Germination of Major Weed Seeds in Response to Temperature and Nitrogen in Bench-Sheko Zone, South Western Ethiopia
Received: 08-Jul-2022 / Manuscript No. ACST-22-68902 / Editor assigned: 11-Jul-2022 / PreQC No. ACST-22-68902 (PQ) / Reviewed: 25-Jul-2022 / QC No. ACST-22-68902 / Revised: 02-Jan-2023 / Manuscript No. ACST-22-68902 (R) / Published Date: 13-Jan-2023
Abstract
Weed seeds and their germination requirements are very diverse. Integration of knowledge of weed germination and seed dormancy status could be used to improve weed control strategies. Weeds especially invasive weeds are a serious challenges causing low crop yield and food insecurity in Ethiopia. Understanding weed germination requirement and optimum temperature is essential to improve weed management practices. Consequently, as assistance in the improvement of control and management practices, the study was carried out with the objectives to: Determine optimum temperature in which major weed seeds germinate and to evaluate effect of nitrogen on germination of major weed seeds. The experiment was conducted at Mizan Tepi university bio medical science laboratory in Southern Ethiopia during 2020. Weed species, Amaranthus spinosus L., Commelina benghalensis L., Guzotia scabra Vis Chiov, Mimosa invisa L. and Parthenium hysterophorus L. which were infest crops in the study area were used in this investigation. Seeds of the weeds were exposed to five temperature regimes viz. 5°C/15°C, 10°C/20°C, 15°C/ 25°C, 20°C/ 30°C and 25°C/35°C for 16 hours followed by for 8 hours. Germination performance of the weed species was determined with and without nitrate (0 and 10 mM) solution. The weed was verified as germinated when the radicle or hypocotyl evidently projected over the seed coat. Weed seeds were punctured with a dissecting needle and placed on blotters moistened with 0.1% tetrazolium solution when they are recognized as ungerminated. The test was laid out in a completely randomized design in factorial arrangement with three replications. The result showed weed seeds germination percentage were affected significantly by alternating temperature, nitrate and their interaction. The highest weed seed germination was recorded at temperature range between 15/25°C-25/35°C with nitrate, but Mimosa invisa L. germinated regardless of nitrate. The highest germination (80.66% and 79.33%) was recorded in Parthenium hysterophorus L. at 15/25°C and 20/30°C with nitrate followed by (79.33%) in Guzotia scabra Vis Chiov at 20/30°C with nitrate. Mimosa invisa L. highest germination was recorded at 25/35°C. Amaranthus spinosus L. germination was highest at 20/30°C and 25/35°C with nitrate. Among weed species lowest germination was recorded in Commelina benghalensis L. Highest percentage of seed dormancy was recorded at lower temperature regardless of nitrate in all species. Among weed species highest dormancy more than 80% recorded in Amaranthus spinosus L. and Commelina benghalensis L. at 10/20°C and 5/15°C with and without nitrate. Except Mimosa invisa L. germination of all species pronounced at higher temperature with nitrate. Therefore the highest weed seed germination was recorded at temperature range between 15/25°C-25/35°C with nitrate which correlated with early spring to late summer temperature of bench sheko zone. It is suggested that applying different management option at their pick emergence period like stimulating weed seed to germinate in flash to reduce weed seed bank and adjusting nitrogen fertilizer application method like placing fertilizer close to crop seed and foliar application of fertilizer is important to reduce weed interference with crop productivity.
Keywords
Weed, Temperature, Nitrate, Germination, Dormancy
Introduction
A weed was defined as a plant that forms populations that are able to enter habitats cultivated, markedly disturbed or occupied by man and potentially depress or displace the resident plant populations which are deliberately cultivated or are of ecological andor aesthetic interest [1].
Agriculture production is still low to meet Ethiopian fast growing population due to several environmental, biological and edaphic factors which interfere with quality and quantity of product or yield. Weeds are one of the main factors which interferes crop productivity due to their direct and in direct competition with crop for growth limiting resources reported that a total loss of wheat yield due to weed competition in Ethiopia was 77%, while, yield losses due to insects and disease range between 51% and 65% respectively [2].
The existence of large weed seed population with varying degree of dormancy allows them to infest agricultural field and results continues yield reduction year to year. Weed seeds exhibit several kinds of dormancy to escape the rigors of the environment and remain viable for longer period without losing their viability and germinate when conditions are most favorable for survival. Parthenium hystherophorus L. invasion in crop lands has resulted in serious seed deposition of the weed in seed bank and weed in seed bank and causes yield loss 70% on sorghum crop [3]. Seed dormancy and longevity, season in which they emerge and seed responsiveness to stimuli varies from weed species to species. Reduction of soil weed seed deposition on soil seed bank depends on understanding of dynamics of seedling emergency. To develop integrated weed management programs detailed understanding of seed germination ecology for the competing species is important [4].
Seed germination ecology helps to recognize and explain plant evolution and ecological adaptation [5]. It determines when plants will emerge and begin to grow and become evident in fields and other habitats [6]. For weed management, all possibilities of mortality at the stage of seedling emergence should be considered [7]. Only a thorough understanding of weed germination can bring about further improvements in current weed management practices [8]. Germination is regulated by a complex interaction of environmental, edaphic, physiological and genetic factors [9]. Germination of weed is mainly controlled by seasonal changes in the dormancy status of buried seeds [10].
Temperature is the most important environmental factor regulating seed germination. It strongly influences both physiological and biochemical processes, including those occurring in germinating seeds [11]. Temperature acts to regulate germination in three main ways [12]. Firstly, it may be involved in the removal of either primary or secondary dormancy. Secondly, temperatures outside of the normal limits for germination may cause the establishment of secondary dormancy. Finally, the temperature at which seeds are incubated determines their capacity for germination and the rate at which this occurs. Temperature range for germination of weed varies from species to species. Some seeds possess specific temperature regulated dormancy mechanisms, whereas other seeds germinate over a wide range of temperature [13]. Seeds need to be subjected to optimum temperature in order to induce germination [14].
Nitrate ion is the most common soil chemical that is known to promote germination [15]. Nitrate is known to be a germination stimulator for a wide variety of plant species, thus it is used as an agent for seed priming [16]. Dormancy of several grass weed species was broken by ammonia [17]. Potassium nitrate in moderate concentrations (about 10 mM) has been used in order to check the effect on germination reported that exogenous nitrogenous compounds were found to stimulate germination in Arabidopsis thaliana [18].
Requirement of germination and period of emergency of most weed varies from species to species and location to location [19]. Weed populations have been reported to have developed different germination behavior as an adaptation to environmental and agronomic conditions [20]. Understanding of weed biology on its germination and dormancy helps to manage weeds by applying various weed management approaches such as preventive measures, intercropping, cover cropping, crop residue as mulches, competitive crop cultivars, optimum planting geometry, optimum sowing time, herbicide tolerant cultivars and herbicides and integrated method.
