Genetic Diversity and Population Structure of Date Palm (Phoenix dactylifera L.) Germplasm from Iran using ISSR Assay
Received: 27-Jun-2022 / Manuscript No. JPGB-22-67774 / Editor assigned: 29-Jun-2022 / PreQC No. JPGB-22-67774(PQ) / Reviewed: 14-Jul-2022 / QC No. JPGB-22-67774 / Revised: 18-Jul-2022 / Manuscript No. JPGB-22-67774(R) / Accepted Date: 23-Jul-2022 / Published Date: 25-Jul-2022
Abstract
Date palm is an important fruit crop cultivated mainly in arid and semiarid regions. The DNA polymorphism among 69 selected Iranian native genotypes belonging to six origin groups and 1 Moroccan genotype was assessed using 10 inter simple sequence repeat (ISSR) primers. Results revealed high genetic variability among studied genotypes. Of the 65 known loci, 46 were polymorphic. Polymorphism information content (PIC) values varied from (6–30 %) with an average 19.4 %,. High number of alleles (Na=1.67), Effective alleles (Ne=1.49), Shanon information index (I=0.40), and polymorphic loci (P= 67.69 %) were detected in “Kerman” population. Based on Nei`s genetic identity, each of the regions of “Morocco”, “Fars” and “Bushehr” populations were located in separate groups, but the rest of the populations were located in the same one. Structure analysis of the studied association panel manifested sevev subpopulations and there was no connection between these subpopulations and their geographical distribution. Likewise, most studied date palm genotypes have mixed genotypes related to their common genetic background. Our finding showed that ISSR markers could recognize male stocks of date palm that are important in crossing programs. This research addresses a more comprehensive picture of Iran's date palms genetic variability and structure. This studied panel can apply parental selection for future breeding programs such as mating designs and mapping populations.
Keywords
Association panel; Genetic variability; PIC; Mixed genotype
Introduction
Date palm (Phoenix dactylifera L.), belonging to the Arecaceae family, is an economically important fruit crop, dioecious, and monocotyledonous. Date palms is a diploid (2n = 2x = 36), and the predicted genome size is estimated to be approximately between 550 and 650 Mbp long [1]. It is cultivated in arid and semiarid regions of the world. Based on historical records and some very narrow germplasm analyses, it has been argued that human selection, clonal propagation, and movement of germplasm by human migration have been the primary forces that have shaped genetic diversity in date palm [2, 3]. This fruit crop is believed to comprise discrete clones of approximately 5,000 cultivars with a limited genetic exchange [4], although it has not been thoroughly verified by molecular marker analysis. Generally based on ripening time (early, mid or late), date palms were grouped into three cultivars. The early-season cultivar has more market able than others [5]. Its fruits possess high nutritional value and contain about 70% sugar, essential vitamins, and minerals, and different value-added products are produced [6]. Date palm is considered a good source of natural antioxidants and anti-mutagenic [7]. In addition to date palm tree is cultivated for fuel, fiber, and shelter for ground crops. Countries including Egypt, Iran, Saudi Arabia, the United Arab Emirates (UAE), Iraq, and Pakistan as leading producers of date palm have accounted for almost 80 % of the world production, amounting to ~5.8 million metric tons [8]. Assessing the genetic diversity of native tree species such as date palms is critical to assisting the conservation of genetic resources, providing a reservoir of genes for developing novel traits associated with yield enhancement, adaptability to climate change, and pest and disease resistance [9]. DNA fingerprinting is mainly exploited for detecting the genetic diversity of plant species and identifying markers linked with specific traits [3, 10]. Several marker systems have been used to study the genetic diversity of date palm. In brief, randomly amplified polymorphic DNA (RAPD) fingerprints have been used to identify date palm accessions in Tunisia [11], Saudi Arabia [12], and Egypt (Soliman et al. 2003; Adawy et al. 2006). Amplified fragment length polymorphic (AFLP) markers have been applied to study the genetic diversity of date palm cultivars in Egypt and California [13, 14]. Inter-simple sequence repeats (ISSRs) are highly discriminative, simple, fast, cost-effective, firm, and reliable in identifying markers. It requires only a small quantity of the DNA sample and does not need prior sequence information to design the primer [15, 16]. ISSR amplification is a comparatively recent technique that can distinguish closely related genotypes and display its suitability for genetic diversity examination.
