Genetic Study of Resistance to Rice Blast in Crosses between Korean and Locally Adapted Rice Genotypes
Received: 21-Feb-2018 / Accepted Date: 12-Mar-2018 / Published Date: 19-Mar-2018 DOI: 10.4172/2329-8863.1000346
Abstract
The rice blast (Magnaporthe grisea) is a serious constraint to rice production in many rice producing countries including Uganda. Yield losses of up to 100% are attributed to the blast disease in different rice growing regions. In addition to these, the inheritance of resistance to the disease has not yet been studied under Ugandan condition.
Experiment was conducted under controlled conditions by using three resistant and four susceptible elite genotypes as parental lines for population development in half dialled mating design with the aim of providing relevant genetic information as a contribution towards the development of rice varieties with resistance to blast disease. A total of 18 crosses were advanced to F2 level. The F2 segregates and their corresponding parents were evaluated in the screen house against single virulent isolate of rice blast pathogen in 4 by 7 alpha lattice design in three replications.
These results showed that there was significant variation for rice blast resistance among genotypes. Significant general and specific combining abilities were observed, indicating that both additive and non-additive gene effects were important for rice blast resistance, although the additive effect was predominant. A High coefficient of genetic determination in the broad sense (0.99) and narrow sense (0.85), were obtained on a genotype mean basis with a high Baker’s ratio of 0.86, indicating primarily additive inheritance among crosses.
The segregation pattern for resistance to rice blast showed single dominant gene in some elite susceptible × resistant crosses, duplicate recessive epistasis in other elite susceptible × resistant crosses and two genes with duplicate dominant epistasis in resistant × resistant crosses. The crosses between susceptible by susceptible genotypes did not show segregation. Overall, resistance was highly heritable, with mainly additive gene action between crosses. Results suggest that simple breeding strategies with selection in early generations would be effective for rice blast resistance.
Keywords: Genetics, Segregation, Rice, Blast, Resistance
Introduction
Development of varieties with improved traits, like resistance to both biotic and abiotic stresses, is very important. Among the biotic stresses, rice blast is the main constraint to rice production. To effectively develop resistant varieties, it is vital to identify the source of resistance, the nature of resistance and the gene action that provides resistance to the disease. Knowledge of these factors helps the breeder to incorporate resistance and to assess whether or not the incorporated resistance is durable and can be expressed in different environments. The inheritance pattern of resistance from the locally adapted genotypes and the Korean genotypes under Ugandan conditions is not yet known and this should be identified for further utilization of these genotypes in resistance breeding. Since the Korean genotypes are new for Ugandan conditions, the inheritance pattern should be known when crossed with the susceptible locally adapted Ugandan genotypes. This is done by analysing the genetic parameters of the parents and their segregating F2 populations.
It is sometimes difficult to get adequate amounts of seed at F1 level, especially in self-pollinating crops like rice and segregation is easily detected in the F2 level. As a result, analysis of the parents and F2 segregants provides an excellent alternative [1]. The general combining ability (GCA) of selected parents and the specific combining ability (SCA) of a parent in a cross with another parent were estimated, as well as the gene actions that control the inheritance of resistance to blast disease and heritability estimates for F2 segregants and their parents. Heritability is estimated by calculating the fraction of genotypic variation to phenotypic variation, and helps to determine the degree to which a trait is transmissible from a parent to its offspring, and the influence of environmental difference on it [2].
Materials and Methods
Selection of parental genotypes
The experiment was conducted at National Crop Resources Research Institute (NaCRRI) Namulonge in Uganda on seven rice varieties from two sources which were selected as parents for population development (Table 1). Three of them were selected from the introduced Korean lines which are resistant for blast and four genotypes were selected from NaCRRI are susceptible to blast, moderate to late maturing, locally adapted and accepted by farmers but, highly attacked by the rice blast disease as presented in Table 1.
| Entry code |
Genotypes | Reaction to blast |
Source |
|---|---|---|---|
| S1 | WAB 1573-22-B-B-FKR 4-2-WAC 1-TGR 3-WAT9-1 | S | NaCRRI |
| S2 | WAC 18-WAT 15-3-1 | S | NaCRRI |
| S3 | WAB2135-WAC B-2-TGR 2-WAT1-1 | S | NaCRRI |
| S4 | FARO × 521-357-H1 | S | NaCRRI |
| R5 | SR33701-HB3330-78 | R | KAFACI |
| R6 | SR33859-HB3324-93 | R | KAFACI |
| R7 | SR33859-HB3324-133 | R | KAFACI |
Table 1: Origin, pedigree and disease reaction of selected genotypes used for population development. S=Susceptible, R=Resistant, NaCRRI=National Crop Resources Research Institute, KAFACI=Korea-Africa Food and Agriculture Cooperation Initiative.
