Research Article Open Access
Geochemical Appraisal of Fluoride Laden Groundwater in Suri I and II Blocks, Birbhum District, West Bengal
Shreya Das and SK Nag*
Department of Geological Sciences, Jadavpur University, Kolkata-700032, India
- *Corresponding Author:
- Nag SK
Department of Geological Sciences
Jadavpur University
Kolkata-700032, India
Tel: 033-2434 9446; 09433919962
E-mail: SisirKNag@gmail.com
Received date: February 25, 2016; Accepted date: May 19, 2016; Published date: May 22, 2016
Citation: Das S, Nag SK (2016) Geochemical Appraisal of Fluoride – Laden Groundwater in Suri I and II Blocks, Birbhum District, West Bengal. J Earth Sci Clim Change. 7:358. doi:10.4172/2157-7617.1000358
Copyright: © 2016 Das S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
The present study has been carried out covering two blocks – Suri I and II in Birbhum district, West Bengal, India. The evaluation focuses on occurrence, distribution and geochemistry in twenty six water samples collected from bore wells spread across the entire study area homogeneously. Quantitative chemical analysis of groundwater samples collected from the present study area have shown that samples from two locations – Gangta and Dhalla contain fluoride greater than the permissible limit prescribed by WHO during both post monsoon and pre monsoon sampling sessions. Significant factor controlling geochemistry of groundwater has been identified to be rock – water interaction processes during both sampling sessions based on the results of Gibb’s Diagrams. Geochemical modeling studies have revealed that fluorite (CaF2) is indeed present as a significant fluoride bearing mineral in groundwaters of this study area. Calcite or CaCO3 is one of the most common mineral with which fluorite remains associated and saturation index calculations have revealed that the calcite – fluorite geochemistry is the dominant factor controlling fluoride concentration in this area during both post and pre monsoon. High fluoride waters have also been found to be of ‘bicarbonate’ type showing increase of sodium in water with decrease of calcium.
Keywords
Groundwater; Fluoride; Geochemistry; Suri
Introduction
Groundwater contamination resulting from presence of high fluoride in groundwater has been encountered in many parts of the world [1,2]. High fluoride in groundwater is usually associated with arid climate conditions where water movement is slow. All over the world more than 260 million people consume water containing more than 1.0 mg/l of fluoride and the majority of these people are based in tropical countries experiencing arid to humid climate type. The World Health Organization has set the permissible limit of fluoride in drinking water at 1.5 mg/l [3]. Though presence of limited amount of fluoride (0.6 – 1.0 mg/l) in drinking water is necessary to prevent dental caries, but excess fluoride consumption ironically leads to fluorosis, which is of two types: dental and skeletal [4-6]. Fluorosis, which is the major irreparable physiological damage caused due to excess consumption of fluoride is spreading at a slow but steady rate [7-9]. Fluorosis is prevalent in some parts of central and western China and caused not only by drinking fluoride in groundwater but also by breathing airborne fluoride released from the burning of fluorideladen coal. Worldwide, such instances of industrial fluorosis are on the rise [10,11]. One of the best-known high fluoride belts on land extends along the East African Rift from Eritrea to Malawi and there is another belt from Turkey through Iraq, Iran, Afghanistan, India, northern Thailand and China [12]. Undesirable amount of fluoride in groundwater i.e. greater than 1.5 mg/l was first reported in the Nellore district in Andhra Pradesh, India in the year 1937 [13]. In 1991 groundwater in 13 states of India including West Bengal, were reported to contain greater than 1.5 mg/l of fluoride [14]. By 1999, the number went up to 17 states, including West Bengal, are reported to have endemic fluorosis [10,11]. All of these states have reportedly encountered endemic fluorosis at various levels [15-17]. Fluoride concentration is also correlated with depth of the water table and increases with increase in depth of the corresponding aquifer [1]. In this chapter, the occurrence, distribution, origin and geochemistry of fluoride in groundwater has been discussed (Table 1).