Moreover, it is exceedingly reproducible, cost-effective, and requires no earlier sequence data [17]. The use of the ISSR marker for detecting the genetic diversity of date palm was reported earlier by many researchers [18, 19]. Despite using vast traditional varieties propagated clonally by offshoots of female trees, little breeding research has been done on native date palm due to its long growth period and a lack of germplasm information. In the current study, we collected 69 date palm genotypes from 6 regions (provinces) and one genotype from Morocco to (i) evaluate the genetic variability of this association panel using ISSR markers (ii) classify of studied regions and identify distinct regions (iii) structure analysis of date palm germplasm using Bayesian approach.
Materials and Methods
Plant material, DNA extraction and quantification
Seventy genotypes were chosen randomly from 7 locations (Bushehr, Fars, Hormozgan, Kerman, Khuzestan, Morocco, Persian Gulf Basin) in Iran (Table 1). The frozen young leaf tissues of date palm collected from each genotype were first cleaned carefully with distilled water to remove the waxy layer. Then, 45 mg of leaf sample was cut into small pieces and ground into a fine powder using liquid nitrogen. Then, DNA was extracted from 45 mg of dried leaf tissue. The samples were ground to a fine powder in a Tissue Lyser II homogenizer (Qiagen) in the presence of steel balls. DNA was extracted using the DNeasy Plant Minikit (Qiagen) following the manufacturer’s instructions. DNA qualification was checked by electrophoresis on 1% agarose gel. The gels were stained in ethidium bromide and visualized under UV light by a NanoDrop spectrophotometer.
| No. | Name | Region (location) | Q matrix | Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | ||||
| 36 | Amme shanbe | Kerman (Kahnuj) | 0.014 | 0.057 | 0.269 | 0.066 | 0.092 | 0.412 | 0.089 | Mixed |
| 37 | Gerd daneh | Hormozgan (Minab) | 0.027 | 0.161 | 0.216 | 0.25 | 0.18 | 0.02 | 0.148 | Mixed |
| 38 | Negar | Kerman (Jiroft) | 0.047 | 0.71 | 0.05 | 0.056 | 0.039 | 0.034 | 0.064 | Mixed |
| 39 | Zardak | Hormozgan (Roodan) | 0.025 | 0.035 | 0.211 | 0.214 | 0.303 | 0.044 | 0.167 | Mixed |
| 40 | Nozohur | Kerman (Jiroft) | 0.036 | 0.022 | 0.156 | 0.059 | 0.112 | 0.555 | 0.06 | Mixed |
| 41 | Mazafati | Kerman (Bam) | 0.036 | 0.027 | 0.094 | 0.332 | 0.201 | 0.026 | 0.284 | Mixed |
| 42 | Tokhmmorghi | Hormozgan (Hajjiabad) | 0.147 | 0.215 | 0.114 | 0.133 | 0.134 | 0.105 | 0.151 | Mixed |
| 43 | Seloni | Kerman (Kahnuj) | 0.05 | 0.03 | 0.102 | 0.404 | 0.195 | 0.031 | 0.186 | Mixed |