Population development
The parental genotypes were planted in buckets measuring 25 cm deep and 30 cm wide, filled with forest soil. In each bucket four seeds were planted, and planting was staggered at four weekly intervals to synchronize flowering dates of the varieties. A half-diallel mating design was used to generate populations.
Since it is difficult to avoid injury to floral parts and to obtain viable seeds with artificial emasculation, as noted by Herrera and Coffman [3], hybridization was done with the aid of a vacuum emasculator in the late morning (10:00 am-12:00 pm) and late afternoon (3:00 pm-5:00 pm) on panicles that had already started flowering. Immature spikelets and any that had already undergone anthesis were cut off at the bottom of the panicle, leaving only the emasculated spikelets in the panicle. After emasculation, panicles were covered with a pollinating bag secured with paper clips to keep out any external pollen. A flowering panicle of the male parent was cut and dusted onto the emasculated panicle, gently tapped onto the receptive stigma and then covered with the pollinating bag. For each cross the date and parent’s name were written on the back of the pollen bag to avoid confusion during harvesting. Mature seeds from successful crosses were harvested and bagged separately according to the cross number.
The harvested F1 seeds were placed in an air-dry oven for seven days at 50ºC in order to break dormancy Herrera and Coffman [3]. The F1 seeds were later surface sterilized by 0.1% Tween 20, followed by 70% ethanol and washed twice with distilled water. Sterilized seeds were placed in sterile Petri-dishes on moistened tissue papers and incubated for 48 hours at 30ºC. Pre-germinated F1 seeds were transferred to small cups until they became strong enough for transplanting. Afterwards, seedlings were transplanted into 25 cm deep by 30 cm wide buckets, filled with forest soil, and kept in the screen house. Morphological markers including plant height, tillering, daysto- flowering and days-to-maturity were used differentiate successful crosses from selfed plants.
Experimental design and inoculation
The F2 segregating populations including parents were evaluated at NaCRRI in the screen house in a 4 × 7 alpha lattice design, replicated three times. A spacing of 20 cm between rows and between blocks and 15 cm between plants was used. All agronomic practices including weeding and fertilizer applications were performed as recommendation.
The plants in the screen house were inoculated by using a virulent races of the pathogen with a hand sprayer until run off at the 3 to 4 leaf stage of the plant [4]. High humidity was maintained by covering the area with a plastic sheet to facilitate infestation. In addition to this, water was sprinkled on the leaves at mid-day till run off for one week, in order to facilitate blast development [5].
Data collection and analysis
Data for leaf blast severity were collected according to IRRI’s standard evaluation system for rice [6]. Evaluation of leaf blast disease was done for each plant four weeks after inoculation. Genotypes with disease severity scores 0-3 were considered resistant, and 5-9 susceptible [6]. The data were analysed by GenStat [7]. Alpha lattice restricted maximum likelihood (ReML) algorithm. The genotypes were considered a fixed effect while blocks and replications were random effects.
In order to select a good combination of parents, coefficient of genetic determination both in broad and in narrow sense, general combining ability and specific combining ability were calculated using Griffing [8] method II model I. The statistical model used was as follows:

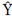 ... is the grand mean, gi and gj are GCA effects of the ith and jth parents respectively, sij is the SCA effect for the crosses between the ith and jth parents, Rk is replication means effects, and eijk is experimental error.
... is the grand mean, gi and gj are GCA effects of the ith and jth parents respectively, sij is the SCA effect for the crosses between the ith and jth parents, Rk is replication means effects, and eijk is experimental error.
To assess the nature of genes involved in rice blast resistance (monogenic or polygenic), a chi-square goodness-of-fit-test was used to determine the deviation of the observed frequencies from the hypothesized frequencies in the segregating F2 populations as described by Steel [9].
