Research Article Open Access
Genomic Control of Upregulation of GRP78 Expression for Promotion of Neurite Elongation and Attenuation of Cell Death via PKA-Mediated Signaling in PC12 Cells
| Ryosuke Yamazoe1, Yoshiki Nishihata2, Kazuma Nakagawa2, Hiroki Aoyama2 and Koji Shimoke1,2* | |
| 1High Technology Research Core (HRC), Kansai University, 3-3-35, Yamate-cho, Suita, Osaka 564-8680, Japan | |
| 2Laboratory of Neurobiology, Department of Life Science and Biotechnology, Faculty of Chemistry, Materials and Bioengineering, Kansai University, 3-3-35, Yamate-cho, Suita, Osaka 564-8680, Japan | |
| Corresponding Author : | Koji Shimoke Laboratory of Neurobiology Department of Life Science and Biotechnology Faculty of Chemistry, Materials and Bioengineering Kansai University, 3-3-35, Yamate-cho Suita, Osaka 564-8680, Japan Tel: +81-6-6368-0853 Fax: +81-6-6330-3770 E-mail: shimoke@kansai-u.ac.jp |
| Received October 08, 2015; Accepted November 17, 2015; Published November 23, 2015 | |
| Citation: Yamazoe R, Nishihata Y, Nakagawa K, Aoyama H, Shimoke K (2015) Genomic Control of Upregulation of GRP78 Expression for Promotion of Neurite Elongation and Attenuation of Cell Death via PKA-Mediated Signaling in PC12 Cells. Clin Pharmacol Biopharm 4:150. doi:10.4172/2167-065X.1000150 | |
| Copyright: © 2015 Yamazoe R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Neurodegeneration occurs due to neuronal cell death and subsequently disrupts neuronal networks. In current medicine, methods for interruption of neuronal cell death and avoidance of disruption of neuronal networks have potential as therapy for neurodegenerative disorders. Development of this therapy requires analysis of the molecular mechanism of neurite extension that leads to network formation. Here, we show that forskolin (fsk), an activator of adenylate cyclase, increases the intracellular cAMP concentration and induces neurite outgrowth in PC12 cells. The effect of fsk on neurite outgrowth was diminished by H89, an inhibitor of protein kinase A (PKA), and by PD98059 and U0126, which are inhibitors of the mitogen-activated protein kinase (MAPK) signaling pathway. With fsk treatment in the presence of tunicamycin, an inducer of cell death, the activity of the glucose-regulated protein 78 (GRP78) promoters was upregulated. Interestingly, this effect was completely abolished by H89 and by PD98059 and U0126. This phenomenon was confirmed using a dominant-negative PKA-expressing PC12 cell line, in which the PKA-mediated signaling pathway was completely eliminated. These lines of evidence suggest that GRP78 promotes neuronal elongation that is regulated by fsk and mediated by PKA.
