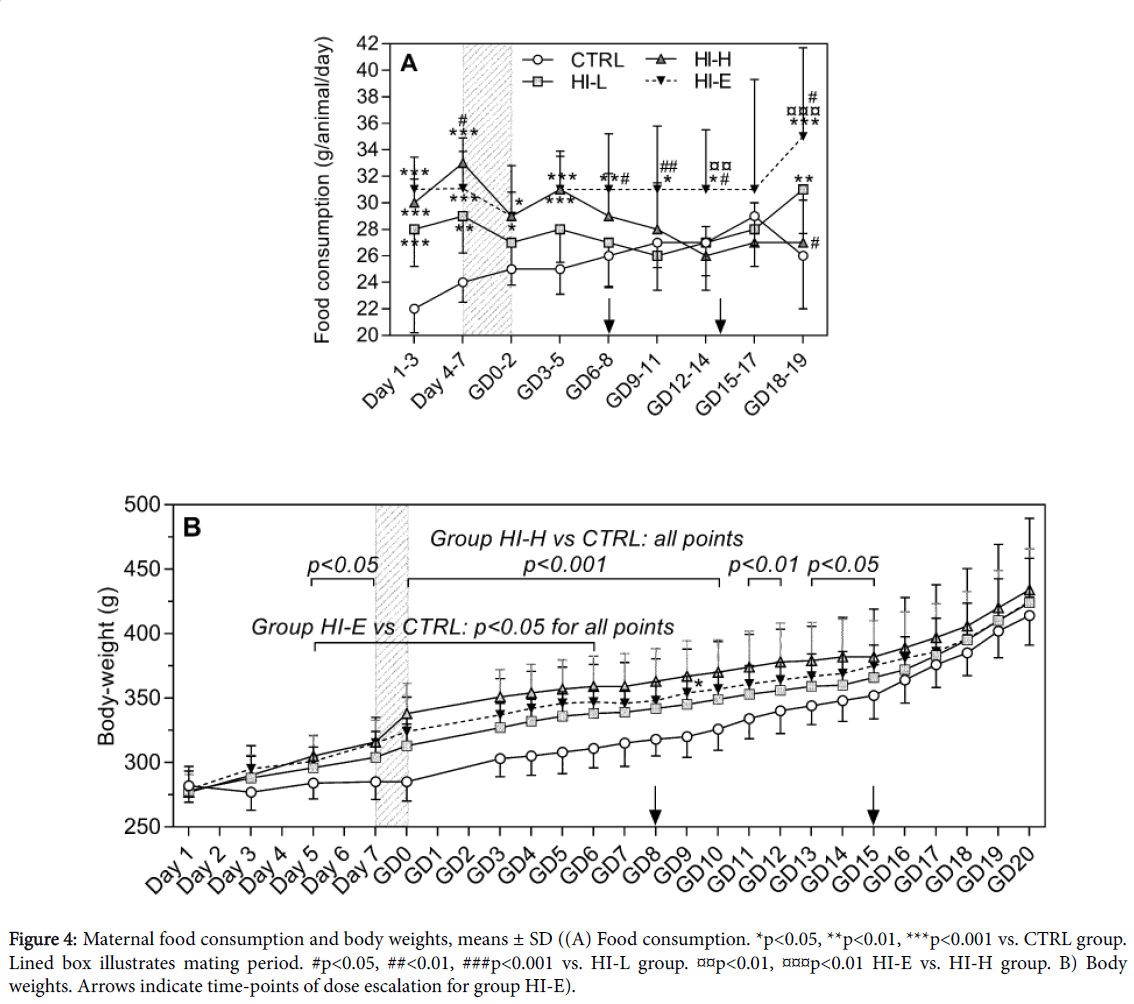
Maternal food consumption and body weight
Food consumption was significantly increased from Day 1 in all insulin-infused groups (Figure 4A). In group HI-L and HI-H this was significant until Day 7 and GD5, respectively, hereafter this effect tapered off. For the HI-E group, this effect was maintained throughout the study. Additionally, body weights were increased following start of insulin-infusion (Figure 4B). This was statistically significant from Day 5 to GD15 in group HI-H and from Day 5 to GD6 in group HI-E, hereafter the differences from the CTRL group were attenuated.
Figure 4: Maternal food consumption and body weights, means ± SD ((A) Food consumption. *p<0.05, **p<0.01, ***p<0.001 vs. CTRL group. Lined box illustrates mating period. #p<0.05, ##<0.01, ###p<0.001 vs. HI-L group. p<0.01, p<0.01 HI-E vs. HI-H group. B) Body weights. Arrows indicate time-points of dose escalation for group HI-E).