Results
Gene action determining rice blast resistance
Analysis of variance of F2 segregating populations evaluated in screen house in season (2016 A) is presented in Table 2. ANOVA revealed significant (P ≤ 0.001) differences among genotypes for rice blast severity, resulting from wide genetic variability of genotypes for resistance to rice blast.
| SOV | df | SS | MS | F-calc | F-prob |
|---|---|---|---|---|---|
| Replications | 2 | 0.45 | 0.22 ns | 0.08 | 0.92 |
| Rep/ block | 18 | 47.16 | 2.62*** | 131.1 | < 0.001 |
| Entries | 24 | 134.72 | 5.61*** | 280.7 | < 0.001 |
| GCA | 6 | 52.49 | 8.75*** | 437.4 | < 0.001 |
| SCA | 18 | 7.19 | 0.40*** | 19.9 | < 0.001 |
| Residual | 30 | 0.74 | 0.02 | ||
| Additive component (s2 GCA) | 1.13 | ||||
| Dominance component (s2 SCA) | 0.38 | ||||
| Bakers ratio | 0.87 | ||||
| CGD–BS | 0.99 | ||||
| CGD–NS | 0.85 | ||||
Table 2: Analysis of variance of F2 segregating rice genotypes with three missing crosses for rice blast severity under screenhouse condition at NaCRRI in 2016 season A.
***Significant at 0.001 probability, ns=Non-significant, SOV=Source of variation, CGD=Coefficient of genetic determination, BS=Broad sense, NS=Narrow sense.
The narrow sense coefficient of genetic determination was 0.85, indicating that 85% of the variation for resistant to leaf blast among genotypes was due to transmissible genetic effects, and 15% was due to environmental effects. The broad sense coefficient of genetic determination was 0.97; indicating 99% of the observed inheritance to rice blast resistance is due to genotypic differences and only 1% due to error or environmental effects. The relative importance of additive to non-additive gene action was 0.87, indicating that 87% of the variation in genotypes is due to additive gene actions.
Estimates of the effects of general combining ability for individual parental lines for rice blast are presented in Table 3. For rice blast disease the desirable GCA effect for parents should be negative. Significant effects (P ≤ 0.001) of GCA and SCA variation among genotypes were observed. The analysis of results showed that the three introduced Korean genotypes used in this experiment, R5, R6 and R7 had highly significant negative GCA effects (-0.91), (-1.02) and (-1.14), respectively, suggesting that these genotypes had good levels of resistance and so are good general combiners for the transfer of resistance to rice blast. On the other hand, highly positive significance (P ≤ 0.001) for GCA effects was obtained on the locally adapted genotypes S1, S2 and S3. This suggested that these genotypes do not have a source of resistance to transfer to the progeny. Generally, GCA effects were much higher than SCA effects, as illustrated by Baker‘s ratio (Table 4), suggesting that gene action was predominantly additive.
| Parents | Parental means | GCA effects |
|---|---|---|
| S1 | 6.2 | 0.90*** |
| S2 | 6.3 | 0.91*** |
| S3 | 6.1 | 1.0*** |
| S4 | 4.2 | 0.24*** |
| R5 | 2.4 | -0.91*** |
| R6 | 2.0 | -1.02*** |
| R7 | 2.1 | -1.14*** |
| S.E GCA[((p/ni)*((p-1)/p(p+2)))* ems/r]1/2 0.03 | ||
Table 3: Summary of GCA effects of rice genotypes to rice blast severity. ***Significant at 0.001 probability, S.E GCA is the standard error for GCA, S=Susceptible, R= Resistant, ni=number of cross combinations.
| Crosses combinations | SCA effects |
|---|---|
| S1 × S2 | -0.07 ns |
| S1 × S3 | -0.38** |
| S1 × S4 | 0.41 ** |
| S1 × R5 | -0.39 ** |
| S1 × R7 | -0.77 *** |
| S2 × S3 | 0.06 ns |
| S2 × S4 | 0.64 **** |
| S2 × R5 | -0.76 *** |
| S2 × R6 | -0.31 * |
| S2 × R7 | -0.77 *** |
| S3 × S4 | 0.74 *** |
| S3 × R6 | -0.90 *** |
| S4 × R5 | -0.40 ** |
| S4 × R6 | -0.37 * |
| S4 × R7 | -0.74 *** |
| R5 × R6 | 0.33 * |
| R5 × R7 | 0.42 ** |
| R6 × R7 | 0.79 *** |
| S.E SCA[(P2+p+2)/(p+1)*(p+2)* ems/r]1/2=0.08 | |
Table 4: SCA effects for resistance to rice blast in F2 segregating populations. *, **, *** Significant at 0.05, 0.01 and 0.001 probability respectively; ns=non-significant; S. E=standard error; P=number of parents; ems=error mean square; r=replications.