Weeds in general on farm land are the major yield reducing factors. In south western Ethiopia particularly in Bench Sheko zone weeds were reported to be among the major constraints of maize, wheat, sorghum, faba bean, field pea and enset production. A recent survey on the importance and distribution of weeds, in South Western Ethiopia, Bench Sheko, Kafa and Sheka zone showed that weeds species like Mimosa invisa L., Parthenium hysterophorus L., Amranthus spinosus L., Commelina benghalensis L. and Guzotia scabra Vis. Chiov are dominant and abundant weeds in crop growing area of those zone.
The efficacy of weed management operations is strongly dependent on their correct timing according to the dynamics of seedling emergence. To predicate time of herbicide application to develop economically feasible and environmentally suitable weed management strategies and to enhance quality and yield of crop knowledge on weed biology especially time and requirement of germination and dormancy period is important. Germination period of most weed species closely correlated with temperature and applied imputes.
Keeping this in view the study was aimed to:
• Determine the optimum temperature at which major weed seeds germinate.
• Evaluate the effect of nitrogen on major weed seeds germination.
Materials And Methods
Description of the experimental site
The experiment was conducted in bio medical science laboratory, at Mizan Tepi University, Bench Sheko Zone. Geographically, the experimental area is located at latitude from 6°46’30’’ to 6°57’0’’N and longitudes from 35°25’30’’ to 35°36’0’’E. The altitude of the experimental area is 1316 meter above sea level. The rainfall pattern of these areas is characterized by bimodal distribution with small rainy season Belg and main rainy season Meher with a high amount of rainfall occurring during the main rainy season of July and August. The study area indicated that total rainfall is 1296 mm and minimum and maximum temperatures are 15.87°C and 29.83°C respectively in Figure 1.
Experimental materials and their description
The dominant and abundant weeds in the arable field of this Zone are Amaranthus spinosus L., Commelina benghalensis L., Guzotia scabra Vis. Chiov, Mimosa invisa L., Parthenium hysterophorus L. were used for this study.
Aluma (Amranthus spinosus L.): Amranthus spinosus L. is Annual or (rarely) short lived perennial life history, herbaceous habit with prostrates to erect stem. It produces 250-292 thousand seeds per plant within 14 weeks. Seeds can remain viable for many years in the soil seed bank. Amranthus spinosus L. germinate in warm conditions and they respond strongly to diurnal temperature fluctuations. Grows well to good soil fertility and organic matter. Through its direct competition, weed grows in crop field, range land, plantation crop and forest that cause total crop failure and significant yield reduction. Amranthus spinosus L. is a principal weed of mangoes, sorghum, soya beans, cowpeas, common beans, vegetables, papayas and sweet potatoes, cassava, bananas, millet and coffee.
Yewhaankur (Commelina benghalensis L.): Commelina benghalensis L. is a widely distributed herbaceous weed that commonly invades agricultural sites and disturbed areas. Grows best in under conditions of highs soil moisture and fertility. It is particularly difficult to control by cultivation, partly because broken pieces of above and below ground stems readily take root. This weed by its nature is very complex, uprooted and every fragments of the weed can grow overnight, if left in the field. A plant can produce 1600 seeds. It is also known as tropical spiderwort, is among the world’s worst weeds, infesting 25 crops in 29 countries.
Metchi (Guzotia scabra Vis. Chiov): Guzotia scabra Vis. Chiov species are probably the most important of all broad leaved weeds in Ethiopia, almost universally distributed at middle and higher elevations. Erect, fast growing to 1 m-2 m (even to 4 m in some localities), but may mature and seed profusely when only 30 cm high in poor soils. Stems are usually covered in short glandular hairs. Opposite leaves, 3 cm-15 cm long is lanceolate, toothed and softly sticky to the touch. Flower heads 2 cm-5 cm across have an involucre of broad, obtuse bracts, yellow ray florets and 1 cm-2 cm long. Grows in many parts of Ethiopia in mid and highlands from 1400 m to 2800 m above sea level. It is the major weed of maize crop in Bench Maji zone.
Konter (Mimosa invisa L.): Mimosa invisa L. have spiny seed pods contain three to four one seeded segments. The seeds are flat, ovate, spiny, 2 mm-2.5 mm long and 0.6 to 1.4 mm thick that are glossy and light brown. Mimosa invisa L. is an important invasive alien weed as it is highly competitive, produces enormous seeds, difficult to control by hand and is an alternative host for nematodes. This weed has been known in Ethiopia, for more than 20 years, it recently has noticed invading the South Western Ethiopia at an alarming rate. Mimosa invisa L. is one of the major social, environmental and economic threats in the surveyed area of South Western Ethiopia. It produces enormous seeds ranging from 8,000 per m2-12,000 per m2 and a single plant can produce 10,000 seeds per annum. The seeds remain dormant for up to 50 years. It is a major weed of annual and perennial crops such as cassava, banana, upland rice, soybeans and maize, plantations such as sugarcane, coconut, rubber and tea and pastures, roadsides, riverbanks and unproductive lands. Crops infested are difficult to weed and harvest because of the thorns. Hence, invasion by Mimosa invisa L. can result in increased production costs, reduced crop yield, loss of crops and biodiversity and reduced land value.
Kinche Arem (Parthenium hysterophorus L.): Parthenium hysterophorus L. weed is an annual herb in the family Asteraceae which is characterized by deep tap root, pale green leaves and an erect stem that becomes woody gradually. At maturity, the plant develops several branches in its top half and may finally reach a height of 1.5 m-2 m. Seed does not have a dormancy period and has capability of germinating at any time when environmental condition is favorable. Seeds persist and remain viable in the soil for reasonably long periods, with a seed bank half-life of approximately six years. Parthenium hysterophorus L. is best suited to areas with an annual summer rainfall greater than 500 mm. Exerts strong allopathic which suppresses the growth and productivity of surround crops. In Ethiopia, Parthenium hysterophorus L. is primarily a weed in sugarcane cropping areas and rangeland areas and is ranked as the most serious weed by farmers.
Potassium Nitrate (KNO3): KNO3 is a major source of nitrogen for many plant species are assimilated via reduction by Nitrate Reeducates (NR) and other enzymes, which ultimately lead to the production of amino acids and nitrogen compounds. In addition to its role as nutrient, nitrates are shown to act as a signal molecule in plant development and metabolism besides assimilation controls.
Treatments and experimental design
The experiments were conducted in dark thermostatically controlled incubators in the laboratory. Germination was determined under the treatments; with and without nitrate solution as factor a free of nitrate solution (5 ml of distilled water) and 10 mM nitrate solution per petridish and in two alternating temperature regimes that mimic diurnal fluctuations.