| Location No. | Location Name | Fluoride (F¯) in mg/l | |
|---|---|---|---|
| Post Monsoon | Pre Monsoon | ||
| AL1 | Abdarpur | 0.26 | 0.31 |
| AL2 | Singur | 0.25 | 0.29 |
| AL3 | Kochujor Primary School | 0.65 | 0.83 |
| AL4 | Lalmohanpur Primary School | 0.63 | 0.79 |
| AL5 | Bonsonka Primary School | 0.81 | 1.02 |
| AL6 | Talibpur High School | 0.40 | 0.47 |
| AL7 | Kubirpur Primary School | 0.30 | 0.31 |
| AL8 | Abinashpur Hospital (Sultanpur) | 0.27 | 0.31 |
| AL9 | Piasala More | 0.49 | 0.56 |
| AL10 | Purandarpur | 0.29 | 0.38 |
| AL11 | Gangta (Beside Mandir) | 1.66 | 2.02 |
| AL12 | Majhigram | 0.79 | 0.91 |
| AL13 | Bhaganbati Primary School | 0.27 | 0.27 |
| AL14 | Dhalla | 2.38 | 2.84 |
| AL15 | Saktipur Primary School | 0.78 | 0.97 |
| AL16 | Ajaypur | 0.25 | 0.28 |
| AL17 | Joka Primary School | 0.40 | 0.45 |
| AL18 | Khatangadi | 0.31 | 0.35 |
| AL19 | Kendulia | 0.22 | 0.24 |
| AL20 | Lataboni Primary School | 0.47 | 0.83 |
| AL21 | Nabagram Primary School | 0.97 | 1.26 |
| AL22 | AamgachiUdayanPathsala | 0.47 | 0.56 |
| AL23 | Gobindopur Unique Club | 1.13 | 1.01 |
| AL24 | Agar | 0.41 | 0.48 |
| AL25 | Ekdala More | 0.56 | 0.71 |
| AL26 | Suri Town | 0.21 | 0.56 |
Table 1: Quantitative chemical analysis results for fluoride in groundwater.
Fluoride occurs both in igneous and metamorphic rocks and the minerals which are part of natural formations and bedrocks of aquifers and contain fluoride are amphiboles, micas, certain clays (illite, chlorite), villiaumite (NaF) [18,19], fluorite (CaF2), fluorapatite, biotite, muscovite (KAl2(AlSi3O10)(F(OH)2), etc. During weathering of these formations and circulation of water around these rocks and soils, fluoride can leach out and dissolve into groundwater and thermal gases. Thus fluoride content of groundwater varies greatly depending on the geological settings and type of rocks. Thus the bedrock mineralogy of aquifers and host rocks most abundant in fluoride minerals can be attributed to be major sources of geogenic fluoride contamination in groundwater [20]. Hydrolysis, dissolution and dissociation reactions that happen over time in natural water systems lead to ion exchange processes which release the fluoride ion into groundwater. Alkaline water systems and minerals containing higher amounts of bicarbonate (HCO3¯) and hydroxyl (OH¯) ions in solution or in solid phase result in presence of free fluoride in water. The fluoride ion is similar to the hydroxyl ion in terms of ionic size and chemical properties and hence can exchange places during displacement reactions in high pH conditions. Besides the natural factors, certain anthropogenic sources which contribute to fluoride in groundwater are mainly the agriculture industry using high doses of phosphatic fertilizers, clay industries and industries using coal for e.g. thermal power plants and brick kilns.
Study Area
The present research work has been carried out in two blocks of the Birbhum District – Suri I and Suri II. Suri is the district headquarter of the Birbhum district and its geographic location is approximately between longitudes 87°25’ E – 87°40’ E and latitudes 23°45’ N – 24°00’ N. The climate of the area during summer is hot and dry with temperatures soaring to 40°C and above, whereas in winter temperatures fall to 10°C or below. The district, on an overall, experiences moderate to high rainfall during the monsoon season. The study area has two main rivers running across its breadth – river Kushkarani in the extreme north and river Bakreshwar in the central and southern parts. River Mayurakshi runs through the Md. Bazaar block and falls just outside the study area lining its northern boundary. The study area is largely comprised of alternating layers of sand and clay, which are soft sediments and part of the Ganga – Kosi formation. Granite gneiss which are hard and foliated type rocks belonging to the Chotanagpur Gneissic complex constitute the north western part of the study area. Hard clays dominate specific parts of the block in the eastern parts of Suri whereas lateritic soils are scattered mainly in the upper parts of Suri. Figure 1 represents the study area map presenting the geology of the area and the sampling location points. Figure 2 presents the distribution of fluoride in groundwater on three levels – global, national and state level.
Methodology
For the present study, two sets of twenty six bore well water samples have been collected in consecutive post monsoon and pre monsoon sessions. For analyzing the samples quantitative chemical analysis methods were adopted in the laboratory. Fluoride ion concentration in water was determined using the ion – selective electrode set – up [19]. Identification of dominant mineral phases and calculation of their saturation indices have been carried out using the USGS Geochemical Model PHREEQC 3.0.3. Simulations have been carried out on two sets of data for each sampling session – one where highest value of fluoride has been recorded and another where the lowest value of fluoride has been recorded. The rest of the interpretations have been made using hydrogeochemical facies in form of the Piper’s Diagram [21,22].