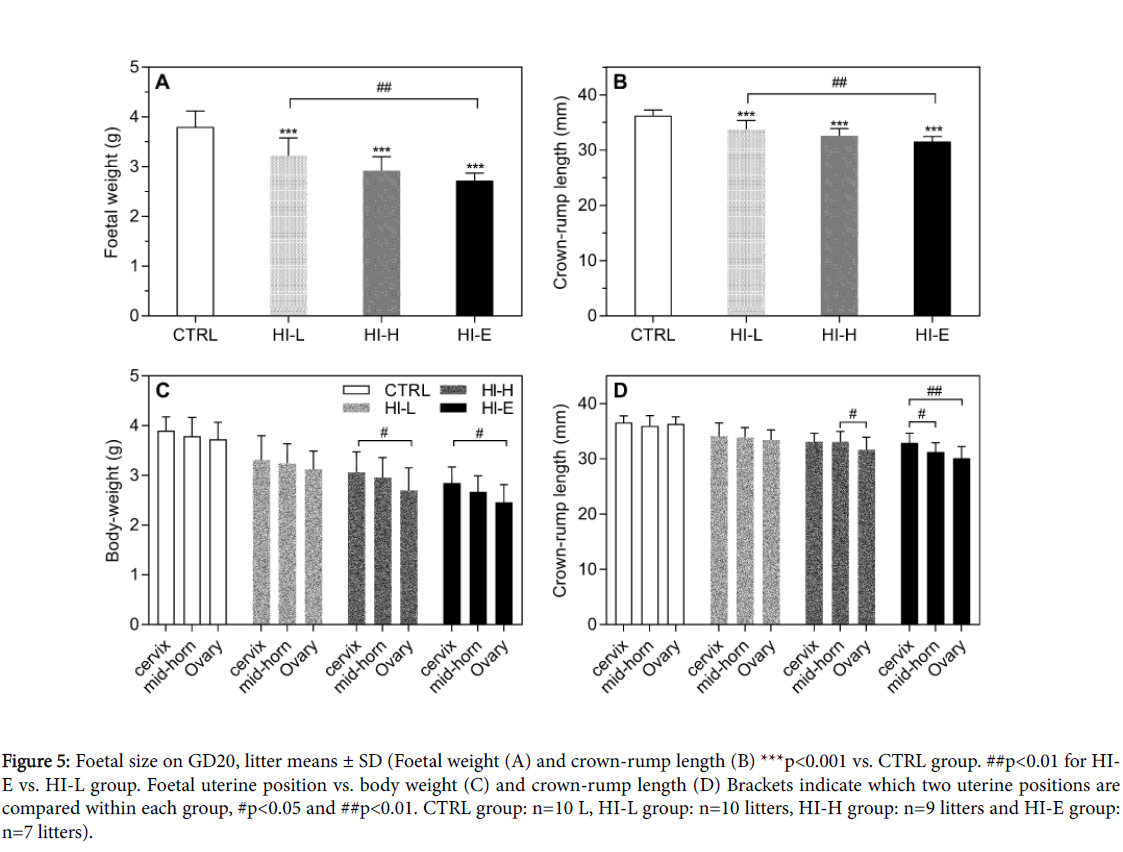
Foetal size
Foetal crown-rump length as well as body weight on GD20 showed an overall effect of group (one-way ANOVA, p<0.0001) for both parameters. Each of the litters from insulin-infused dams had significantly decreased crown-rump length and body weight compared to the CTRL group (Figures 5A and 5B). Both parameters showed a dose-response. There was an overall effect of uterine position on both body weight and crown-rump length generally with significantly decreased size of foetuses adjacent to the ovary compared to cervix and/or the mid-horn position in group HI-H and HI-E, with the same tendency in the HI-L group (Figures 5C and 5D). In control animals there was no statistical effect of uterine position on foetal size.
Figure 5: Foetal size on GD20, litter means ± SD (Foetal weight (A) and crown-rump length (B) ***p<0.001 vs. CTRL group. ##p<0.01 for HIE vs. HI-L group. Foetal uterine position vs. body weight (C) and crown-rump length (D) Brackets indicate which two uterine positions are compared within each group, #p<0.05 and ##p<0.01. CTRL group: n=10 L, HI-L group: n=10 litters, HI-H group: n=9 litters and HI-E group: n=7 litters).
Histopathologic changes in maternal pancreas
Histopathological examination of maternal pancreas showed increased incidence of minimal islet cell atrophy in the in each of the insulin-infused groups versus the CTRL (Table 3). There were no significant differences between the insulin-infused groups.
| Terminal sacrificed | Premature sacrificed | Compiled incidence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | CTRL | HI-L | HI-H | HI-E | CTRL | HI-L | HI-H | HI-E | CTRL | HI-L | HI-H | HI-E |
| Total no. examined | 0/10 | 6/10* | 8/9*** | 7/7*** | 0/1 | 0/1 | 0/1a | 03-Apr | 0/11 | 06-Nov | 8/10a | 10-Nov |
| Incidence (%) | 0% | 60% | 89% | 100% | 0% | 0% | 0% | 75% | 0% | 55% | 80% | 91% |
Table 3: Incidence of minimal islet cell atrophy in maternal pancreas (a: One animal from this group was not sampled by mistake; *p = 0.05, ***p<0.001; versus CTRL group).
Litter data
Litter data as assessed by the number of corpora lutea, implantation sites and live young showed no differences between groups (data not shown).