With five levels; as factor B as follows:
• 5°C for 16 hours followed by 15°C for 8 hours.
• 10°C for 16 hours followed by 20°C for 8 hours.
• 15°C for 16 hours followed by 25°C for 8 hours.
• 20°C for 16 hours followed by 30°C for 8 hours.
• 25°C for 16 hours followed by 35°C for 8 hours.
While, the five weed species were treated as factor C. The light period correspond with 8 hours at the higher temperature, as it was phased mostly under natural conditions. Temperature experiments were performed in order to determine the optimum temperature for seed germination of each weed species. The experiments were laid out in a CRD in factorial arrangement with three replications. Each treatment had 50 seeds per petri dish.
Experimental procedure and management
The selected weed species seed were collected by hand from highly infested arable fields at their maturity from the mother plants from Bench Sheko zone. Each of the weed seeds were air dried for three days and threshed by hand. Seeds were stored at room temperature and incubated in darkness before the launching of the experiments.
For each treatment, visually acceptable and uniform sound seeds were evenly placed on layers of filter paper in 9.5 cm diameter petridish. The petri dishes were enclosed in clear plastic bags containing wet paper towels to retard change in solution concentration due to evaporation. The solutions: Potassium Nitrate (KNO3), Disodium Hydrogen Phosphate (Na2HPO4), Potassium Di Hydrogen Phosphate (KH2PO4), tetrazolium and clearing solution. lactic acid 20 parts, phenol 20 parts, glycerin 40 and water 20 parts were used in the experiment prepared based on their standard solution preparation rules.
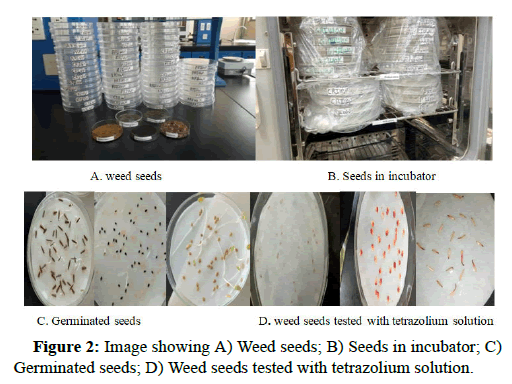
Germinated seeds were counted and removed daily for a period of 20 days of any experiment. The seeds were recorded as germinated when the radical or hypocotyls visibly protruded through the seed coat. Ungerminated seeds were punctured with a dissecting needle and placed on blotters moistened with 0.1% tetrazolium solution. The seeds were incubated for a further three hours at 35°C. Later on, they dissected and those showing a distinctly red embryo were scored as viable dormant seeds. Seeds already found rotten during puncturing and seeds looking normal but showed no red embryo after incubation with tetrazolium solution were recorded as dead seed in Figure 2.
Data collected
Numbers of weed seeds germinated per plate within each replication, with 10 mM KNO3 solution and without nitrate solution and also on the five regimes of temperature, weed seeds reaction with tetrazolium solution were periodically recorded.
Germination Percentage (GP): It had been calculated by the following formula: GP=(NT × 100)/N Where NT: Proportion of germinated seeds in each treatment for the final measurement, and N: Number of seeds used in bioassay.
Dormant seed (DO): It is calculated by DO=(DT × 100)/N where; DT=Proportion of dormant seeds in each treatment after final measurement D and N=Number of seeds used in bioassay.
Dead seed (DE): Seeds used in bioassay germinated seed dormant seed=dead seed.
A combination of standard germination test and tetrazolium test of the same sample had been provided information on the germination percentage, dormant seed as well as the percentage of dead seeds of that sample. For each treatment, the averaged data have been taken over three replications and the mean was tested for treatment differences.
Data analysis
The collected experimental data were subjected to analysis of variance using the SAS version. 9.3. Simple descriptive statistics, like percentages, were used to evaluate the weed seed germination, dormancy and decay. Treatment means were compared using the Least Significance Difference (LSD) test at 5% level of probability using the procedure as described in Gomez and Gomez.
Results And Discussion
Effects of alternating temperature and nitrate on the germination of major weed seeds
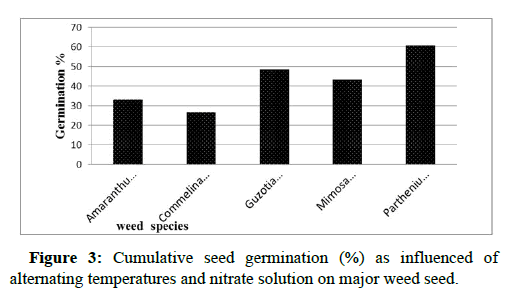
Analysis of variance showed that germination percentage of major weed species was highly significantly (P<0.001) affected by temperature, nitrate and species interaction in Appendix Table 1. Among weed species highest germination (80.66% and 79.33%) was recorded in Parthenium hysterophorus L. at 15°C/25°C and 20°C/30°C with nitrate followed by in Guzotia scabra Vis. Chiov (79.33%) at 20° C/30°C with nitrate. Germination of all weed species was lowest at lowest temperature both with and without nitrate solution. But germination percentage varies with species at lowest temperature. Lowest germination among weed species at lowest temperature was recorded in Amaranthus spinosus L. and Commelina benghalensis L. at 5°C/15°C. In Amranthus spinosus L. no germination was recorded at 5°C/15°C in both treatments. In Commelina benghalensis L. 1.33% and no seed germinated recorded at 5°C/15°C with and without nitrate.
The weed species showed highly significant differences (p ≤ 0.001) in their percentage. Germination is averaged over species, temperature and nitrate the highest germination among species was recorded in Parthenium hysterophorus L. while lowest germination among weed species was recorded in Commelina benghalensis L. The lowest germination of Commelina benghalensis L. might due to presence of other factor which limits seed to reach maximum germination. Similarly stated that inability of some species to reach a high seed germination percentage is most likely due to the presence of hard seeds coat in the seed lot Appendix Table 2. The current result corroborated with the findings of who reported that Commelina benghalensis L. has an impermeable seed coat that restricts germination, but piercing the seed coat alleviates this condition.
The possible reason for variation in germination response of species under different level of temperature and nitrate might due to genetic variation between species, variation in level of dormancy, variation in size of the seed, variation in their adaptability in wide and narrow range of temperature. Similarly reported that both the level and rate of germination varied with species, indicating that each species attains their physiological or environmental requirements for germination at different times correspondingly. Reported that each plant species has an optimal temperature at which its seed has been germinate with its maximum potential.