Results and Discussions
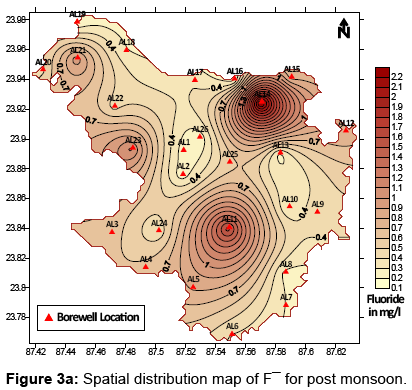
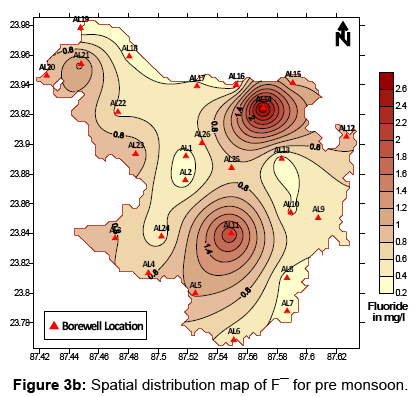
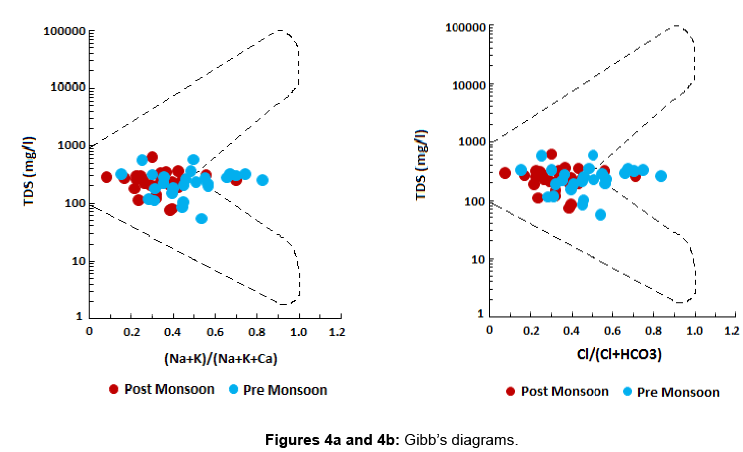
Presence of fluoride in groundwater of a particular area as previously mentioned depends majorly on the geological setting and lithology of the study area. The spatial and temporal distribution pattern of fluoride ion in groundwater of the study area has been presented in Figures 3a (post – monsoon) and 3b (pre – monsoon). A part in the study area where high fluoride has been reported is dominated by hard clays which are known to adsorb the fluoride ion strongly. The F¯ ion replaces the OH¯ ion easily due to their similar ionic radii [23], thus enhancing chances of presence of high fluoride in water circulating in clay dominated regions. Granitic rocks are a typical source of fluoride rich rocks. They have been reportedly found to contain much higher fluoride than any other rock type. Fluoride content in granitic rocks can range anywhere between 500 and 1400 mg/kg [24,25]. The dominant fluoride bearing minerals in these rocks are fluorite [CaF2], villiaumite [NaF], fluoroapatite [Ca5(PO4)43F], biotite [K(Mg,Fe)2(AlSi2O10)(F,OH)2], etc. Whether or not dissolution and leaching of these various minerals into groundwater is a major source of fluoride content in water can be confirmed when the factors controlling hydrogeochemistry of groundwater are explored. Of the many controlling factors – evaporation, precipitation and rock water interaction are some of the preliminary factors. Whichever particular factor controls the overall hydrogeochemistry of an area, can be identified with the help of the Gibb’s Diagram [26]. In Figures 4a and 4b the Gibbs’s diagrams for post and pre monsoon sessions have been presented. From the Gibb’s diagrams it can be clearly interpreted that rock water interaction is a dominant factor affecting the hydrogeochemistry of the study area during post monsoon as well as pre monsoon. Hence, dissolution and leaching of fluoride bearing minerals into groundwater from the rocks lining the aquifers holding groundwater at optimal conditions can be attributed to be a significant source of fluoride ion in groundwater.
The fluorine bearing minerals in granites and metamorphic rocks: fluorite, apatite, fluorapatite, cryolite, micas and amphiboles [27-30] undergo dissociation or displacement reactions to release fluoride into water. Of all the above minerals mentioned, fluorite is the most common fluorine bearing mineral found in granitic terrains [31,32] which is a dominant feature in the chosen area of this research. To evaluate which particular mineral/minerals of the above mentioned ones might be contributing to fluoride levels in groundwater of this area, the use of the geochemical model PHREEQC 3.0.3 was made. The model helps to speciate chemical analysis results entered into its data tab. Simulations done over the raw quantitative data leads to results presenting phases (major minerals) predicted to be present in water and or SI of these phases.