The specific combining ability of crosses is shown in Table 4. For rice blast severity, except for two crosses (S1 × S2 and S2 × S3), all the crosses revealed significant SCA effects. The most desirable SCA effects were obtained in cross S3 × R6 (-0.9) followed by crosses S2 × R7 (-0.77) and S1 × R7 (-0.77). Three crosses had positive and undesirable SCA effects R6 × R7 (0.79), S3 × S4 (0.74) and S2 × S4 (0.64).
Segregation patterns of the F2 populations of a cross between Korean accessions and locally adapted rice genotypes for resistance to rice blast
The pattern of segregation in the successful F2 populations studied showed that the mode of gene action governing rice blast resistance varied, depending on the parents involved. The detailed segregation of F2 populations is also presented in Table 5. In order to see the segregation pattern of resistance to rice blast, a chi-square good-nessof- fit test was carried out. Results showing test of fit for 3:1, 15:1 and 9:7 segregation pattern are presented in Table 5.
| Crosses | Number of plants | Observed | Χ2 under different model ratios | |||
|---|---|---|---|---|---|---|
| R | S | 3 to 1 | 15 to 1 | 9 to 7 | ||
| S1 × S2 | 72 | 0 | 72 | - | - | - |
| S1 × S3 | 76 | 0 | 76 | - | - | - |
| S1 × S4 | 75 | 0 | 75 | - | - | - |
| S1 × R5 | 79 | 48 | 31 | 6.86** | 132.06*** | 0.97 ns |
| S1 × R7 | 83 | 64 | 19 | 0.88 ns | 35.35*** | 17.56 *** |
| S2 × S3 | 75 | 0 | 75 | - | - | - |
| S2 × S4 | 74 | 0 | 74 | - | - | - |
| S2 ×R5 | 80 | 46 | 34 | 21.05*** | 206.80*** | 1.24 ns |
| S2 × R6 | 75 | 54 | 21 | 0.36 ns | 60.55*** | 7.56** |
| S2 × R7 | 81 | 59 | 22 | 0.55 ns | 33.76*** | 16.42*** |
| S3 × S4 | 91 | 0 | 91 | - | - | - |
| S3 × R6 | 81 | 62 | 19 | 0.55ns | 31.43*** | 15.87*** |
| S4 × R5 | 76 | 45 | 31 | 12.84*** | 1.20*** | 23.14*** |
| S4 × R6 | 73 | 57 | 16 | 0.31ns | 31.35*** | 13.56*** |
| S4 × R7 | 73 | 55 | 18 | 0.04 ns | 48.73*** | 9.32** |
| R5 × R6 | 71 | 65 | 6 | 11.75*** | 0.10 ns | 38.12*** |
| R5 × R7 | 73 | 70 | 3 | 17.57*** | 0.61 ns | 47.38*** |
| R6 × R7 | 89 | 82 | 7 | 15.82*** | 0.04 ns | 49.53*** |
Table 5: Segregation of F2 populations for chi-square good ness of fit test for rice blast resistance in the crosses between Korean and locally adapted rice varieties grown at NaCRRI. **,***Significant at 0.01 and 0.001 probability respectively, ns=non-significant, R=Resistant, S=Susceptible.