The performance of weed seeds germination with the application of nitrate solution had a significant effect. Effect of 10 mM potassium on seed germination might be due to the consumption as signal molecule in seed to enhance synthesis of gibberellin hormone which synthesis α amayles enzyme force cell wall and cell membrane degradation which allows radicle parturition after cell elongation. The result was similar with the findings of who reported that liquid ammonia, which is used as a nitrogen fertilizer in various parts of the world, has been shown to break dormancy in seeds of Avena fatua and several other grass weed species in Figure 3.
Amranthus spinosus L.: The germination percentage of Amranthus spinosus L. was highly significantly affected by main effect of temperature, nitrate and their interaction Appendix Table 3. Maximum germination percentage (74%) was recorded at 20°C/30° C with application of 10 mM potassium nitrate followed by 70.66% at 25°C/35°C (Table 1). The lowest germination percentage (4% and 2%) was recorded at 10°C/20°C with and without nitrate but no germinated seed was recorded at 5°C/15°C in both treatments. The maximum germination of Amranthus spinosus L. at higher temperature with nitrate might be due to effectiveness of temperature to speed up seed water imbibition, metabolic activity, signaling and nutritive effect of nitrate on weed seed germination.
The result was in line with that of who reported that Amranthus spinosus L. seeds responded positively under an alternating temperature regimes at 20°C, 25°C and 30°C and 35°C and but at lower temperature (15°C) no germination of weed seeds occur. Similarly reported that warmer temperatures and increased nitrogen availability increases seed germination, growth and establishment in five species. Alike result was reported by who reported that Amaranthus retroflexus L. has enhanced by 98% with the application of potassium nitrate in the field of Amaranths also reported that redroot pigweed (Amaranthus retroflexus L.) seed germination was stimulated by 10 ppm-100 ppm of ammonium nitrate or urea with fluctuating temperature.
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate 10 mM | 0 | 4 | 33.33 | 74 | 70.66 | 36.4 |
| No nitrate | 0 | 2 | 31.33 | 56.66 | 58 | 29.33 |
| Mean | 0 | 3.33 | 32.33 | 65.33 | 64.33 | |
| LSD (0.05) | 2.32 | |||||
| CV (%) | 4.34 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 1: Interaction effects of alternating temperatures and nitrate on Amranthus spinosus L. germination.
Commelina benghalensis L.: Analysis of variance indicated that germination percentage of Commelina benghalensis L. was highly significantly (P<0.001) affected by main effect of temperature, nitrate and their interaction Appendix Table 4. Incubation of seed at 20°C/30° C and 25°C/35°C with nitrate solution gave the highest germination percentage (64.66%) and (63.33%) respectively. Application of nitrate solution increase germination by 22.66 and 23.33% at 20°C/30°C and 25°C/35°C alternating temperature treatments compared to control. The interaction effect of alternating temperature with nitrate solution shows there was significant impact than main effects for the germination of Commelina benghalensis L. weed seeds. Germination was highly hindered at 10°C/20°C and 5°C/15°C. The lowest germination of (1.33% and 0%) was recorded at 5°C/15°C with and without nitrate solution.
The possible reason, for highest germination of Commelina benghalensis L. at highest temperature with nitrate might due to effect of high temperature on seed dormancy breaking and the effect of nitrate on stimulating seed germination. The result was in accordance with who reported that greater than 70% of Commelina benghalensis L. seed germinated best at temperature of 30°C.
Effectiveness of nitrate on Commelina benghalensis L. weed germination in the filed was reported by the author said that Germination response of Commelina benghalensis L. in field were high with application of 130 kg N ha-1-170 kg N ha-1 compared to control treatment. The effect of nitrate containing compound on breaking weed seed dormancy were also reported by who reported that Nitrogen containing compounds are considered to be effective in breaking seed dormancy and inducing seed germination of Chenopodium album L. Similarly reported that Potassium nitrate improved germination of Myagrum perfoliatum a broad leaved weed by 71% compared to control 2.9% (Table 2).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate 10 mM | 1.33 | 6.66 | 21.33 | 64.66 | 63.33 | 31.467 |
| No nitrate | 0 | 8.66 | 19.33 | 42 | 40.66 | 22.133 |
| Mean | 0.66 | 7.667 | 20.33 | 53.33 | 52.00 | |
| LSD (0.05) | 2.062 | |||||
| CV (%) | 4.56 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of variation; ns=non-significant | ||||||
Table 2: Interaction effects of alternating temperatures and nitrate on Commelina benghalensis L. germination.
Guzotia scabra Vis. Chiov: Germination percentage of Guzotia scabra Vis. Chiov was highly significantly (p<0.001) affected by main effect of temperature, nitrate and the interaction of both factors Appendix Table 5. Maximum germination percentage (79.33%) was recorded at 20°C/30°C with nitrate solution. At 15°C/25°C alternating temperature 74.66% germination percentage was recorded with the application of nitrate solution, which was significantly higher than the germination recorded in control treatment at similar temperature. The germination of Guzotia scabra Vis. Chiov decreased at 25°C/35°C, 10°C/20°C and 5°C/15°C alternating temperature regime.
The lowest germination percentage 8% and 8.66% was recorded at 5°C/15°C with and without nitrate. The lowest germination percentage at highest and lowest temperature might due to low metabolic and low water imbibition of seed at lower temperature and synthesis of germination inhibiting hormones at higher temperature.
The result was corroborated by who reported that high temperature up regulates ABA biosynthesis genes and down regulate catabolism genes in Leptochloa chinensis weed. The result was in accordance with who reported that maximum germination percentage of Guzotia abyssinica was recorded at alternating temperature of 20°C/30°C. Similarly reported that the interaction of temperature and potassium nitrate highly significantly affects germination of broad leaved weed seeds germination (Table 3).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 8 | 44 | 74.66 | 79.33 | 52.66 | 51.73 |
| No nitrate | 8.66 | 41.33 | 61.33 | 66.66 | 51.33 | 45.86 |
| Mean | 8.33 | 42.66 | 68.00 | 73 | 52.00 | |
| LSD (0.05) | 1.75 | |||||
| CV (%) | 2.1 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 3: Interaction effect of alternating temperature and nitrate on Guzotia scabra Vis. Chiov germination.
Mimosa invisa L.: Analysis of variance showed that germination percentage of Mimosa invisa L. was highly significantly (p<0.001) affected by main effect of temperature. However both main effect of nitrate and their interaction was not significant Appendix Table 6. The highest germination percentage (78%) was recorded at 25°C/35°C, which was suggestively better than germination recorded at 20°C/30°C, 15°C/25°C, 10°C/20°C and 5°C/15°C. The lowest germination (7.66%) was recorded at 5°C/15°C. But germination recorded with and without nitrate was not significantly different in all level of temperature Appendix Table 7. The maximum germination percentage at the highest temperature treatment may indicate that Mimosa invisa L. seed require higher temperature to germinate timely.