The saturation index values obtained from PHREEQC simulations carried out on two sets of data for each sampling session – one where highest value of fluoride has been recorded and another where the lowest value of fluoride has been recorded, have been presented in Table 2.
| Location No. | Post Monsoon | Pre Monsoon | ||
|---|---|---|---|---|
| SI fluorite | SI calcite | SI fluorite | SI calcite | |
| AL1 | -2.77 | -0.01 | -2.96 | -0.97 |
| AL2 | -2.65 | -0.23 | -2.69 | -0.36 |
| AL3 | -1.26 | 1.32 | -1.37 | 0.54 |
| AL4 | -1.73 | 2.12 | -1.77 | 1.23 |
| AL5 | -1.39 | 1.03 | -1.64 | 1.12 |
| AL6 | -1.99 | 1.22 | -2.35 | 0.29 |
| AL7 | -2.49 | 1.82 | -2.52 | 0.39 |
| AL8 | -2.43 | 1.83 | -2.42 | 1.33 |
| AL9 | -2.38 | 2.15 | -2.16 | 0.39 |
| AL10 | -2.03 | 1.2 | -2.16 | 0.45 |
| AL11 | -1.04 | 2.05 | -0.79 | 1.83 |
| AL12 | -1.51 | 1.36 | -1.17 | 1.16 |
| AL13 | -2.13 | 0.99 | -2.16 | 0.54 |
| AL14 | -1.34 | 1.86 | -1.06 | 1.23 |
| AL15 | -1.53 | 0.8 | -1.52 | 1.57 |
| AL16 | -2.52 | 0.6 | -2.48 | 0.38 |
| AL17 | -2.59 | 2.11 | -2.28 | 1.24 |
| AL18 | -2.28 | 1.69 | -1.38 | 1.29 |
| AL19 | -2.79 | 2.35 | -2.51 | 0.49 |
| AL20 | -1.86 | 1.69 | -1.52 | 0.38 |
| AL21 | -1.3 | 0.91 | -1.35 | 0.29 |
| AL22 | -2.04 | 0.67 | -1.95 | 0.2 |
| AL23 | -1.33 | 1.73 | -1.57 | -0.15 |
| AL24 | -2.05 | 2.03 | -2.27 | -0.04 |
| AL25 | -2.07 | 2.32 | -1.89 | 0.57 |
| AL26 | -2.52 | 0.82 | -1.74 | -0.03 |
Table 2: Saturation index values of fluorite and calcite.
The common mineral bearing fluoride during both sampling sessions has been found to be fluorite (CaF2). Fluoride ion takes relatively long time to leach out into groundwater due to its low solubility. In such conditions its occurrence is predominantly controlled by free calcium ions (Ca2+) sourced into groundwater majorly from the common mineral, calcite (CaCO3) (Jacks et al.). The dissociation reactions and solubility products of fluorite (equations 1 & 3) and calcite (equations 2 & 4) can be demonstrated by the following equations [27]:
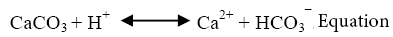 (1)
(1)
Where, the equilibrium constant K calcite(K1) = {[Ca2+] [HCO3¯]}/ [H+]…. Equation (2)
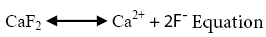 (3)
(3)
Where, the equilibrium constant K fluorite(K2) = {[Ca2+] [F¯]2 (4)
Coupling the above equations Handa formulated equation 5 as follows:
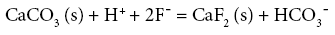 (5)
(5)
A ratio of the two equilibrium constants can be presented as:
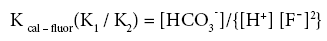 (6)
(6)
From the above formulations it can be interpreted that at a homogeneous pH range, (defined by the H+ ion concentration) where water is of bicarbonate type, fluoride concentration in water tends to rise with a dip in the calcium concentration.
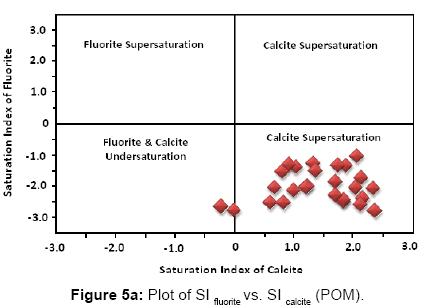
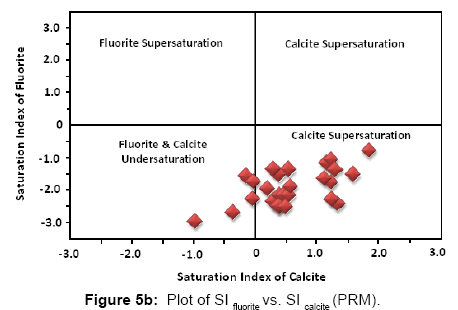
As according to the geochemical model used fluorite has been found to be the dominant fluoride bearing mineral in groundwater, hence the saturation indices of fluorite and calcite have been calculated for each sample collected over both the sampling sessions. Figures 5a and 5b below present the graphical distribution of SI values of fluorite and calcite. Saturation index (S.I.) (equation 7) of a chemical compound is calculated using the following standard formula:
S.I. = Log10 Q/K (7)
Where: Q is the ionic activity product of a mineral, in this case - fluorite or calcite;
K is the equilibrium constant of the mineral.