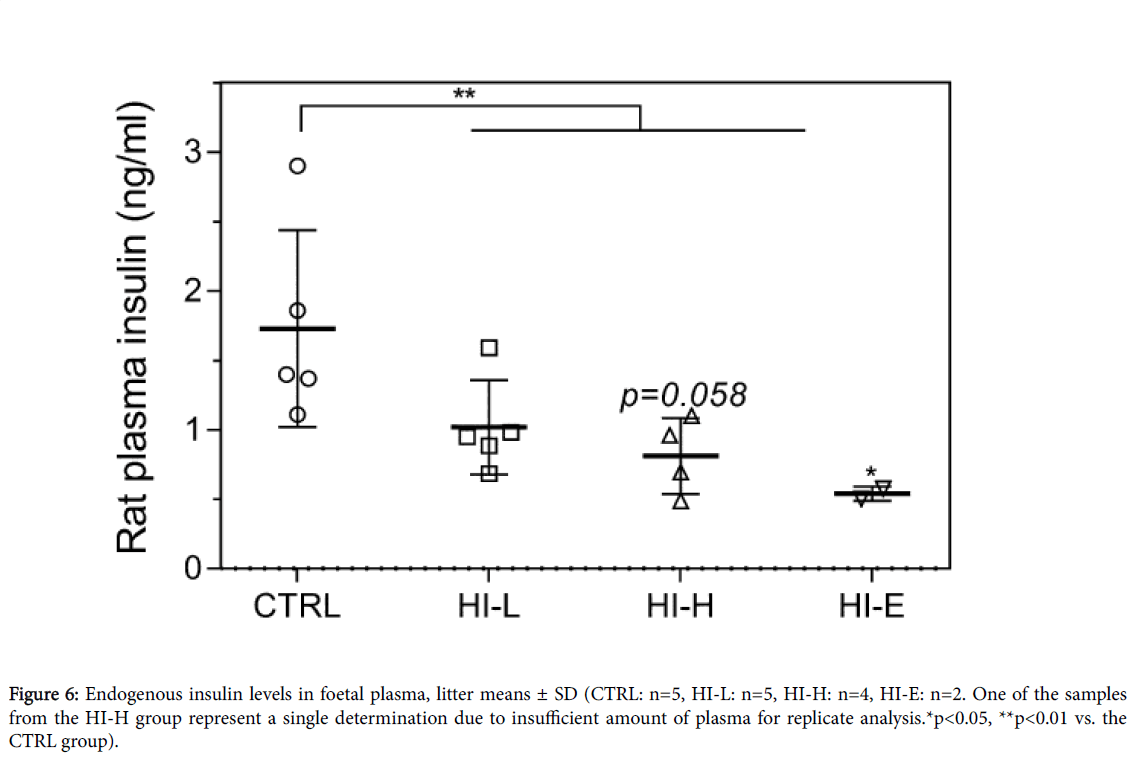
Endogenous insulin levels in foetal plasma
Maternal insulin-infusion caused an overall significant decrease in foetal endogenous insulin plasma levels (Figure 6), with a significant decrease in the HI-E group and a decrease in the HI-H group, which approached a significant decrease (p=0.058). Levels were reduced by 41%, 53%, and 69% versus CTRL group in the HI-L, HI-H, and HI-E group, respectively, suggesting a dose-response.
Fetal skeletal changes
Skeletal malformations, such as misshapen cranial bones, kinked ribs, and short long bones were evident in each of the insulin-dosed groups (Tables 4A and 4B). Furthermore, all insulin-dosed groups were affected with delayed/decreased ossification of several bones (Figure 4B). Incidences of foetuses affected were generally significantly higher in insulin-dosed animals versus CTRL group. A dose-response was present for the malformations.