| 44 | Lasht | Kerman (Kahnuj) | 0.019 | 0.021 | 0.324 | 0.15 | 0.205 | 0.056 | 0.224 | Mixed |
| 45 | Nobar | Hormozgan (Fin) | 0.018 | 0.062 | 0.124 | 0.242 | 0.21 | 0.027 | 0.316 | Mixed |
| 46 | Posht nabi | Hormozgan (Fin) | 0.022 | 0.026 | 0.126 | 0.32 | 0.123 | 0.033 | 0.35 | Mixed |
| 47 | Shahani | Kerman (Orzuiyeh) | 0.039 | 0.446 | 0.082 | 0.125 | 0.134 | 0.046 | 0.129 | Mixed |
| 48 | Najmeh | Kerman (Kahnuj) | 0.072 | 0.015 | 0.212 | 0.079 | 0.151 | 0.33 | 0.14 | Mixed |
| 49 | Krut | Kerman (Bam) | 0.608 | 0.02 | 0.061 | 0.125 | 0.041 | 0.083 | 0.062 | Mixed |
| 50 | Halileie | Kerman (Shahdad) | 0.192 | 0.056 | 0.183 | 0.145 | 0.177 | 0.05 | 0.196 | Mixed |
| 51 | Zeinabi | Kerman (Kahnuj) | 0.081 | 0.031 | 0.13 | 0.166 | 0.085 | 0.284 | 0.223 | Mixed |
| 52 | Lilgouni | Kerman (Kahnuj) | 0.027 | 0.046 | 0.235 | 0.138 | 0.337 | 0.059 | 0.158 | Mixed |
| 53 | Peirizi | Fars (Lar) | 0.026 | 0.027 | 0.148 | 0.309 | 0.163 | 0.03 | 0.297 | Mixed |
| 54 | Gand gorbeh | Kerman (Jiroft) | 0.472 | 0.05 | 0.095 | 0.146 | 0.076 | 0.049 | 0.111 | Mixed |
| 55 | Farz | Kerman (Kahnuj) | 0.023 | 0.057 | 0.188 | 0.063 | 0.124 | 0.458 | 0.086 | Mixed |
| 56 | Maktoub | Hormozgan (Roodan) | 0.165 | 0.018 | 0.123 | 0.054 | 0.063 | 0.538 | 0.039 | Mixed |
| 57 | Souzdan | Kerman (Jiroft) | 0.123 | 0.009 | 0.151 | 0.247 | 0.197 | 0.115 | 0.158 | Mixed |
| 58 | Yaghuti | Kerman (Bam) | 0.395 | 0.023 | 0.082 | 0.197 | 0.099 | 0.055 | 0.149 | Mixed |
| 59 | Khosh kang | Kerman (Kahnuj) | 0.028 | 0.024 | 0.091 | 0.387 | 0.251 | 0.017 | 0.202 | Mixed |
| 60 | Nesfei | Hormozgan (Roodan) | 0.019 | 0.013 | 0.272 | 0.076 | 0.147 | 0.343 | 0.13 | Mixed |
| 61 | Halileie | Kerman (Shahdad) | 0.058 | 0.034 | 0.321 | 0.082 | 0.276 | 0.024 | 0.204 | Mixed |
| 62 | Gorbani | Kerman (Kahnuj) | 0.032 | 0.019 | 0.094 | 0.342 | 0.108 | 0.034 | 0.371 | Mixed |
| 63 | Kabkab | Bushehr | 0.05 | 0.022 | 0.178 | 0.196 | 0.296 | 0.017 | 0.241 | Mixed |
| 64 | Morad sang | Kerman (Orzuiyeh) | 0.047 | 0.294 | 0.096 | 0.16 | 0.159 | 0.031 | 0.214 | Mixed |
| 65 | Galami | Hormozgan (Roodan) | 0.037 | 0.023 | 0.106 | 0.18 | 0.096 | 0.308 | 0.249 | Mixed |
| 66 | Sahlaki | Kerman (Kahnuj) | 0.024 | 0.017 | 0.075 | 0.33 | 0.134 | 0.014 | 0.407 | Mixed |
| 67 | Zohrei | Kerman (Kahnuj) | 0.017 | 0.025 | 0.073 | 0.267 | 0.069 | 0.252 | 0.297 | Mixed |
| 68 | Makou | Kerman (Kahnuj) | 0.016 | 0.039 | 0.089 | 0.29 | 0.106 | 0.144 | 0.317 | Mixed |
| 69 | Khsoueie | Kerman (Kahnuj) | 0.017 | 0.041 | 0.04 | 0.036 | 0.026 | 0.795 | 0.044 | Pale blue |
| 70 | Kheli get | Kerman (Kahnuj) | 0.063 | 0.033 | 0.081 | 0.309 | 0.107 | 0.079 | 0.328 | Mixed |
DNA fingerprinting by ISSR primers