The interaction of a mineral with water (precipitation or dissolution of ions into water) can be explained based on its saturation index value [33-35]. SI values define the following phases:
When SI > 0, the mineral lies in super saturated domain (precipitates from the aqueous medium)
When SI = 0, the mineral is in equilibrium with the aqueous medium
When SI < 0, the mineral lies in under saturated domain (dissolves into aqueous medium)
According to the SI values majority of the samples of the study area are under saturated w.r.t. fluorite and super saturated w.r.t. calcite during both post monsoon (Figure 5a) and pre monsoon (Figure 5b). Generally groundwater is under – saturated with respect to fluorite but in some cases they are saturated or over saturated with respect to both fluorite and calcite [36,37]. Cases where both calcite and fluorite saturation occurs in groundwater with high fluoride have also been reported previously (Brindha and Elango). At a couple of locations calcite lies in the under – saturated zone during both sampling sessions. If relative increase of fluoride in groundwater is observed even when calcite mineral lies in the under – saturated domain, it might be due to the Ion exchange phenomenon (where calcium (Ca) or magnesium (Mg) in groundwater is exchanged with sodium (Na) or potassium (K) in the aquifer material). Chloro – alkaline indices (CA1 and CA2, defined by equations 8 and 9), commonly known as indices of Base Exchange [38,39] are used as indicators to determine whether ion exchange or reverse ion exchange takes place in groundwater.
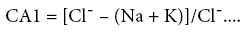 (8)
(8)
 (9)
(9)
Where, concentrations of all ions have been expressed in meq/l.
When there is an exchange between Na and/or K in groundwater with Mg and/or Ca in the aquifer material, both of the indices are positive, indicating reverse ion exchange. When the reverse of this process occurs, the indices have a negative value, indicating ion exchange [40,41]. In Table 3 below the chloro – alkaline index (CA1 and CA2) values of both post monsoon and pre monsoon sessions have been presented.
| Location No. | Post Monsoon | Pre Monsoon | ||
|---|---|---|---|---|
| CAI1 | CAI2 | CAI1 | CAI2 | |
| AL1 | 0.43 | 0.12 | 0.062 | 0.032 |
| AL2 | 0.33 | 0.27 | 0.271 | 0.182 |
| AL3 | 0.35 | 0.09 | 0.430 | 0.581 |
| AL4 | -0.57 | -0.03 | -1.901 | -0.234 |
| AL5 | -0.13 | 0.00 | 0.185 | 0.036 |
| AL6 | -2.26 | -0.05 | -3.181 | -0.284 |
| AL7 | 0.36 | 0.03 | -0.998 | -0.224 |
| AL8 | -0.55 | -0.02 | -0.401 | -0.066 |
| AL9 | -1.37 | -0.09 | -1.858 | -0.357 |
| AL10 | 0.85 | 0.17 | 0.831 | 0.624 |
| AL11 | -0.95 | -0.07 | -0.972 | -0.213 |
| AL12 | -1.08 | -0.04 | -0.970 | -0.136 |
| AL13 | -0.08 | -0.01 | 0.791 | 2.388 |
| AL14 | -1.58 | -0.10 | -4.364 | -0.532 |
| AL15 | -0.55 | -0.04 | -0.428 | -0.114 |
| AL16 | -0.10 | -0.01 | -0.018 | -0.004 |
| AL17 | -1.49 | -0.07 | -2.408 | -0.295 |
| AL18 | -0.03 | 0.00 | 0.134 | 0.060 |
| AL19 | -0.13 | -0.02 | 0.273 | 0.220 |
| AL20 | 0.40 | 0.15 | 0.345 | 0.179 |
| AL21 | -0.48 | -0.04 | -0.604 | -0.168 |
| AL22 | -0.04 | 0.00 | 0.250 | 0.086 |
| AL23 | 0.29 | 0.03 | 0.290 | 0.231 |
| AL24 | -0.79 | -0.05 | -0.967 | -0.436 |
| AL25 | -0.97 | -0.03 | -1.406 | -0.123 |
| AL26 | 0.64 | 0.18 | 0.534 | 0.338 |
Table 3: Chloro – alkaline index (CA1 and CA2) values.