| Dose groups | CTRL | HI-L | HI-H | HI-E | |
|---|---|---|---|---|---|
| Total no. of foetuses (litters) examined | 141 (10) | 132 (10) | 124 (9) | 85 (7) | |
| Cranial | Unossified area(s) | 0 | 00-Jan | 1 | 0 |
| Sutural bone | 1 | 1 | 0 | 0 | |
| Interparietal fissure(s) | 1 | 0 | 0 | 0 | |
| Misshapen basisphenoid | 0% | 12 (5) | 13 (5) | 40 (7) | |
| Vertebral abnormality | Thoracic | 0 | 01-Jan | 1 | 1 |
| Caudal | 0 | 0 | 0 | 1 | |
| Cartilaginous centrum | Unfused | 0 | 0 | 1 | 0 |
| Ribs | Medially thickend/kinked | 1 | 7 (3) | 11 (2) | 10 (3) |
| Branched | 0 | 0 | 1 | 0 | |
| Fused/partially fused | 0 | 2 | 1 | 2 | |
| Sternebrae | Misaligned ossification sites | 1 | 0 | 0 | 0 |
| Costal cartilage | Absent | 0 | 1 | 0 | 1 |
| Additional | 0 | 0 | 1 | 0 | |
| Hole in xiphoid | 0 | 1 | 0 | 1 | |
| Appendicular | Short long bones | 0 | 30 (3) | 36 (4) | 19 (5) |
| Tail | Kinked | 0 | 0 | 0 | 1 |
| Total no. of foetuses (litters) affected by one or more of the above | 4(3) | 40***### (6) | 50*** (8) | 53***## (7) | |
| Incidence | 3% (30%) | 30% (-60%) | 40% (89%) | 62% (100%) | |
| Rib and vertebral configuration | |||||
| 1st rib | Short | 0 | 0 | 1 | 0 |
| 13th rib | Short | 2 | 0 | 0 | 0 |
| Short/with costal cartilage | 0 | 1 | 0 | 0 | |
| Number of 14th rib | Short supernumerary | 7 (4) | 11 (5) | 21 (7) | 19 (5) |
| Full supernumerary | 0 | 0 | 0 | 3 | |
| Total no. of foetuses (litters) affected by one or more of the above | 7 (4) | 11## (5) | 21 ** (7) | 19 *** (5) | |
| Incidence | 5% (40%) | 8% | 17% (78%) | 22% (71%) | |
| -50% | |||||
| Dose groups | CTRL | HI-L | HI-H | HI-E | |
| Total no. of foetuses (litters) examined | 141 (10) | 132 (10) | 124 (9) | 85 (7) | |
| Delayed/incomplete/unossified | |||||
| Cranial | Cranial centres | 14 (4) | 64 (7) | 61 (7) | 48 (7) |
| Presphenoid | 0% | 89 (9) | 86 (8) | 65 (7) | |
| Hyoid | 12(4) | 46 (10) | 52 (7) | 62 (7) | |
| Tympanic annulus | 0 | 2 (1) | 1 (1) | 3 (3) | |
| Vertebrae | Cervical | 3 (2) | 34 (6) | 46 (6) | 36 (7) |
| Thoracic | 2 (1) | 48 (6) | 57 (7) | 69 (7) | |
| Lumbar | 0 | 36 (5) | 48 (5) | 45 (7) | |
| Sacrocaudal | 2 (2) | 94 (10) | 76 (8) | 63 (7) | |
| Caudal | 0 | 49 (6) | 59 (7) | 54 (6) | |
| Sternebrae | 5th and/or 6th | 88 (10) | 131 (10) | 122 (9) | 85 (7) |
| other | 4 (3) | 117 (10) | 96 (9) | 85 (7) | |
| Total | 90 (10) | 132 (10) | 122 (9) | 85 (7) | |
| Ribs | Any | 1 (1) | 9 (3) | 6 (2) | 8 (3) |
| Appendicular | Pelvic bones | 2 (1) | 100 (10) | 81 (8) | 71 (7) |
| Long bones | 0 | 26 (3) | 12 (3) | 9 (3) | |
| Metacarpals | 0 | 75 (9) | 65 (7) | 52 (7) | |
| Metatarsals | 0 | 80 (9) | 73 (8) | 83 (7) | |
| Scapula(e) | 0 | 1 (1) | 0 | 0 | |
| Total no. of foetuses affected by one or more of the above | 94 (10) | 132*** (10) | 123*** (9) | 85*** (7) | |
| Incidence | 67% (100%) | 100% | 99% (100%) | 100% | |
| #### | -100% | ||||
Table 4: Summary of findings at skeletal examinations of foetuses (Foetal skeletons stained for calcified bone and cartilage were evaluated for extent of ossification and malformations A) Skeletal abnormalities given as number of foetues (litters) affected, B) changes to skeletal ossification given as number of foetuses (litters) affected. No. of foetuses involved: **p<0.01 and ***p<0.001 vs. CTRL, ##p<0.01 and ###p<0.001 vs. HI-H group). Placental .
Placental and liver measurements
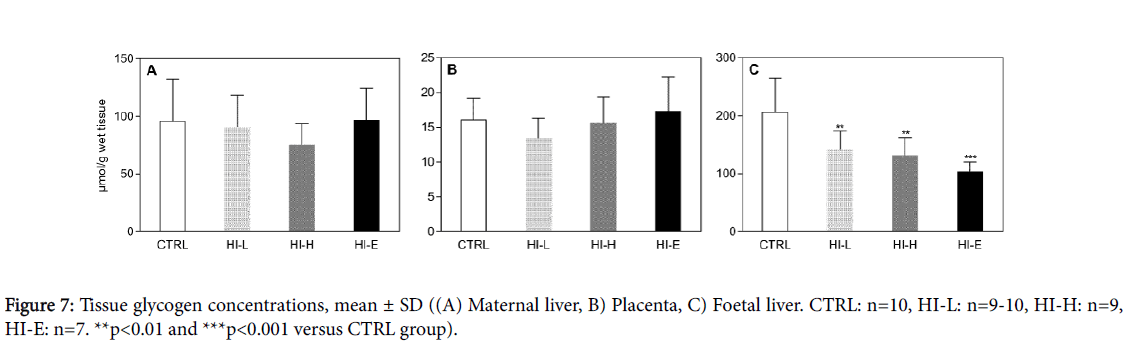
Absolute and relative maternal liver weights were unchanged by insulin-dosing (Table 5). In contrast in the foetuses, absolute pooled litter liver weights were decreased in insulin-dosed groups, with no significant dose-response, whereas relative litter liver weights remained unaffected. Tissue concentration of glucose stored as glycogen was not different between groups in maternal liver or placental tissue, respectively (Figure 7A and B). In contrast, foetal liver glycogen concentration was decreased by 32%, 36%, and 50% in HI-L, HI-H, and HI-E groups, respectively, versus the CTRL group with no significant dose-response (Figure 7C). In line with this, foetal to maternal ratio of liver glycogen concentration changed from 2:1 to 1:1.