Ten of 12 anchored ISSR primers were given reproducible bands used in current research. The main characteristics of using the ISSR primers are summarized in (Table 2). Polymerase chain reaction (PCR) was carried out in a total reaction mixture of 25 μl of the mixture contained 4 ng.μl genomic DNA, 1U of Taq DNA Polymerase, 1 × PCR buffer, 2 mM of each dNTPs, 2.5 mM MgCl2 and 4 μM of each primer) [20]. DNA amplification was performed in a 96-well thermal cycler (Veriti®, California, USA). Under the following conditions: initial denaturation at 94°C for 5 min, 32 cycles (denaturation 94°C for 45 s, annealing temperature depending on primer for 45 s, extension 72°C for 2 min), final extension 72°C for 7 min. The ISSR-PCR products were separated on 2.5% agarose gel, stained with ethidium bromide and visualized under UV.
| Primer Sequence |
Number of loci | Polymorphic loci | Monomorphic loci | PIC value(%) | |
|---|---|---|---|---|---|
| P1 | 8 | 7 | 1 | 26 | |
| P2 | 5 | 4 | 1 | 29 | |
| P3 | 5 | 4 | 1 | 10 | |
| P4 | 10 | 8 | 2 | 17 | |
| P5 | 5 | 3 | 2 | 16 | |
| P6 | 6 | 4 | 2 | 20 | |
| P7 | 6 | 6 | 0 | 20 | |
| P8 | 7 | 3 | 4 | 20 | |
| P9 | 6 | 1 | 5 | 6 | |
| P10 | 7 | 6 | 1 | 30 | |
| Total | 65 | 46 | 19 | 19.4 | |
Data analysis
The PCR amplification products were scored for the presence (1) or absence (0) of each band marker across all 70 date palm genotypes, and the data were used to construct a binary data matrix. Software GenAlEx version 6.503 [21] was used to analyze genetic diversity parameters related to each population, including the number of different alleles (Na), the number of effective alleles (Ne), Shannon’s Information Index (I), expected heterozygosity (He) and percentage of polymorphic loci (PL%) as well as pairwise population matrix of Nei genetic identity. Population structure was analyzed using a modelbased Bayesian approach in the software Structure, version 2.3.4 [22]. Five independent runs were performed, setting the sub-populations (K) from 1 to 10, burn-in time and MCMC (Markov Chain Monte Carlo) replication number to 500,000, and a model for admixture and correlated allele frequencies. Delta K (ΔK) was used to represent the K value based on the second-order rate of change in the likelihood [23]. Inferred ancestry estimates of individuals (Q matrix) were derived for the selected population [22]. Trait-marker association analysis was performed using a mixed linear model (MLM) approach in TASSEL 2.1, accounting for population structure and kinship relatedness (Q+K model). Both kinship coefficients and linkage disequilibrium (LD) were calculated via TASSEL 2.1.