Geochemistry of fluoride in groundwater can also be assessed by correlating its concentration in water to the type of the water it is in association with. Delineation of “Water types” can be carried out using the Piper’s trilinear diagram. Sodium bicarbonate type waters are typical of high fluoride waters. Correlation analysis studies carried out in such cases have shown that fluoride in most cases bears a positive correlation with both sodium and bicarbonate and a negative correlation with calcium; which particular trend has also been observed in groundwaters of the present study area. In order to relate concentration of fluoride in groundwater to the groundwater type the samples in the quantitative analysis data sets for both post monsoon and pre monsoon sessions were divided into three categories according to their corresponding fluoride levels. The limits of the fluoride values considered for each category was as follows:
• Less than 0.6 mg/l
• 0.6 mg/l – 1.5 mg/l
• Greater than 1.5 mg/l
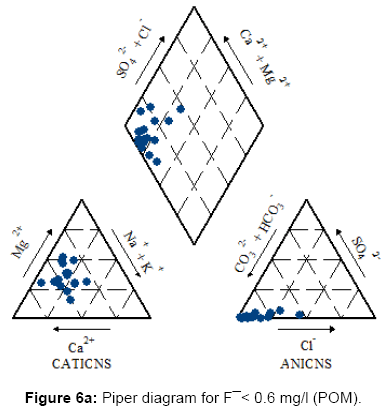
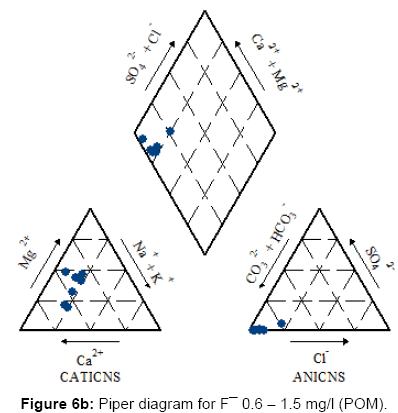
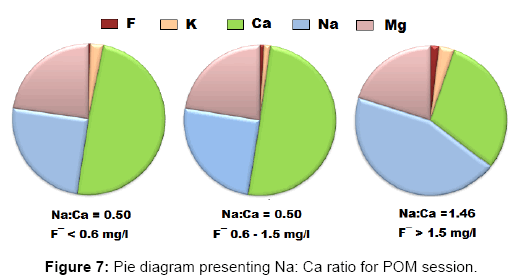
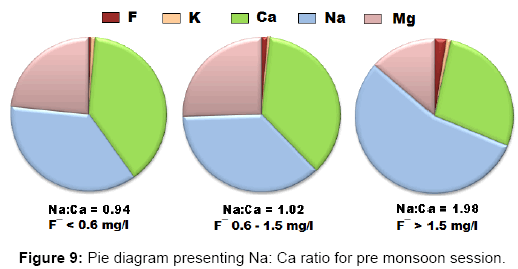
For each class a Piper trilinear diagram was plotted to interpret the overall water type and a pie diagram was present the ratio of Na: Ca (sodium: calcium) ratio which increases gradually as fluoride concentration in water increases [42].
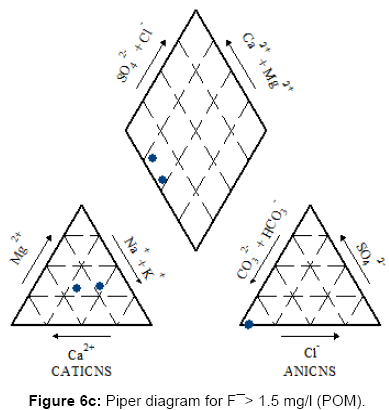
Figures 6a, 6b, 6c and 7 present the Piper diagrams and pie diagrams for the three fluoride data classes for post monsoon session respectively.
From the Piper trilinear diagrams of the post monsoon session depict that water with high fluoride is of Na-HCO3 (sodium bicarbonate) type whereas water containing low quantities of fluoride is mostly of Ca-Mg-HCO3 (mixed) type. The three corresponding pie diagrams present the gradual rise in Na: Ca distribution with rise in fluoride concentration.
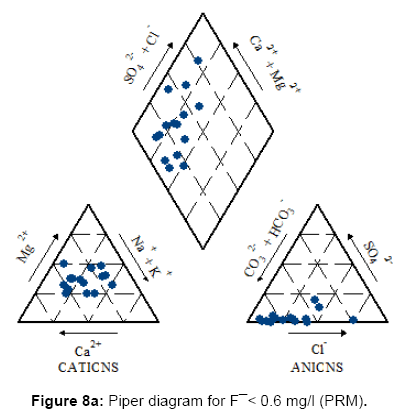
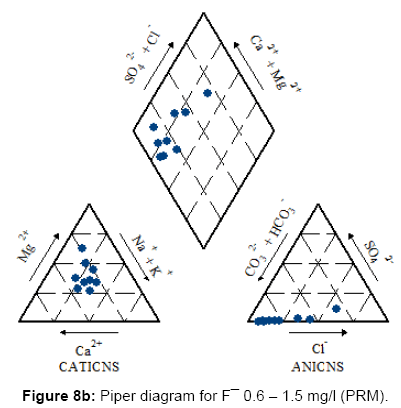
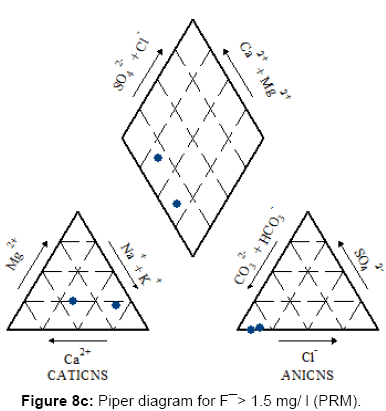
Figures 7, 8a, 8b, 8c and Figure 9 present the Piper diagrams and pie diagrams for the three fluoride data classes for pre monsoon session.