| Keywords |
| neurites; survival; PC12; GRP78; gene expression; genomics; epigenetics |
| Introduction |
| The endoplasmic reticulum (ER) is an important organelle with a crucial role in protein biosynthesis. Disrupted quality control in biosynthesis of proteins leads to cell death due to accumulation of unfolded proteins [1-4]. Tunicamycin (Tm), an inhibitor of glycosylation of newly biosynthesized proteins, is a well-known inducer of cell death [6-8]. Disruption of the homeostatic balance of the Ca2+ concentration in the ER also induces cell death [9-12]. In both cases, accumulation of unfolded proteins in the ER is ultimately the cause of cell death. |
| Glucose-regulated protein 78 (GRP78) refolds protein structures and attenuates cell death. Thus, GRP78 can be a molecular target in drug therapy for neurodegenerative diseases, for instance, Alzheimer’s or Parkinson’s disease, and there is a need to understand the promoter mechanisms associated with GRP78 upregulation. Two sensor proteins on the ER membrane are involved in this process: Ire1, which has an RNase domain that cleaves pre-XBP-1 mRNA to spliced XBP-1 mRNA and the translated product binds to the GRP78 promoter region; and ATF6, which is cleaved by site-1 and site-2 proteases to form a transcriptional factor (p50 ATF6) for GRP78. OASIS is a sensor of unfolded proteins that also upregulates GRP78 expression, but this effect is limited only to glial cells [13]. |
| In this study, we show that forskolin (fsk), an activator of adenylate cyclase, attenuates Tm-mediated cell death and promotes elongation of neurites in pheochromocytoma 12 (PC12) cells. PC12 cell is a developmental model neuron to study neurite extension system and formation of neuron-like networks through intracellular signaling pathway(s) [14]. Then, we discover that fsk-mediated PKA signaling upregulates GRP78 to bear neurites via an effect on the GRP78 promoter region by using PC12 cells. Analyses using inhibitors for PKA (H89) and MEK1 (PD98059 or U0126) showed that PKA activates MAPK signaling to upregulate GRP78. These findings were confirmed using a dominant-negative PKA-expressing stable transformant PC12 cell line (DN-PKA-PC12). Overall, the results suggest that upregulation |
| of GRP78 is important for attenuation of Tm-induced cell death and promotion of neurite outgrowth in PC12 cells. |
| Materials and Methods |
| Cell culture |
| PC12 and DN-PKA-PC12 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1% (v/v) penicillin-streptomycin, 5 % (v/v) heat-inactivated precolostrum newborn calf serum and 5% (v/v) heat-inactivated horse serum [8]. For measurement of cell viability, PC12 cells were seeded on collagencoated 96-well plates at 1×105 cells/cm2. The next day, the medium was changed to serum-free DMEM and 1.0 μg/ml Tm was added without or with inhibitors. For other assays, cell density was denoted in each section in Materials and Methods. |
| Measurement of neurite length |