Results
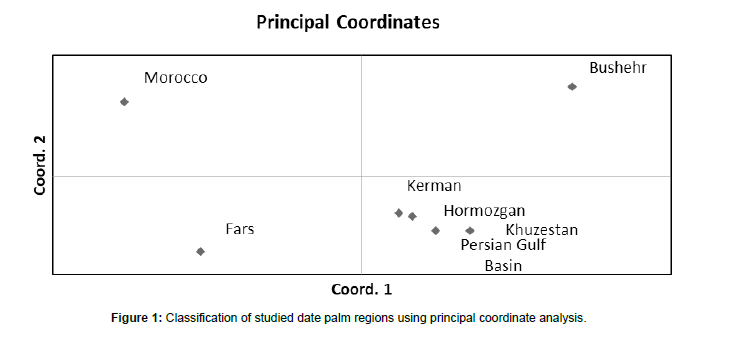
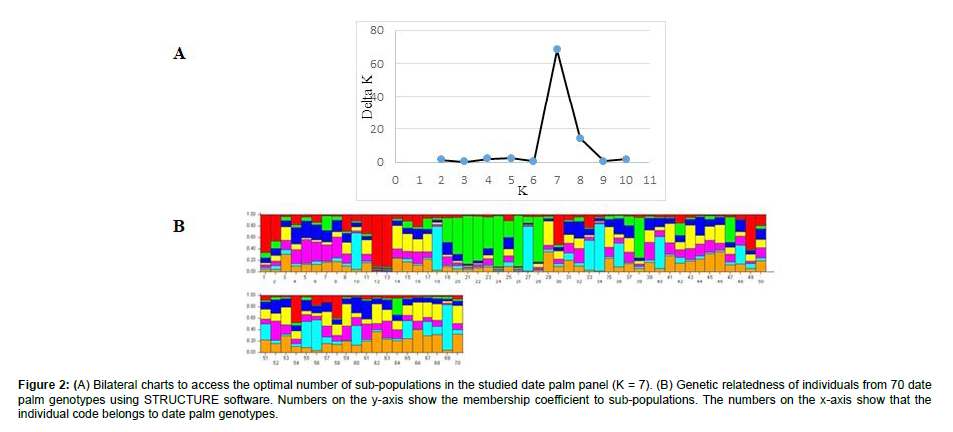
In this research, genetic diversity among 70 date palm genotypes was investigated using 10 ISSR markers and high molecular genetic variability was observed among the studied genotypes. Of the 65 genes studied loci, 45 loci were polymorphic and 19 were monomorphic (Table 2). In this regard, primer P4 with 10 detected loci possessed a maximum number of amplified loci. Resulted that P10 with a PIC value of 30 followed by primers P2 (PIC = 29) and P1(PIC = 26) can effectively be utilized in evaluating date palm genetic variation (Table 2). According to Nei genetic identity (Table 3), the maximum similarity was seen between regions “Hormozgan” and “Kerman”; meanwhile, the minimum value was detected among “Fars” and “Bushehr” regions. According to PCoA analysis of studied populations, regions of “Kerman”, “Hormozgan”, “Khuzestan” and “Persian Gulf Basin” were clustered in the same group and each of the regions of “Bushehr”, “Morocco” and “Fars” located in a separate group (Figure 1). Regarding ISSR marker information of each studied regions, the minimum and maximum values of Na and Ne were possessed in the “Bushehr” and “Kerman” regions, respectively (Table 4). In the current research, the maximum values of He and I were detected for the region of “Kerman”. The average percentage of polymorphic loci (PL%) over regions was 34.73% and region “Kerman” had a maximum value of polymorphic loci (67.69%) (Table 4). In order to understand the genetic structure of the association panel, a modelbased Bayesian approach in the STRUCTURE software was used to assign each genotype to the corresponding subgroup. The group of 70 date palm genotypes was partitioned into seven subgroups (Figure 2A). These subgroups included Red (Diri and Deglet nour genotypes), Green (5 male rootstock genotypes), Pale blue (Halileie and Khasoueie genotypes) and Mixed (the rest of the studied genotypes) based on each genotype Q value (Figure 2B and Table 1).