During the pre-monsoon session water containing low quantities of fluoride are Ca-Mg-HCO3, Ca-Mg-Cl-SO4 and Ca-Mg-SO4 (mixed) types whereas water with high fluoride is Na-HCO3 (sodium bicarbonate) type. The corresponding pie diagrams present the rise in Na: Ca ratio with increase in fluoride concentration.
Conclusion
Fluoride occurrence in the study area has been found to be a localized phenomenon as excess fluoride (>1.5 mg/l) was reported in two out of twenty six locations. Major parts of the study area are dominated by granite gneisses and recent layers of alluvium as evident from lithology map of the area. Pockets of hard clays are also common on the eastern parts of the blocks. Average fluoride concentration in groundwater rises during pre-monsoon in comparison with post monsoon. In between these sampling sessions aquifer reserves are not recharged because monsoon precedes both sampling sessions. Hence there is very less chance of increase in fluoride concentration of groundwater due to any other source besides the aquifer materials. Association of fluoride bearing minerals like apatite, fluorapatite and fluorite with the lithology type the present study area bears, is quite common and geochemical modeling studies have revealed that fluorite (CaF2) is indeed present as a significant fluoride bearing mineral in groundwaters of this study area. Calcite or CaCO3 is one of the most common mineral with which fluorite remains associated and saturation index calculations have revealed that the calcite – fluorite geochemistry is the dominant factor controlling fluoride concentration in this area during both post and pre monsoon. High fluoride waters have also been found to be of ‘bicarbonate’ type showing increase of sodium in water with decrease of calcium.
Acknowledgement
The author (S. Das) gratefully acknowledges the financial support from UGC, New Delhi and Jadavpur University also for providing the fellowship to her under UGC Meritorious Scheme. The other author (S. K. Nag) is thankful to Jadavpur University for providing financial assistance in conducting the field work related to this work.
References
- Brunt R, Vasak L, Griffioen J (2004) Fluoride in groundwater: Probability of occurrence of excessive concentration on global scale. International Groundwater Resources Assessment Centre Report. SP 2004-2.
- Edmunds WM, Smedley P (2006) Fluoride in natural waters. Essentials of Medical Geology, Elsevier Academic Press: 301-329.
- WHO (2010) Guideline for drinking water quality (4thedn) World Health Organization, Geneva
- Choubisa, SL, Sompura K, Bhatta SK, Choubisa DK, Pandya H, et al. (1996) Prevalence of fluorosis in some villages of Dungarpur district of Rajasthan. Ind J Environ Health 38: 119-126.
- IPCS (2002) Fluorides. Geneva, World Health Organization. International Programme on Chemical Safety (Environmental Health Criteria) 22: 38.
- Yadav JP, Lata S, Kataria SK, Kumar S (2009) Fluoride distribution in groundwater and survey of dental fluorosis among school children in the villages of the Jhajjar District of Haryana, India. Environ Geochem Health 31: 431-438.
- Agarwal V, Vaish AK, Vaish P (1997) Groundwater quality: Focus on fluoride and fluorosis in Rajasthan. Current Science 73: 743-64.
- Brindha K, Elango L (2011) Fluoride in groundwater: Causes, implications and mitigation measures. Monroy SD (ed.), Fluoride Properties, Applications and Environmental Management pp.111-136.
- Hussain I, Arif M, Hussain J (2011) Fluoride contamination in drinking water in rural habitations of Central Rajasthan, India. Environ Monit Assess 184: 5151-5158.
- UNICEF (1999) Fluoride in water: An overview. Waterfront – A UNICEF Publication on Water, Environment, Sanitation and Hygiene.
- UNICEF (1999) State of the art report on the extent of fluoride in drinking water and the resulting endemicity in India, Fluorosis Research & Rural Development Foundation for UNICEF, New Delhi.
- WHO (2006) Fluoride in drinking water, World Health Organization, London and Seattle.
- Short HE, Mcrobert GR, Bernard TW, Mannadinayar AS (1937) Endemic fluorosis in the Madras Presidency. Indian Journal of Medical Research. 25: 553-561.
- Mangla B (1991) India’s dentists squeeze fluoride warnings off tubes. New Scientist 131: 16.