| PC12 cells were plated in 24-well plates at 0.2×104 cells/cm2 and cultured in DMEM supplemented with 5% (v/v) fetal bovine serum (FBS), 5% (v/v) heat-inactivated horse serum, and 0.1% (v/v) penicillinstreptomycin solution. Adherent cells were treated with reagents in DMEM supplemented with 0.1% (v/v) penicillin-streptomycin solution for 24 h. The plated cell density and the serum concentration were lower than those of cell viability assay because enough space for the observation of neurite outgrowth and suppression of mitogenic effect were needed for this assay. After treatment, the cells were fixed with 4% paraformaldehyde for 30 min. For analysis of neurite outgrowth about 200 cells/well were randomly photographed using a phase-contrast microscope (Biozero BZ-9100, Keyence, Japan). Neurite lengths were measured using BZ-H1C software (Keyence, Japan). |
| Transient transfection and luciferase assay |
| PC12 cells were seeded on 6-well plates at 0.5×105 cells/cm2. The next day, the medium was changed to fresh DMEM for cell culture. A GRP78 promoter-containing plasmid (-457/Luc), with a firefly luciferase gene and the promoter sequence up to -457 of rat GRP78 was dissolved in 50 μl of OPTI-MEM (Gibco BRL). The TK plasmid, containing a renilla luciferase gene and a thymidine kinase promoter, was mixed with -457/Luc. The quantity of total DNA for transfection was 2.0 μg (-457/Luc TK = 4:1). In addition, 1 μl of Lipofectamine2000 (Invitrogen, USA) was dissolved in 50 μl of OPTI-MEM and incubated for 5 min. The two transfection solutions, the DNA solution and the Lipofectamine2000 solution were then mixed and incubated for 20 min. The mixture was added to the culture medium of PC12 or DNPKA- PC12 cells. After 2 days, the cells were used for a luciferase assay (Promega, USA). -457/Luc were gifts from Prof. A.S. Lee (University of Southern California). |
| Statistical Analysis |
| Results for experimental groups are expressed as the mean ± SEM. Differences among groups were examined by one-way ANOVA, with P<0.05 taken to indicate a significant difference. All analyses were performed using SigmaStat 3.0. |
| Results and Discussion |
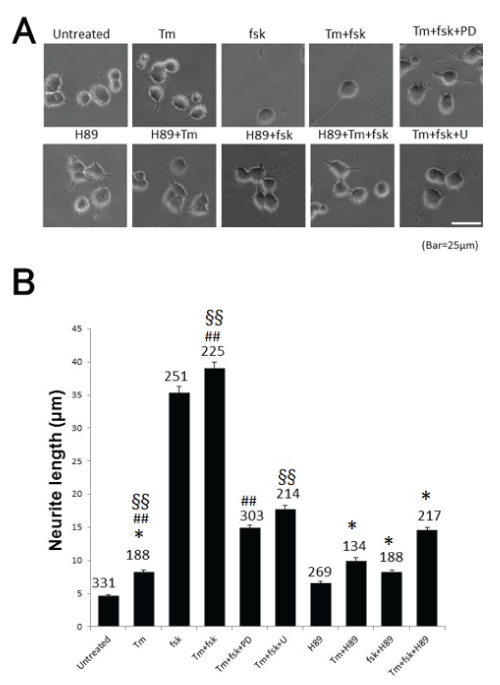
| In this study, we found that GRP78 expression is regulated via PKA-mediated signaling. Neurite outgrowth was markedly induced by forskolin (fsk), an activator of adenylate cyclase, which activates PKAmediated signaling. As shown in Figure-1A, fsk or tunicamycin (Tm) plus fsk elongated neurites up to 45 μm on PC12 cells, and this effect was diminished by H89, a specific PKA inhibitor, and PD98059 (PD) or U0126 (U) which are inhibitors of MAP kinase signaling. Lengths of neurites are shown in Figure-1B. Cell counts are also given in the figure. These data suggest that PKA elongates neurites in the presence of fsk alone and with fsk plus Tm, a cell death-inducer, as a mimic of pathophysiological conditions in the brain. MAP kinase signaling also clearly contributed to neurite outgrowth in PC12 cells, based on the effects of PD and U, indicating that at least two