| Dose groups | CTRL | HI-L | HI-H | HI-E | |
|---|---|---|---|---|---|
| n | 10 | 10 | 9 | 7 | |
| Maternal liver | |||||
| Weight, absolute (g) | 15.27 (1.48) | 16.09 (1.93) | 15.56 (1.35) | 16.62 (2.08) | |
| Weight, relative to BW | 0.037 (0.002) | 0.038 (0.002) | 0.036 (0.002) | 0.039 (0.004) | |
| Triglyceride | 10.78 (2.91) | 8.79 (1.21) | 11.91 (2.45)# | 8.31 (2.37)¤ | |
| Cholesterol | 9.68 (1.44) | 9.41 (1.03) | 9.77 (0.89) | 9.06 (0.51) | |
| FFA | 2.74 (0.63) | 2.74 (0.70) | 3.28 (0.56) | 3.25 (0.37) | |
| Placenta | |||||
| Weight, absolute (g) | 0.54 (0.058) | 0.49 (0.060) | 0.47 (0.030) | 0.51 (0.049) | |
| Triglyceride | 4.60 (0.54) | 3.72 (0.56)** | 4.46 (0.53)# | 3.85 (0.36)a) | |
| Cholesterol | 11.38 (0.80) | 10.89 (1.28) | 11.17 (0.68) | 10.89 (0.95) | |
| FFA | 2.61 (0.28) | 2.63 (0.35) | 2.58 (0.19) | 2.67 (0.25) | |
| Foetal liver (pooled litter) | |||||
| Weight, absolute (g) | 4.462 (0.779) | 3.405 (0.845)* | 3.458 (0.823) | 2.700 (1.039)** | |
| Weight, relative to BW | 0.084 (0.011) | 0.080 (0.004) | 0.086 (0.008) | 0.082 (0.011) | |
| Triglyceride | 5.22 (2.53) | 3.72 (0.55) | 4.00 (0.70) | 4.39 (0.86) | |
| Cholesterol | 8.11 (0.53) | 8.48 (0.47) | 8.26 (0.54) | 8.48 (0.30) | |
| FFA | 2.57 (0.20) | 2.61 (0.16) | 2.57 (0.09) | 2.58 (0.19) | |
Table 5: Tissue lipid concentrations, means (± SD) (µmol/g wet tissue; *p<0.05 and **p<0.01 vs. CTRL group. #p<0.05 vs. HI-L group. ¤p<0.05 vs. HI-H group; a: p=0.0506 vs. CTRL group).
Tissue lipid concentrations are presented in Table 5. Cholesterol and FFA concentrations were maintained in maternal liver and placental tissue, whereas triglyceride concentrations showed some changes in both tissues. In maternal liver, triglyceride concentration was higher in the HI-H group vs. the HI-L and HI-E group, respectively; none of the groups had significantly different levels from the CTRL group. In placenta, triglyceride concentration was significantly decreased in group HI-L (by 19%) versus the CTRL group, and approached a significant decrease in the HI-E group (by 16%, p=0.0506). In foetal livers, cholesterol, FFA, and triglyceride concentrations were unchanged by maternal insulin-infusion.
Discussion
Initiation of insulin-infusion induced a persistent drop in maternal blood glucose levels by approximately 50% of control values, while foetal blood glucose levels on GD20 were decreased by approximately 10% in insulin-infused groups. Maternal hypoglycaemia resulted in decreased foetal size and skeletal changes comprising malformations as well as decreased ossification.
The decrease in foetal skeletal ossification and growth likely reflects a delay in development due to decreased availability of glucose following maternal hypoglycaemia rather than the maternal hyperinsulinaemia. Intra-uterine growth restriction as well as decreased skeletal ossification in foetuses is also seen with maternal food-restriction in rats, which induces maternal hypoinsulinaemia and hypoglycaemia accompanied by foetal hypoglycaemia [17-20]. As insulin has been shown to cross the rat placenta in small amounts [21-23], foetal plasma level of human insulin was also measured in the present study. As there was no detectable foetal exposure to human insulin, endogenous insulin plasma levels were also measured showing foetal hypoinsulinaemia.