The result was similar to who demonstrated that high temperature can produce cracks on the seed coat which in turn can increase seed germination. Similarly reported that when non-scarified seed of Mimosa invisa L. were exposed to high temperatures for 5 minutes, germination increased as temperature increased from 25°C to 120°C but declined to zero at 200°C. also reported that 88% and 100% germination of two mimosa species Mimosa aculeaticarpa and Mimosa luisana recorded at 30°C and 25°C, respectively.
Non-significance of nitrate to Mimosa invisa L. weed seed germination may due to difference in seed size among species.
Similarly reported that large seeds have many advantages over small seeds and usually have greater percent germination. Possibly, the variations found for Mimosa invisa weed seeds germination are more closely related to the temperature reduction. It is important to note the temperature is one of the environmental factors which more limit the seed germination due to imbibition and metabolic processes, determining the range and germination capacity and interfering on the water absorption speed on the biochemist reactions (Table 4).
| Temperature(°C) | Germination percentage |
|---|---|
| 5/15 | 7.667 |
| 10/20 | 25.667 |
| 15/25 | 37.667 |
| 20/30 | 68.667 |
| 25/35 | 78 |
| LSD | 2.55 |
| Nitrate (N) | |
| 0 | 43.33 |
| 10mm | 43.6 |
| LSD | ns |
| TE | <0.0001 |
| N | ns |
| TE*N | ns |
| CV | 4.48 |
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns= non-significant | |
Table 4: Effect of alternating temperature and nitrate on Mimosa invisa L. germination.
Parthenium hysterophorus L.: Analysis of variance indicated that Germination of Parthenium hysterophorus L. was highly significantly (P<0.0001) affected by main effect of temperature, nitrate and interaction of two factors Appendix Table 8. Highest germination of 80.66% and 79.33% recorded at 15°C/25°C and 20°C/30°C with 10 mM KNO3 with no significant difference between two mean. Germination of Parthenium hysterophorus L. significantly decreased at lower and higher temperature. But still highest germination of (67.33% and 63.33%) was recorded at 25°C/35°C with and without nitrate. But lowest germination of 34% germination were recorded at 5°C/15°C without nitrate. Germination recorded at four level of alternating temperature with and without nitrate in Parthenium hysterophorus L. was more than 50%. More than 50% seed germination at four level of alternating temperature and positive response of nitrate might indicate that the broad adaptability and increasing invasiveness of weed especially in fertile soil which may require continuous management efforts.
According to Tamado, temperature regimes ranging from 12°C/2°C to 35°C/25°C (day and night) were all suitable for the germination of Parthenium hysterophorus L. in Ethiopian seed. However, low temperature in high altitudes (>2500 m) areas might reduce its growth and reproduction as it does not normally occur in these areas. Similarly reported on weed seeds of Emmenanthe penduliflora treated with 10 mM KNO3 under daily light/dark resulted in maximum promotion of germination percentage (Table 5).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 36 | 57.33 | 80.66 | 79.33 | 67.33 | 64.13 |
| No nitrate | 34 | 54.66 | 67.33 | 66.66 | 63.33 | 57.2 |
| Mean | 35 | 56 | 74 | 73 | 65.33 | |
| LSD (0.05) | 1.78 | |||||
| CV (%) | 1.71 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)= Coefficient of Variation; ns=non-significant | ||||||
Table 5: Interaction effect of alternating temperature and nitrate on Parthenium hysterophorus L. germination.
Effects of alternating temperature and nitrate on the dormancy of major weed seeds
Seed dormancy is a common character of many species that favorably help the plants to survive under difficult circumstances.
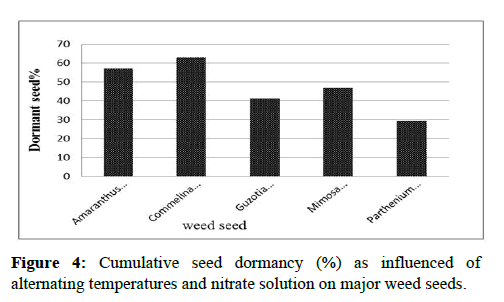
Analysis of variance indicated that Seed dormancy was highly significantly (P<0.001) affected by main effect of temperature, nitrate, species and interaction of three factors. Among weed species highest dormancy was recorded in Commelina benghalensis L. followed by Amaranthus spinosus L. while lower dormancy was recorded in Parthenium hysterophorus L. (Figure 4). Maximum percentage of dormant seed 90.66% and 88.66% was recorded in Commelina benghalensis L. with and without nitrate at 5°C/15°C. Similarly 90.66%and 89.66% in Amaranthus spinosus L. was recorded at lower temperature of 5°C/15°C in control and with nitrate respectively Appendix Table 9.
Level of dormancy varies from species to species but different percent of dormant seed was recorded from all weed species at all treatment. Except Parthenium hystherophorus L. More than 80% seed dormancy was recorded at 5°C/15°C in four species While lowest percentage of dormant seed (10% and 10.66%) recorded in Parthenium hysterophorus L. at 15°C/25°C and 20° C/30°C with nitrate followed by in Guzotia scabra Vis. Chiov (11.33%) at 20°C/30°C with nitrate. In Mimosa invisa also lowest dormancy (12.66%) recorded at 25°C/35°C. Non-stimulatory effect of KNO3 at lower temperature might due to dependency of nitrate on temperature to enhance seed germination reported that, deeply dormant seeds are not responsive to light and nitrate, but as deep dormancy is relieved sensitivity and responses to different signals (nitrate and light) occur progressively in Appendix Table 10. Lower dormancy of weed at their optimum temperature with nitrate might due to role of temperature on breaking seed dormancy and additive role of nitrate on relieving dormancy by increasing synthesis of gibberellin hormone. The result was in accordance with who reported that the depth of seed dormancy was inversely correlated to seed nitrate content.
But all seeds placed at favorable environment might not germinate equally at the same incubation period due to different dormancy level between seed lot and presence of other factor such differences in germination behavior between seeds from an individual plant may be due to variations in the microenvironment experienced by seeds in different parts of an inflorescence further added that germination events may not necessarily lead to emergence of the primary root (radicule) and germination. When conditions are apparently favorable for germination events such as imbibition, respiration, nucleic acid and protein synthesis as well as a host of other metabolic events may proceed while cell elongation does not occur. This failure in cell elongation and radicle protrusion is due to germination inhibitors within the seed that prevent seed from germinating. Higher dormancy of weed seed at undesirable temperature might indicate seed may stay dormant for long period until environment is suitable for germination (Figure 4).