signaling pathways are involved in neurite elongation. These pathways also promote cell survival because cells treated with fsk remained fresh and alive (Figure- 1A) [15,16]. Both may contribute to rescue from cell death because Tm is well known to induce ER stress-mediated cell death by accumulation of unfolded proteins. Thus, the two pathways may be key targets in therapy for neurodegenerative disease. This possibility is also proposed by using Parkinson’s disease model [17]. However, some hereditary diseases, for instance, huntinton’s disease cannot be cured because responsible gene is continued to express. |
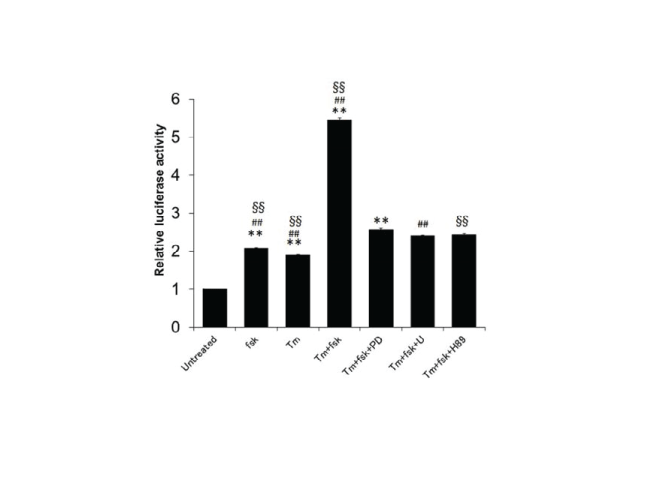
| Interestingly, we discovered that fsk activated the GRP78 promoter and that this effect was decreased by H89, PD, and U (Figure-2). The promoter activity of GRP78 has been shown to be mediated through epigenetic regulation [18,19]. We did not find evidence for this activity, but histone modification or DNA methylation may be involved in neurite elongation and cell survival via upregulation of GRP78 expression. This is likely because GRP78 is a chaperone protein that attenuates ER stress-mediated cell death by reducing the number of unfolded proteins in the ER. |
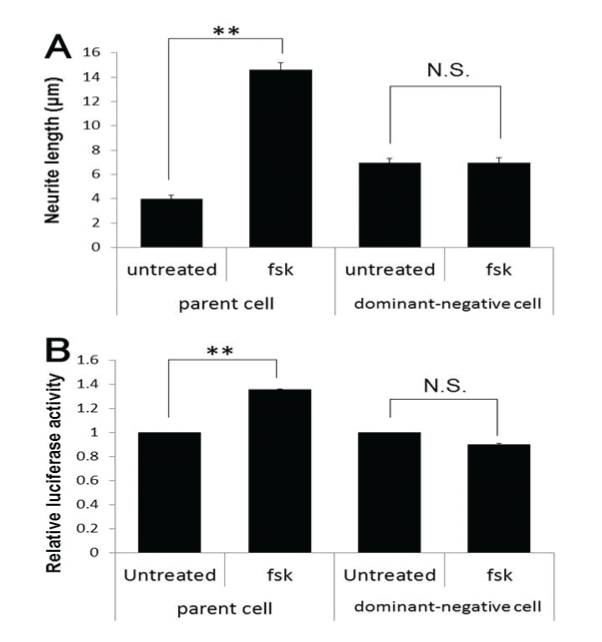
| Next, we confirmed that fsk-induced neurite elongation is mediated through PKA, using DN-PKA-PC12 cells, a dominant-negative PKAexpressing stable transformant of PC12 cell line. Neurites could not be elongated in these cells, whereas the parent cells were normal (Figure-3A), indicating that PKA is necessary for fsk-induced neurite elongation. DN-PKA-PC12 cells also had reduced GRP78 promoter activity (Figure-3B). Thus, downstream molecules of PKA leading to upregulation of GRP78 protein should be clarified for further study. |
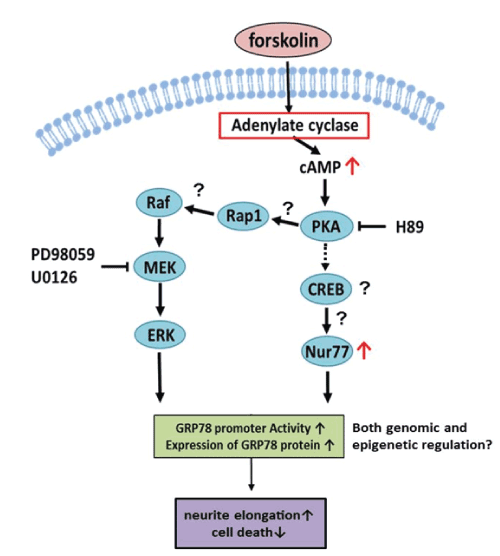
| The effects of fsk on neurite elongation and cell survival are summarized in Figure-4. PKA activates Rap1 to activate MAP kinase signaling [20] and fsk induces expression of Nur77 [21]. Thus, upregulation of GRP78 may activate both the PKA-Nur77 and MAP kinase signaling pathways via fsk-induced PKA-mediated signaling. This is consistent with the elimination of neurite elongation and reduced GRP78 promoter activity in DN-PKA-PC12 cells. It is striking that GRP78 can elongate neurites via fsk because GRP78 is a chaperone protein that is not directly related to neurite elongation. Further studies of this new function of GRP78 will be necessary for development of therapy against neurodegenerative diseases. |
| Acknowledgements |
| We thank Prof. A.S. Lee (University of Southern California) for luciferase assay plasmids. This work was supported by SENRYAKU (2013-2017) and KAKENHI (25340104) grants-in-aid from MEXT (Ministry of Education, Culture, Sports, Science and Technology of Japan), and by a Kansai University Grant-in- Aid for Encouragement of Scientists (2014). |
| References |
References
- Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110: 1389-1398.
- Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, et al. (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J BiolChem 277: 21836-21842.
- Rao RV, Ellerby HM, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11: 372-380.
- Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, et al. (2004) Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J BiolChem 279: 177-187.
- Adams JM, Cory S. (2002) Apoptosomes: engines for caspase activation. CurrOpin Cell Biol 14: 715-720.
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, et al. (2004) Involvement of Caspase-4 in endoplasmic reticulum stress-induced apoptosis and abeta-induced cell death. J Cell Biol 165: 347-356.
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, et al. (2000) Caspase-12 mediates endoplasmic reticulum specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98-103.
- Shimoke K, Amano H, Kishi S, Uchida H, Kudo M, et al. (2004) Nerve growth factor attenuates endoplasmic reticulum stress-mediated apoptosis via suppression of caspase-12 activity. J Bioche. 135: 439-446.
- Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, et al. (2004) Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12 NeurosciLett. 357: 127-130.
- Lytton J, Westlin M, Hanley MR. (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J BiolChem 266: 17067-17071.
- Nguyen HN, Wang C, Perry DC. (2002) Depletion of intracellular calcium stores is toxic to SH-SY5Y neuronal cells. Brain Res 924: 159-166.
- Takadera T, Ohyashiki T, (1998) Apoptotic cell death and CPP32-like activation induced by thapsigarginand their prevention by nerve growth factor in PC12 cells. BiochimBiophysActa 1401: 63-71.
- Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, et al., (2005) Oasis, a CREB/ATF family member, modulates UPR signalling in astrocytes. Nat Cell Biol 7:186-194.
- Liu X, Wang X, Lu J, (2015) Tenuifoliside A promotes neurite outgrowth in PC12 cells via the PI3K/AKT and MEK/ERK/CREB signaling pathways. Mol Med Rep 12: 7637-7642.
- Shimoke K, Utsumi T, Kishi S, Nishimura M, Sasaya H, et al. (2004) Prevention of endoplasmic reticulum stress-induced cell death by brain-derived neurotrophic factor in cultured cerebral cortical neurons. Brain Res 1028: 105-111.
- Maruoka H, Shimoke K. (2013) Mechanisms of neurotrophic activities via low-molecular-weight compounds: post transcriptional regulation in PC12 cells and neurons. Clinic Pharmacol Biopharmaceutics. S1 001-003.
- Chong CM, Ma D, Zhao C, Franklin RJ, Zhou ZY, et al. (2015) Discovery of a novel neuroprotectant, BHDPC, that protects against MPP+/MPTP-induced neuronal death in multiple experimental models. Free RadicBiol Med 89: 1057-1066.
- Baumeister P, Luo S, Skarnes WC, Sui G, Seto E, et al. (2005) Endoplasmic reticulum stress induction of the GRP78/Bip promoter: activating mechanisms mediated by Yy1 and its interactive chromatin modifiers. Mol Cell Biol 25: 4529-4540.
- Xiao Y, Huang W, Zhang J, Peng C, Xia M, et al. (2015) Increased plasma S-adenosylhomocysteine-accelerated atherosclerosis is associated with epigenetic regulation of endoplasmic reticulum stress in apoE-/- mice. ArteriosclerThrombVascBiol 35: 60-70.
- Ravni A, Vaudry D, Gerdin MJ, Eiden MV, Falluel-Morel A, et al. (2008) A camp-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. MolPharmacol 73: 1688-1708.
- Davis IJ, Lau LF. (1994) Endocrine and neurogenic regulation of the orphan nuclear receptors nur77 and nurr-1 in the adrenal glands. Mol Cell Biol 14: 3469-3483.
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 13774
- [From(publication date):
November-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 12964
- PDF downloads : 810
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