This is in line with the studies mentioned above where maternal food-restriction also resulted in foetal hypoinsulinaemia [17,18] and with results from a study with short-term maternal insulin-induced hypoglycaemia [24]. Therefore, it seems that the maternal hypoglycaemia leading to foetal hypoglycaemia is the cause of the foetal changes rather than the insulin itself. Although, IGF-I is the main growth factor involved in foetal growth and skeletal development [25], we cannot rule out that the decrease in endogenous insulin level in the foetuses is involved in the growth restriction and skeletal changes, as insulin is a well-known growth factor and stimulates bone formation [26-28]. But, it should be kept in mind that foetal hypoinsulinaemia is probably induced secondary to foetal hypoglycaemia as pancreatic insulin production is regulated by blood glucose level [29].
Both maternal and foetal liver glycogen concentrations were measured to assess mobilisation of hepatic glucose stores, reflecting the overall metabolic pressure on the dam and litter, as glycogen stores are typically decreased in response to hypoglycaemia [30,31]. Surprisingly, glycogen concentration in maternal livers was maintained in the present study. This may represent an adaption to the steady-state hypoglycaemia possibly by increase in other metabolic routes of glucose production, such as compensatory increase in gluconeogenesis or break-down of muscle glycogen.
Several counter-regulatory responses to help maintain blood glucose levels were observed in the dams. An increase in food consumption was evident, increasing the exogenous supply of glucose and thus attenuating the blood glucose lowering effect of insulin leading to tolerable levels of hypoglycaemia that did not cause clinical signs. This is emphasised by the fact that, while a dose-response was not seen on blood glucose levels, it was reflected in the food consumption. Higher doses necessitate a higher compensatory response; therefore, the animals increase their food consumption further. Moreover, the HI-E group was the only group, where compensatory increase in food consumption was maintained throughout the study, whereas it tapered off from GD6 in the two remaining insulin-infused groups. This was probably due to a decrease in insulin sensitivity, which is a known to develop as gestation progresses in the rat [32-34]. Additionally, in line with the persistent hypoglycaemia, pancreatic islets were atrophic in insulin-infused dams indicating a decrease in production of endogenous insulin, which has also been shown previously in non-pregnant growing rats infused with human insulin [12]. This decrease in insulin production is a typical counter-regulatory response to a low blood glucose level [29], as also reflected by the decrease in plasma insulin level in the foetuses. In contrast to maternal liver glycogen stores, litter hepatic glycogen concentration decreased by 30 to 50% of controls in insulin-infused groups. Again, this suggests a hypoglycaemia-driven decrease in materno-foetal glucose transfer in line with the notion that the dam is prioritised at expense of the foetus during low nutrient availability. This may also in part explain the unchanged maternal liver glycogen concentration. Additionally, it could also reflect that the foetus is more sensitive to hypoglycaemia compared to the dams, possibly due to limited potential for counter-regulation. The foetus cannot increase its intake of exogenous glucose and therefore has to rely on endogenous sources such as hepatic glycogen, when maternal glucose transfer is decreased. In the present study, foetal liver glycogen concentration was 200% of maternal levels in the controls, similar to previous reports [18, 35], and the liver constituted 8% of total body weight in litters versus 4% in the dams. This emphasises that hepatic glycogen is probably a very important glucose reservoir in foetuses. In line with this, foetal hepatic glycogen depletion has been associated with foetal growth restriction, as seen in the present study, following maternal short-term insulin-induced (5-6 days) or fasting-induced (3-4 days) maternal hypoglycaemia [18,36]. The foetal growth restriction observed in our study, was reflected both by a delay in skeletal ossification as well as decreased body weight and crown-rump length, presumably also caused by a decrease in materno-foetal glucose transfer, which is a known effect of maternal insulin-induced hypoglycaemia in rats [37].
The present study revealed a significant effect of uterine position on foetal size in group HI-H and HI-E, foetal size decreased with increasing distance from the cervical blood supply. Higher foetal weight in the cervical versus the ovarian end has been reported previously in non-diabetic rats by others [38,39]. Such differences are interpreted to be due to the differences in blood flow and thus nutrient supply. This could also explain the effect of foetal position seen in the present study, as foetuses “last in line” to the blood glucose supply from the maternal circulation would most likely receive less glucose and consequently have a more constrained growth. However, differences in blood supply alone cannot explain the size differences, as the uterine blood supply has been shown to be higher to foetuses at each end of the uterine horn compared to the mid-horn position in rats [40]. This should lead to the smallest foetuses being the ones in the mid-horn position and not the foetuses adjacent to the ovaries. However, blood is not the only foetal source of maternal glucose; the amniotic fluid contains glucose of maternal origin and is known to be a major source of glucose to the rat embryo prior to the establishment of the chorioallantoic circulation [41,42]. The amniotic fluid has a glucose concentration of about 1.5 mM [43], which is relatively high compared to the foetal blood glucose level <1 mM seen later in gestation. This amniotic glucose can be transported into the embryo by passive diffusion maintained by high-affinity glucose transporters [41]. It is known that testosterone can be transported from the amniotic fluid to the rat foetus by crossing the membranes of the amniotic sac [44]. This occurs in a highly directional fashion from the ovarian end to the cervical end of the uterine horn with a three-fold higher amniotic fluid concentration of testosterone between cervical and ovary adjacent foetuses [44]. This is thought to be mediated by movement of intraluminal fluid in the uterine horns from the ovarian to the cervical ends [44]. If the same can be said regarding intraluminal glucose movement in the uterine horns during the embryonic period, this could also be a mechanism favouring glucose delivery to the embryos closest to the cervix supporting the embryonic growth.