Amranthus spinosus L.: Analysis of variance indicated that Amranthus spinosus L. seed dormancy was highly significantly (P<0.001) affected by temperature, nitrate and interaction of two factors. Highest dormancy of Amranthus spinosus L. was recorded at two lower temperature levels. Maximum dormancy of (90% and 90.66%) at 5°C/15°C and (86.66% and 87.33%) at 10°C/20°C was recorded with and without nitrate. Dormancy decrease with increase temperature from 10/20°C to higher temperature. Lowest dormancy of 16% followed by 19.33% was recorded at 20°C/30°C and 25°C/35°C with 10 mM KNO3 (Table 6). This may due to that nitrate may weaken seed dormancy if seed is exposed to suitable temperature. The result was in line with that of who reported that temperature influences the induction and relief of dormancy in Appendix Table 11.
| Temperature (°C) | ||||||
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 90 | 86.66 | 56.66 | 16 | 19.3 | 53.73 |
| No nitrate | 90.66 | 87.33 | 58.66 | 34 | 32 | 60.533 |
| Mean | 90.333 | 87 | 57.66 | 25 | 25.667 | |
| LSD (0.05) | 2.63 | |||||
| CV (%) | 2.85 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 6: Interaction effect of alternating temperature and nitrate on Amranthus spinosus L dormancy.
Commelina benghalensis L.: Analysis of variance indicated that Commelina benghalensis L. seed dormancy was highly significantly (P<0.001) affected by main effect of temperature, nitrate and the interaction of two factors. Highest dormancy of (88.66% and 90.66%) was recorded at 5°C/15°C followed by (83.33% and 81.33%) at 10° C/20°C with and without nitrate respectively (Table 7). While lower dormancy (25.33% and 26%) was recorded at 20°C/30°C and 25°C/35°C with nitrate compared to control (47.33% and 48.66%) recorded at 20°C/30°C and 25°C/35°C nitrate. this might due to effectiveness of higher temperature and stimulatory effect of 10 mM KNO3 to break seed dormancy. The result was in accordance with who reported that Increase of minimum temperature increase seed dormancy while dormancy alleviation occurs through widening of the thermal range with a decrease in minimum temperature required for germination (Appendix Table 12).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 88.66 | 83.33 | 68.66 | 25.33 | 26 | 58.4 |
| No nitrate | 90.66 | 81.33 | 71.33 | 47.33 | 48.66 | 67.86 |
| Mean | 89.667 | 82.333 | 68.333 | 34.667 | 37.33 | |
| LSD (0.05) | 2.06 | |||||
| CV (%) | 2.1 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation ns=non-significant | ||||||
Table 7: Interaction effect of alternating temperature and nitrate on Commelina benghalensis L. dormancy.
Guzotia scabra Vis. Chiov: Guzotia scabra Vis. Chiov weed seed dormancy was highly significantly (P<0.001) affected by main effect of temperature, nitrate and interaction of two factors. Highest percentage of (82%) dormant seed was recorded at lowest temperature at 5°C/15°C with and without nitrate. Dormancy in Guzotia scabra Vis. Chiov decrease with increase in temperature with and without nitrate but dormancy recorded with nitrate was lower than control at higher temperature. Lowest number of dormant seed (11.33%) was recorded at 20°C/30°C with nitrate followed by (14.66%) at 15°C/25°C with nitrate. Dormancy at higher temperature increased to some extent. At 25°C/35°C 37.33% and 38% dormancy recorded with and without nitrate respectively with no significant difference between two mean (Table 8).
The highest dormancy at lower and higher temperature might due to low seed water imbibition and metabolic activity of seed at lower temperature and inhibition of germination regulating hormone at higher temperature. But dormancy at higher temperature was less affected than at lower temperature. The significant effect of nitrate may due to role of nitrate on breaking seed dormancy by increasing metabolic activity and synthesis of geberline hormone. The result was in accordance with who reported that the depth of seed dormancy was inversely correlated to seed nitrate content.
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 82 | 45.3 | 14.66 | 11.33 | 37.33 | 38.13 |
| No nitrate | 82 | 49.33 | 28.00 | 23.33 | 38 | 44.13 |
| Mean | 82 | 47.33 | 21.33 | 17.33 | 37.66 | |
| LSD (0.05) | 2.21 | |||||
| CV (%) | 3.15 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 8: Interaction effect of alternating temperature and nitrate on Guzotia scabra Vis. Chiov dormancy.
Mimosa invisa L.: Analysis of variance indicated that seed dormancy of Mimosa invisa L. was highly significantly (P<0.001) affected by main effect of temperature. However main effect of nitrate and interaction of both factors was not significant. Seed dormancy of Mimosa invisa L. increase with decrease incubation temperature from 25°C/35°C to 5°C/15°C. Maximum dormant seed (83%) was recorded at 5°C/15°C. While lower dormant seed (12.66%) was recorded at 25/35°C (Table 9). The result was in line with that of who reported that seasonal dormancy pattern of Sisymbrium officinale was simulated on the basis of the dual role of temperature, on the one hand regulating dormancy and on the other hand, affecting germination in Appendix Table 13.
| Temperature (°C) | DO |
|---|---|
| 5/15 | 83.000 |
| 10/20 | 64.333 |
| 15/25 | 53.000 |
| 20/30 | 21.667 |
| 25/35 | 12.667 |
| LSD | 3.02 |
| Nitrate (N) | |
| 0 | 47.067 |
| 10mm | 46.800 |
| LSD | ns |
| TE | <0.001 |
| N | ns |
| TE*N | ns |
| CV | 5.32 |
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation;ns=non-significant | |
Table 9: Effect of alternating temperature and nitrate on Mimosa invisa L. dormancy (in %).
Parthenium hysterophorus L.: Analysis of variance indicated that Seed dormancy in Parthenium hysterophorus L. was highly significantly (P<0.001) affected by main effect of temperature, nitrate and interaction of two factors highest percentage of dormant seed (56%) was recorded at incubation temperature at 5°C/15°C without nitrate followed by (54%) at 5°C/15°C with nitrate solution. Nitrate inhibited seed dormancy at all level of temperature but more reduced at 15°C/25°C and 20°C/30°C and lowest dormancy (10% and 10.66%) was recorded at two temperature levels with nitrate respectively. While dormancy recorded without nitrate was 24% and 22.66% at 15°C/25°C and 20°C/30°C incubation temperature (Table 10).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 54 | 32 | 10 | 10.66 | 22.66 | 25.86 |
| No nitrate | 56 | 35.3 | 24 | 22.66 | 26.66 | 32.9 |
| Mean | 55 | 33.66 | 17 | 16.66 | 24.66 | |
| LSD (0.05) | 2.69 | |||||
| CV (%) | 5.35 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 10: Interaction effect of alternating temperature and nitrate on Parthenium hysterophorus L. dormancy.