Discussion
Combining ability for resistance to rice blast
Understanding the mode of inheritance of resistance to rice blast is essential to facilitate the resistance breeding. Since the inheritance of resistance to rice blast depends on the genotype involved in the crossing, the pathogen race and environmental condition, it is important to assess the pattern of inheritance in every new putative source before the start of the breeding work. The analysis of data from this study showed significant differences among the progenies tested with their parents. The results indicated that both additive and nonadditive gene actions were involved in the inheritance of blast resistance. However, the additive portion was greater than the nonadditive, suggesting that additive gene effects contribute more to rice blast resistance. Similar studies were reported by Mulbah [10]. The low GCA values obtained in the introduced Korean genotypes (SR33701- HB3330-78, SR33859-HB3324-93 and SR33859-HB3324-133) indicated their importance in contributing resistance to rice blast in crosses involving them. However, the locally adapted genotypes showed positive GCA effects, suggesting their poor contribution for resistance to rice blast when crossed with other parents. Locally adapted parent S4 showed relatively lower positive GCA as compared to other susceptible parents. Parents with high negative GCA effects are potentially superior, and may be included in breeding programs to select new inbred lines in advanced generations [10]. In addition to this, the genotypes showed high negative GCA effects have also showed good agronomic performance for different traits. As a result, after extensive testing in different environments and for different seasons would be used for production purpose.
The proportion of additive to non-additive gene effects for rice blast resistance was high, as estimated by Baker’s ratio of 0.87 (obtained at 28 days after inoculation), implying that additive genes effects were more important than non-additive [11]. Thus, it helps to predict the performance of the progenies from the GCA values of the parents, allowing for few targeted crosses to be made in order to obtain the desired combination of resistance with other traits [12]. The high Baker’s ratio also implies that selection in early generations can be effective. Therefore, methods such as pedigree selection, modified pedigree, or mass selection can be useful.
A high narrow sense coefficient of genetic determination was obtained, suggesting that 85% of the inheritance to rice blast resistance was governed by additive genes and transmissible to the progeny and suggested that phenotypic selection would be effective [11]. A high broad sense coefficient of genetic determination (99%) for inheritance to rice blast resistance was observed in this study. This showed that the proportion of genotypic to environmental factors is very high. This coincides with reports on heritability of rice blast resistance on F1 populations of 96.6% by Manneh [13].
Evaluation of F2 segregation patterns
The eighteen successful F2 segregating populations responded differently depending on the parents involved in the cross. Except the crosses between S3 × S4, R5 × R6 and R6 × R7, other crosses showed lower F2 severity means and showed skewed distributions toward the resistant parents suggesting the presence of a dominant component of resistance to rice blast Hayashi and Yoshida [14].
All the crosses between resistant parents showed some susceptible individuals. This inconsistency among the resistant by resistant crosses suggested that the resistant parents involved in the cross might have different alleles for resistance. In this study the cross between susceptible by susceptible (S1 × S2, S1 × S3, S1 × S4, S2 × S3, S2 × S4, S2 × S3 and S3 × S4) did not show segregation, but the F2 means were less than either the mid-parent or either susceptible parent. This indicates that none of these parents had any resistance genes. These crosses suggest presence of recessive genes for susceptibility. This result contrasts with the report by Niyongabo et al. [15] that the cross between susceptible by susceptible genotypes showed duplicate dominant epitasis for most of the crosses.
The cross between susceptible by resistant parents showed that only six crosses of S1 × R7, S2 × R6, S2 × R7, S3 × R6, S4 × R6 and S4 × R7 were conformed to the 3:1 ratio, suggesting the presence of at least one dominant gene. This study coincides with the report by Rahim et al. [16] and Rajashekara et al. [17] that the inheritance of blast resistance gene in F2 populations derived from the cross between susceptible and resistant varieties showed a segregation ratio of 3R:1S, in both the normal and reciprocal crosses. On the other hand, three crosses, (S1 × R5, S2 × R5 and S4 × R5) fitting a 9:7 phenotypic ratios, suggested the involvement of two complementary dominant genes (duplicate recessive epistasis). In contrast, three crosses R5 × R6, R5 × R7 and R6 × R7 fit a 15:1 ratio, suggesting the involvement of two independent dominant genes. This result is in conformity with those obtained from the F2 populations from cross between genotype Bluebellel and ramatulasi segregating in the ratio of 15: 1 to race IB-1 [18]. In this study, the F2 distribution analysis and the chi-square test suggest the presence of single gene; two complementary dominants and two independent dominant genes are responsible for inheritance of rice blast resistance.