In addition to hepatic glycogen levels, lipid concentration was also measured in livers as an indication of general nutrient status and as a reflection of potential usage of liver triglyceride and FFAs for gluconeogenesis. Maternal and foetal hepatic concentration of lipids was maintained. Unchanged foetal hepatic lipid concentrations suggest that adaptive contribution of gluconeogenesis from lipids was not an important counter-regulatory response. In fact, others have found an increase in foetal hepatic lipid concentrations following maternal fasting-induced hypoglycaemia [45,46]. This is thought to represent a prioritisation of maintenance of the supply of lipids for foetal brain development, with is largely dependent on adequate levels of lipids [45,46].
Conclusion
In conclusion, persistent maternal hypoglycaemia throughout gestation was accomplished in the present study. Maternal hypoglycaemia resulted in foetal hypoglycaemia, reduced foetal growth and skeletal changes such as decreased ossification and specific skeletal malformations (bent ribs, misshapen cranial bones, short long bones). Foetal hypoinsulinaemia was most likely secondary to the maternal hypoglycaemia causing a decrease in placental glucose transfer to the foetus and consequently foetal hypoglycaemia. The foetal adaptive response included mobilisation of the high glycogen stores in the liver and decreased growth. In contrast, maternal hepatic glycogen levels were maintained, suggesting that the dam is prioritised at expense of the foetus. Additionally, it may reflect that the foetus is more sensitive to hypoglycaemia than the dam, as it cannot compensate to the same extent, e.g. by increasing intake of exogenous glucose. The present study contributes with novel information regarding the foetal effects to persistent maternal hypoglycaemia in the non-diabetic rat. This will aid the interpretation of findings in embryo-foetal development studies with longer-acting insulin analogues allowing distinguishing between foetal effects of toxicological origin and those due to the persistent hypoglycaemia.
References
- Ornoy A (2016) Effect of maternal diabetes on the embryo, fetus and children: Congenital anomalies, genetic and epigenetic changes and developmental outcomes, pp. 1542-9768.
- Tanigawa K (2014) Skeletal malformations in rat offspring. Long-term effect of maternal insulin-induced hypoglycemia during organogenesis. Diabetes 40: 1115-1121.
- Buchanan TA, Schemmer JK, Freinkel N (1986) Embryotoxic effects of brief maternal insulin-hypoglycemia during organogenesis in the rat. J Clin Invest 78: 643-649.
- FDA (1994) Guideline for Industry: Detection of toxicity to reproduction for medicinal products ICH-S5A.
- FDA (2008) Guidance for industry. Diabetes Mellitus: Developing drugs and therapeutic biological for treatment and prevention.
- EMA (2001) Points to consider document on the non-clinical assessment of the carcinogenic potential of insulin analogues.
- Smithberg M, Runner MN (1963) Teratogenic effects of hypoglycemic treatments in inbred strains of mice. Am J Anat 113: 479-489.
- Lichtenstein H, Guest GM, Warkany J (1951) Abnormalities in offspring of white rats given protamin zinc insulin during pregnancy. Proc Soc Exp Biol Med 78: 398-402.
- Gaccioli F (2013) Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis 4: 101-115.
- Jensen VF (2016) Histopathological nerve and skeletal muscle changes in rats subjected to persistent insulin-induced hypoglycemia. J Toxicol Pathol 29: 17-30.
- Jensen VFH (2017) Chronic hyperinsulinaemic hypoglycaemia in rats is accompanied by increased body weight, hyperleptinaemia and decreased neuronal glucose transporter levels in the brain. Int J of Endocrinol p: 14.
- Jensen VFH (2017) Toxicological Effects during and following persistent insulin-induced hypoglycaemia in healthy euglycaemic rats. Basic Clin Pharmacol Toxicol.
- Plum A, Agersø H, Andersen L (2000) Pharmacokinetics of the Rapid-acting insulin analog, insulin as part, in rats, dogs and pigs and pharmacodynamics of insulin as part in pigs. Drug Metabol Disposit 28: 155-160.
- EU D (2004) Directive 2004/10/EC of the European Parliament and of the Council of 11 February 2004 on the harmonisation of laws, regulations and administrative provisions relating to the application of the principles of good laboratory practice and the verification of their applications for tests on chemical substances.
- OECD (1998) OECD principles on good laboratory practice.
- UK G (2004) The Good laboratory practice regulations.
- Gruppuso PA, Brautigan DL (1989) Induction of hepatic glycogenesis in the fetal rat. Am J Physiol 256: 49-54.
- Girard JR (1977) Fetal metabolic response to maternal fasting in the rat. Am J Physiol 232: 456-463.