Effects of alternating temperature and nitrate on the major weed seeds decay
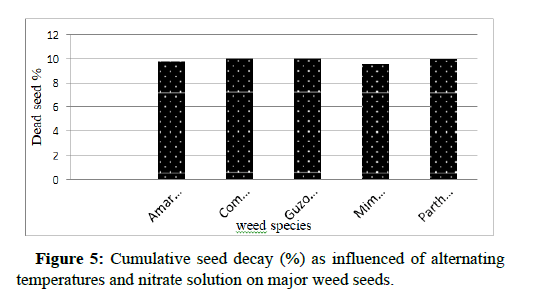
Analysis of variance indicated that seed decay of all weed species was not significantly affected by temperature, nitrate and interaction of two factors. Dead seed percentage recorded in all species at different level of temperature treated with and without nitrate was less than 10.2% and was not statistically different (Figure 5). Lowest percentage and non-significance of weed seed decay might indicate high viability and persistence of weed seed at undesirable environment (Tables 11-15).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 15-May | 20-Oct | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 10 | 10 | 10 | 10 | 10 | 9.866 |
| No nitrate | 9.33 | 9.33 | 10 | 9.33 | 10 | 9.733 |
| Mean | 9.66 | 9.66 | 10 | 9.66 | 10 | |
| LSD (0.05) | ns | |||||
| CV (%) | 6.8 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 11: Interaction effect of alternating temperature and nitrate on Amranthus spinosus L. decay.
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 10.00 | 10.00 | 10.00 | 10.66 | 10.66 | 10.13 |
| No nitrate | 9.33 | 10.00 | 9.33 | 10.00 | 10.66 | 10.00 |
| Mean | 9.667 | 10.00 | 96.667 | 10.333 | 10.667 | |
| LSD (0.05) | ns | |||||
| CV (%) | 8.2 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 12: Interaction effect of alternating temperature and nitrate on Commelina benghalensis L. decay (in %).
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 10.00 | 10.66 | 10.667 | 9.33 | 10.00 | 10.133 |
| No nitrate | 9.33 | 9.33 | 10.667 | 10.00 | 10.667 | 10 |
| Mean | 9.66 | 10.00 | 10.667 | 9.667 | 10.333 | |
| LSD (0.05) | ns | |||||
| CV (%) | 9.13 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 13: Interaction effect of alternating temperature and nitrate on Guzotia scabra Vis. Chiov decay.
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 9.33 | 10.00 | 9.33 | 9.33 | 10.00 | 9.6 |
| No nitrate | 9.33 | 10.00 | 9.33 | 10.00 | 9.33 | 9.6 |
| Mean | 9.33 | 10.00 | 9.33 | 9.667 | 9.667 | |
| LSD (0.05) | ns | |||||
| C V (%) | 9.57 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 14: Interaction effect of alternating temperature and nitrate on Mimosa invisa L. decay.
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Nitrate level | 5/15 | 10/20 | 15/25 | 20/30 | 25/35 | Mean |
| Nitrate10 mM | 10.00 | 10.66 | 10.00 | 10.00 | 10.00 | 10.33 |
| No nitrate | 10.00 | 10.00 | 8.66 | 10.00 | 10.00 | 9.86 |
| Mean | 10.00 | 10.33 | 9.33 | 10.00 | 10.00 | |
| LSD (0.05) | ns | |||||
| CV (%) | 9.43 | |||||
| Note: Means in column followed by the same letters are not significantly different at 5% level of significant; LSD (5%)=Least Significant Difference at CV (%)=Coefficient of Variation; ns=non-significant | ||||||
Table 15: Interaction effect of alternating temperature and nitrate on Parthenium hysterophorus L. decay.
Conclusion
Weeds are one of yield reducing factor which compete with crop for nutrient, water and surface. In Bench Sheko Zone different weed species were recorded in arable field. The range of yield reduction due to crop weed competition depends on type and nature of weeds infested in crop lands.
Five major weed species which highly infested in Bench Sheko Zone are problematic due to their unique property. Their prolific nature leads continuous accumulation of seed in the soil seed bank which allows infestation of weed and reduction of yield year to year. Their extensive root system increase competition for growth limiting resource with crop species. In addition, allele chemical production through falling leaf and root exudate and Creating of root at stem nod and reemergence of weed after weeding is another property of weed species which hinder germination and growth of crop. All this property makes those weed species as dominant abundant and problematic weeds in crop growing area in Bench Sheko Zone.
However, variations in their emergency period and germination requirement makes difficult to control those weed species. Seeds in the soil represent the passive weed population that remain viable for extended periods of time and able to re-infest agricultural lands. Without understanding critical time of emergency it is difficult to control those weeds and results continues reduction of quality and yield of crops year to year. Understanding weed biology especially germination period and germination requirement is important to increase those weed management options.
Keeping this in view laboratory experiment was conducted to determine the optimum temperature in which major weeds germinate and to evaluate effect of nitrate on major weed seed germination. Seeds of the five weeds species were exposed to five temperature regimes viz. 5°C/15°C, 10°C/20°C, 15°C/ 25°C, 20°C/ 30°C and 25°C/35°C for 16 hours followed by for 8 hours. Germination performance of the weed species was determined with and without nitrate (0 mM and 10 mM) solution. Weed was verified as germinated when the radicle or hypocotyl evidently projected over the seed coat. Weed seeds were punctured with a dissecting needle and placed on blotters moistened with 0.1% tetrazolium solution when they are recognized as ungerminated. Treatment were laid out using complete randomized design and replicated three times.
The result showed even though level of germination was not constant in all species, germination responses was associated with combined nitrate and different level of temperature. Germination of weed species increase with increase in temperature and nitrate increased germination of all weed species except Mimosa. Among weed species highest germination was recorded in Parthenium hysterophorus L. while lowest germination was recorded in Commelina benghalensis L. Highest germination recorded in Parthenium hysterophorus L. at 15/25°C and 20/30vC with nitrate followed by Guzotia scabra Vis. Chiov at 20/30°C with nitrate. In Mimosa invisa L. highest germination was recorded at 25/35°C regardless of nitrate. Amranthus spinosus Lalso germinated highly at 20/30°C and 25/35°C with nitrate. The lowest germination among weed species was recorded in Commelina benghalensis L. at different level of temperature. But nitrate promoted germination of Commelina benghalensis L. at 20/30°C and 25/35°C.
Germination of all weed species was lowest at lower temperature regardless of nitrate. But Parthenium hysterophorus L. was less affected at wide range of temperature and has more than 50% germination at four temperature level with and without nitrate. This might indicate germination ability of weed in different season and it might require continues management efforts. Optimum temperature for germination of weed species is 20/30°C.Which mimic with diurnal temperature fluctuation of spring in Bench Sheko Zone.