- FRRDF (1999) State of the art report on the extent of fluoride in drinking water and the resulting endemicity in India. Fluorosis Research and Rural Development Foundation; Scientific and technical information compiled for UNICEF, New Delhi.
- Yadav S, Khan TI, Gupta S, Gupta AB, Yadava RN (1999) Fluorosis in India with special reference to Rajasthan. Proceedings of the International Conference on Water, Environment, Energy, Socioeconomics and Health Engineering (WEESHE), Seoul National University.
- Yadav JP, Lata S (2004) Fluoride levels in drinking water sources in rural area of block Jhajjar District Jhajjar, Haryana. J Water Works Asso 36: 131-136.
- Boyle DR, Chagnon M (1995) An incidence of skeletal fluorosis associated with groundwaters of the Maritime Carboniferous Basin, Gaspé Region, Quebec, Canada. Environ Geochem Health 17: 5–12.
- Apambire, WB, Boyle DR, Michael FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol 33: 13-24.
- Chae GY, Seong TM, Bernard K, Kyoung Ho K, Seong Yong K (2007). Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385: 272-283.
- APHA (American Public Health Association) (1995). Standard methods for examination of water and waste water. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington DC, USA.
- Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Earth and Space Science News 25: 914-928.
- Hitchon B (1995) Fluorine in formation water, Alberta basin, Canada. Applied geochemistry 10: 357-367.
- Koritnig S (1978) Fluorine. Wedepohl KH (ed.) Handbook of Geochemistry, Vol. II/1. Springler-Verlag, Berlin, 9C1-B9O3.
- Krauskopf KB, Bird DK (1995) An introduction to geochemistry. McGraw-Hill Int, Singapore 647.
- Gibbs RJ (1970) Mechanisms for controlling world’s water chemistry. Science 170: 1088-1090.
- Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Groundwater 13: 275-281.
- Pickering WF (1985) The mobility of soluble fluoride in soil. Environ Pollut 9: 281-308.
- Subba Rao N, Devdas DJ (2003) Fluoride incidence in groundwater in area of Peninsula India. Environ Geology 45: 243-251.
- Zhang B, Hong M, Zhao Y, Lin X, Dong J (2003) Distribution and risk assessment of fluoride in drinking water in the western plain region of Jilin province, China. Environ Geochem Health 25: 421-431
- Deshmukh AN, Valadaskar PM, Malpe DB (1995) Fluoride in environment: A review, Gondwana. Geol Mag, 1-19.
- Shah MT, Danishwar S (2003) Potential fluoride concentration in drinking water of Naranji area, North frontier province Pakistan. Environ Geochem Health 25: 475-481.
- Al-amry AS (2009) Hydrogeochemistry and origin of fluoride in groundwater of Hidhran & Alburayhi Basin, Northwest Taiz City, Yemen. Delta J Sci 33: 10-20.
- Dey RK, Swain SK, Mishra S, Sharma P, Patnaik T, et al. (2011) Hydrogeochemical processes controlling the high fluoride concentration in groundwater: A case study at the Boden block area, Orissa, India. Environ Monit Assess 184: 3279-91
- Sreedevi PD, Ahmed S, Made B, Ledoux E, Gandolfi JM (2006) Association of hydrogeological factors in temporal variations of fluoride concentration in a crystalline aquifer in India. Environ Geol 50: 1-11.
- Srinivasa Rao N (1997) The occurrence and behaviour of fluoride in the groundwater of the Lower Vamsadhara River basin, India. Hydrol Sci 42: 877-892.
- Smedley PL, Nicolli HB, Macdonald DMJ, Barros AJ, Tullio JO (2002) Hydrogeochemistry of arsenic and other inorganic constituents in ground waters from La Pampa, Argentina. Appl Geochem 17: 259-284.
- Schoeller H (1965) Qualitative evaluation of ground water resources. Water Resources Series No. 33 UNESCO Methods and techniques of groundwater investigation and development 44-52.
- Schoeller H (1977) Geochemistry of groundwater. Kovalevsky (eds.) Paris UNESCO. Groundwater studies - An international guide for research and practice 1-18.
- Arveti N, Sarma MRS, Aitkenhead-Peterson J, Sunil K (2011). Fluoride incidence in groundwater: A case study in Talupula, Andhra Pradesh, India. Environ Monit Assess 172: 427–443.
- Rajmohan N, Elango L (2004) Identification and evolution of hydro-geochemical processes in the groundwater environment in area of the Palar and Cheyyar River Basins South India. Environmental Geology 46: 47-61.
- Beg MK, Srivastava SK, Carranza EJM, De Smeth JB (2011) High fluoride incidence in groundwater and its potential health effects in parts of Raigarh District, Chhattisgarh, India. Research Communications, Current Science 100: 750-754
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 13267
- [From(publication date):
June-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12247
- PDF downloads : 1020