- Fleeman TL (2005) The effects of feed restriction during organogenesis on embryo fetal development in the rat. Birth Defects Res B Dev Reprod Toxicol 74: 442-449.
- Ariyuki F (1987) Growth retardation induced in rat fetuses by maternal fasting and massive doses of ergocalciferol. J Nutr 117: 342-348.
- Goodner CJ, Freinkel N (1961) Carbohydrate metabolism in pregnancy. IV. Studies on the permeability of the rat placenta to I-131 insulin. Diabetes 10: 383-392.
- Clark CM, Cahill GF, Soeldner JS (1968) Effects of exogenous insulin on the rate of fatty acid synthesis and glucose C-14 utilization in the twenty-day rat fetus. Diabetes 17: 362-368.
- Widness JA (1983) Impermeability of the rat placenta to insulin during organogenesis. Teratology 28: 327-332.
- Ogata ES, Paul RI, Finley SL (1987) Limited maternal fuel availability due to hyperinsulinemia retards fetal growth and development in the rat. Pediatr Res 22: 432-437
- Agrogiannis GD (2014) Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review). Mol Med Rep 10: 579-584.
- Griffen SC (1987) Insulin exerts metabolic and growth-promoting effects by a direct action on the liver in vivo: Clarification of the functional significance of the portal vascular link between the beta cells of the pancreatic islets and the liver. Proc Natl Acad Sci U S A 84: 7300-7304.
- Cornish J, Callon KE, Reid IR (1996) Insulin increases histomorphometric indices of bone formation in vivo. Calcif Tissue Int 59: 492-495.
- Yano H, Ohya K, Amagasa T (1994) Effects of insulin on in vitro bone formation in fetal rat parietal bone. Endocrine J 41: 293-300.
- Chen L (1989) Effects of hypoglycemia and prolonged fasting on insulin and glucagon gene expression. Studies with in situ hybridization. J Clin Invest 84: 711-714.
- Fau MK, Sonne B (1989) Glucose turnover during insulin-induced hypoglycemia in liver-denervated rats. Am J Physiol 248: 327-332.
- Obici S (2008) Liver glycogen metabolism during short-term insulin-induced hypoglycemia in fed rats. Cell Biochem Funct 26: 755-759.
- Martin A (1986) Glucose tolerance tests and "in vivo" response to intravenous insulin in the unanaesthesized late pregnant rat and their consequences to the fetus. Diabete Metab 12: 302-327.
- Lopez-Luna P (1998) Aerobic exercise during pregnancy reverts maternal insulin resistance in rats. Med Sci Sports Exerc 30: 1510-1514.
- Munoz C, Lopez-Luna P, Herrera E (1995) Glucose and insulin tolerance tests in the rat on different days of gestation. Biol Neonate 68: 282-291.
- Diamant YZ, Shafrir E (1978) Placental enzymes of glycolysis, gluconeogenesis and lipogenesis in the diabetic rat and in starvation. Comparison with maternal and foetal liver. Diabetologia 15: 481-485.
- Higham FC, Pillay D, Bailey E (1984) The effect of maternal diet on maternal and fetal hepatic and brown adipose tissue lipogenesis and blood and tissue metabolites. J Dev Physiol 6: 153-158.
- Herrera E (1985) Relationship between maternal and fetal fuels and placental glucose transfer in rats with maternal diabetes of varying severity. Diabetes 34: 42-46.
- Norman NA, Bruce NW (1979) Fetal and placental weight relationships in the rat at days 13 and 17 of gestation. J Reprod Fertil 57: 345-358.
- Fau-Bruce NN, Bruce NW (2001) Fetal and placental weight relationships in the albino rat near term. Teratology 19: 245-250.
- Even MD (1994) Differences in blood flow to uterine segments and placentae in relation to sex, intrauterine location and side in pregnant rats. J Reprod Fertil 102: 245-252.
- Matsumoto K (1995) Abundant expression of GLUT1 and GLUT3 in rat embryo during the early organogenesis period. Biochem Biophys Res Commun 209: 95-102.
- Shepard TH, Tanimura T, Park HW (1997) Glucose absorption and utilization by rat embryos. Int J Dev Biol 41: 307-314.
- Park HW, Shepard TH (1992) Volume and glucose concentration of rat amniotic fluid: Effects on embryo nutrition and axis rotation. Teratology 49: 465-469.
- Even MD, Dhar MG, vom Saal FS (1992) Transport of steroids between fetuses via amniotic fluid in relation to the intrauterine position phenomenon in rats. J Reprod Fertil 96: 709-716.
- Bertrand PC, O'Kusky JR, Innis SM (2006) Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr 136: 1570-1575.
- Schiefermeier M, Yavin E (2002) n-3 Deficient and docosahexaenoic acid-enriched diets during critical periods of the developing prenatal rat brain. J Lipid Res 43: 124-125.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3316
- [From(publication date):
June-2017 - Dec 18, 2024] - Breakdown by view type
- HTML page views : 2616
- PDF downloads : 700