Conclusion
Effective sources of resistance from incorporated genotypes depends on the gene action they contribute and this study showed that three modes of inheritance controlled the genes resistant to rice blast; namely one dominant gene, two complementary dominants and two independent dominant genes. Genotypes SR33859-HB3324-133, SR33859-HB3324-93 and SR33701-HB3330-78 were considered suitable for future resistance breeding, since they have high negative GCA effects for rice blast resistance. The inheritance of rice blast resistance is mainly controlled by additive gene effects, although a small contribution of non-additive effects was found.
Conflict of Interest
There is no conflict of interest among the authors
Acknowledgments
The authors gratefully acknowledge Alliance for Green Revolution in Africa (AGRA) project for the financial support and the National Crop Resources Research Institute (NaCRRI), Uganda for hosting the research work. In line with this, Fogera National Rice Research and training center acknowledged for hosting the senior author for his internship.
References
- Viana J, Cruz C, Cardoso A (2001) Theory and analysis of partial diallel crosses. Parents and F2 generations. Genet Mol Biol 22: 627–634.
- Allard R (1960) Principles of plant breeding. Wiley, New York, pp: 83–108.
- Herrera R, Coffman (1974) Emasculation of rice by vacuum extraction. Proc Crop Sci Soc Philipp 5: 12–14.
- Talukder M, Leifert C, Price A (2000) Mapping QTLs for partial resistance to blast in rice.Proceedings of the Fourth International Rice Genetics Symposium. In: Kush E, Brar D, Hardy B (eds.) Department of Plant and Soil Science, University of Aberdeen, Aberdeen Philippines.
- Koutroubas SD, Katsantonis D, Ntanos DA, Lupotto E (2009) Blast fungus inoculation reduces accumulation and remobilization of pre-anthesis assimilates to rice grains. Phytopathol Mediterr 48: 240–252.
- IRRI (2014) Standard evaluation system for rice. International Rice Research Institute. Los Banos, Manila, Philippines.
- Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2009) An introduction to GenStat for Windows. VSN International, Waterhouse Street, Hemel Hemstead, UK.
- Griffing B (1956) Concept of general and specific combining ability in relation to diallel crossing system. Aust J Biol Sci 9: 463–493.
- Steel RG, Torrie J, Dickey D (1997) Principles and procedures of statistics: A biometrical approach. New York: McGraw-Hill.
- Mulbah S, QuaquaShimelis HA, Laing MD (2015) Combining ability and gene action of three components of horizontal resistance against rice blast. Euphytica 206: 805–814.
- Fehr WR (1987) Prniciples of cultivar development, theory and technique. Macmillan publishing company, London 1: 536.
- Hammoud SA (2012) Gene action and relative importance of some agronomic and biotic stress traits affecting genetic divergence in rice. J Plant Production 3: 2971–2992.
- Manneh B (2010) Gene action and combining ability for agronomic traits and biotic stress tolerance in rice, pp: 22–26.
- Hayashi K, Yoshida H (2009) Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruit- ment of a retrotransposon as a promoter. Plant Sci 57: 413– 425.
- Niyongabo F, Lamo J, Asea G, Edema R (2010) Genetic studies of resistance to rice blast in upland rice Resume 2008–2010.
- Rahim HA, Bhuiyan AR, Saad A, Azhar M (2013) Identification of virulent pathotypes causing rice blast disease ( Magnaporthe oryzae ) and study on single nuclear gene inheritance of blast resistance in F 2 population derived from Pongsu Seribu 2 × Mahshuri. Australian Journal of Crop Science 7: 1597–1605.
- Rajashekara H, Ellur RK, Khanna A, Nagarajan M, Krishnan SG (2014) Inheritance of blast resistance and its allelic relationship with five major R genes in a rice landrace. Vanasurya 67: 365–369.
- Philippi MC, Prabhu A (1996) Inheritance of blast resistance in rice to two Pyrincularia grisea races, IB-1 and IB-9. Brazilian J genetics 19: 599–604.
Citation: Zewdu Z, Edema R, Lamo J (2018) Genetic Study of Resistance to Rice Blast in Crosses between Korean and Locally Adapted Rice Genotypes. Adv Crop Sci Tech 6: 346. DOI: 10.4172/2329-8863.1000346
Copyright: © 2018 Zewdu Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4419
- [From(publication date): 0-2018 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 3594
- PDF downloads: 825