In contrast to seed germination seed dormancy was highest for all weed species at lowest temperature with and without nitrate. Among weed species maximum dormancy more than 88% was recorded in Amranthus spinosus L and Commelina benghalensis L. at 5/15°C regardless of nitrate. Maximum dormancy recorded in Parthenium hysterophorus L. was 56% at 5/15°C without nitrate. Which was lowest dormancy than dormancy recorded in other weed species at similar temperature 5/15°C. Dormancy of all species was lowest at their optimal temperature with nitrate except Mimosa. The lowest dormant seed among weed species was recorded in Parthenium hysterophorus L. at 15/25°C and 20/30°C with nitrate followed by Guzotia scabra Vis. Chiov at 20/30°C with nitrate. Lowest dormant seed in Mimosa invisa L. was recorded at 25/35°C regardless of nitrate. Amranthus spinosus and Commelina benghalensis L. has lowest dormant seed at 20/30°C and 25/35°C with nitrate. The stimulatory effect of nitrate and emergency of weeds was higher when seed placed at suitable environment. But seed decay was not significantly affected by temperature and nitrate solution. This might indicate persistence and high viability of seed at different fluctuating temperature and deep dormancy of seed at undesirable temperature.
With respect to seed germination in the natural environment, a reduction in seed dormancy as a consequence of exposure to higher temperature and nitrate may have an important influence on when and where, seed germination will occur. Determining date of seed germination and their emergence is important for future crop-weed competition and the ability of the weed to replenish the seed bank. With this in mind, the ability of this weed species to germinate in response to temperature and nitrate is consistent except mimosa.
In conclusion it can be said that results from this study showed that different control methods might be needed for different weed species at time of their emergency. Because fields are most often infested with several weed species, knowledge with regard to germination requirements obtained in this study will certainly help to improve weed control in field crops in Bench Sheko Zone and other place with similar agro ecologies. Practice applying different control measure manipulating soil to bring seed to the soil surface to improve their germination and killing at seedling, applying herbicide chemical, Practices that avoid large pulses of soluble N early in crop development, such as delayed or split N applications or use of slow releasing N sources, such as mature compost, can delay weed emergence and reduce weed density in the crop.
Many studies dealing with the effect of temperature on germination of weed seeds have been conducted, but literature on the effect of temperature and nitrate in regulating seed germination and breaking seed dormancy of tested weed species is poorly understood. A detailed knowledge regarding the environmental conditions required for weed seed germination is an important prerequisite for the development of integrated and biological weed control strategies so more research work is needed to clear the existing ambiguities.
Acknowledgement
We thank mizan Tepi University for facilitating and providing necessary materials during conducting research. We also acknowledge the staff members, especially laboratory technicians at mizan Tepi University for their help by providing required laboratory material.
References
- Chauhan BS, Manalil S, Florentine S, Jha P (2018) Germination ecology of Chloris truncata and its implication for weed management. Weed Res 14: 95-102. [Crossref][Googlescholar][Indexed]
- Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14: 1-16. [Googlescholar]
- Gardarin A, Durr C, Colbach N (2011) Prediction of germination rates of weed species: Relationships between germination speed parameters and species traits. Ecol Model 222: 626-636. [Crossref][Googlescholar]
- Chauhan BS, Johnson DE (2009) Germination ecology of spiny (Amaranthus spinosus) and slender Amaranth (A. viridis): Troublesome weeds of direct seeded rice. Weed Sci 57: 379-385. [Googlescholar]
- Ali HH, Tanveer A, Nadeem MA, Asghar HN, Javaid MM, et al. (2013) Germination ecology of Rhynchosia capitata: An emerging summer weed in Asia. Planta Daninha 31: 249-257. [Googlescholar]
- Radosevich SR, Holt JS, Ghersa CM (1996) Weed ecology implications for management. 2nd edition, John Wiley, USA.
- Batlla D, Luis Benech-Arnold R (2007) Predicting changes in dormancy level in weed seed soil banks: Implications for weed management. Crop Prot 26: 189-197. [Crossref][Googlescholar]
- Hills PN, Van Staden J (2003) Thermoinhibition of seed germination. S Afr J Bot 69: 455-461. [Crossref][Googlescholar]
- Bewley JD, Black M (1985) Seeds: Physiology of development and germination, Plant Cell Environment, Wiley Online Library, USA, pp. 356.
- Shim SI, Moon JC, Jang CS, Raymer P, Kim W, et al. (2008) Effect of potassium nitrate priming on seed germination of sea shore Paspalum. Hort Science 43: 2259-2262. [Googlescholar]
- Booth BD, Murphy SD, Swanton CJ (2003) From seed to seedling. Weed ecology in natural and agricultural systems, CABI Publishing, Wallingford, UK, pp. 81-99.
- Khan MA, Shaheen K, Ali HH, Bakhtiar G, Ali R, et al. (2019) Effect of environmental factors on the germination and growth of Parthenium hysterophorus and Rumex crispus. Pak J Bot 51: 2195-2202. [Googlescholar]
- Loddo D, Sousa E, Masin R, Calha IM, Zanin G, et al. (2014) Germination response of local Southern European populations of Datura stramonium at a range of constant temperatures. Weed Res 54: 356-365. [Crossref][Googlescholar]
- Chauhan BS (2012) Weed ecology and weed management strategies for dry seeded rice in Asia. Weed Technol 26: 1-3. [Googlescholar]
- Masin R, Vasileiadis VP, Loddo D, Otto S, Zanin G, et al. (2011) A single time survey method to predict the daily weed density for weed control decision making. Weed Sci 59: 270-275. [Googlescholar]
- Steckel LE, Sprague CL, Stoller EW, Wax LM (2004) Temperature effects on germination of nine Amaranthus species. Weed Sci 52: 217-221.[Googlescholar]
- Kumari P, Sahu PK, Soni MY, Awasthi P (2014) Impact of Parthenium hysterophorus L. invasion on species diversity of cultivated fields of Bilaspur (CG) India. Agricul Sci 5: 1-11. [Googlescholar]
- Webster TM, Burton MG, Culpepper AS, York AC, Prostko EP, et al. (2005) Tropical spiderwort (Commelina benghalensis): A tropical invader threatens agroecosystems of the southern United States. Weed Technol 19: 501-508. [Googlescholar]
- Mulatu W (2011) An invasive alien weed giant sensitive plant (Mimosa diplotricha sauvalle) invading Southwestern Ethiopia. Afr J Agric Res 6: 127-131.[Googlescholar]
- Navie SC, Panetta FD, McFadyen RE, Adkins SW (1998) Behaviour of buried and surface-sown seeds of Parthenium hysterophorus. Weed Res 38: 335-341.[Googlescholar]
Citation: Muluneh G, Tsadik MG (2023) Germination of Major Weed Seeds in Response to Temperature and Nitrogen in Bench-Sheko Zone, South Western Ethiopia. Adv Crop Sci Tech 11:554
Copyright: © 2023 Muluneh G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1447
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1097
- PDF downloads: 350