| Bushehr | Fars | Hormozgan | Kerman | Khuzestan | Morocco | Persian Gulf Basin | |
|---|---|---|---|---|---|---|---|
| Bushehr | 1.000 | ||||||
| Fars | 0.799 | 1.000 | |||||
| Hormozgan | 0.89 | 0.899 | 1.000 | ||||
| Kerman | 0.888 | 0.9 | 0.983 | 1.000 | |||
| Khuzestan | 0.855 | 0.846 | 0.908 | 0.918 | 1.000 | ||
| Morocco | 0.799 | 0.867 | 0.853 | 0.864 | 0.815 | 1.000 | |
| Persian Gulf Basin | 0.864 | 0.869 | 0.927 | 0.921 | 0.89 | 0.828 | 1.000 |
Region |
Na | Ne | I | He | %PL |
|---|---|---|---|---|---|
| Bushehr | 1.154 | 1.152 | 0.13 | 0.089 | 21.54% |
| Fars | 1.246 | 1.196 | 0.167 | 0.115 | 27.69% |
| Hormozgan | 1.6 | 1.475 | 0.376 | 0.262 | 60.00% |
| Kerman | 1.677 | 1.493 | 0.406 | 0.279 | 67.69% |
| Khuzestan | 1.308 | 1.235 | 0.192 | 0.132 | 32.31% |
| Persian Gulf Basin | 1.323 | 1.255 | 0.201 | 0.139 | 33.85% |
| Grand Mean over Loci and regions | 1.299 | 1.258 | 0.21 | 0.145 | 34.73% |
| Na = No. of Different Alleles Ne = No. of Effective Alleles = 1 . (p^2 + q^2) I = Shannon's Information Index = -1* (p * Ln (p) + q * Ln(q)) He = Expected Heterozygosity = 2 * p * q %PL = percentage of polymorphic loci |
|||||
Discussion
The date palm can be assumed as an important fruit crop due to its great potential to generate income in extreme environmental conditions and unproductive lands. As well as, there are vast and worthy cultivars and genotypes of it have been planted in southern regions of Iran for many years [24]. Accordingly, characterization of date palm germplasm from Iran is a prerequisite for its improvement through breeding programs. This study implemented ISSR markers to fingerprint date palm genotypes from six regions (provinces) of Iran, accompanied by a genotype from Morocco. The current study implies that ISSR markers are effective tools to discriminate various date palm genotypes. Several studies reported the efficiency of ISSR markers in fingerprinting and distinguishing date palm genotypes (Srivashtav et al. 2013; Marsafari and Mehrabi 2013; Purayil et al. 2018; Abdelaziz et al. 2020). In this research, the percent of polymorphism revealed by ISSR markers was 70.76% (46 loci out of 65 detected loci), meanwhile previously reported as 28.6% by Hussein et al. [25], 64.1% by Adawy et al [14], and 95% by Marsafari and Mehrabi (2013).
The most important parameter proposed to calculate for a molecular marker data set is the polymorphism information content (PIC) value that measures the ability of a marker to detect polymorphisms and has enormous importance in selecting markers for genetic studies [26]. Herein, P10 among studied primers possessed the highest PIC value and will be effectively recommended to evaluate date palm germplasm. The average PIC (19.4) observed in the present work is comparable with previous reports by Purayil et al. (2018) and Abdelaziz et al. (2020), with values of 0.23 and 0.45, respectively. Despite low PIC values, these ISSR markers can be utilized to estimate relationships between varieties based on their geographic origin [27]. The pairwise Nei genetic identity between studied regions was calculated and then PCoA analysis revealed four major clusters for studied regions. In this regard, the region “Morocco” has been constructed as a separate class which is logical. There was no coincidence between geographical distribution and Nei genetic identity in other studied regions. For instance, the regions “Bushehr” and “Fars” have also been located separately; they were geographically near each other. Oppositely, regions that were far from together (“Kerman”, “Hormozgan”, “Khuzestan” and “Persian Gulf Basin”) were located in the same group (Figure 1). Similar to our findings, in a study of desert date (Balanites aegyptiaca Del.) genetic variation, five geographically distant populations was located in the same group (Abdelaziz et al. 2020). Overall, moderate genetic differentiation was detected among studied regions in this research. This finding is similar to what was reported for oil palm by using an SSR [28]. Among studied regions, the “Kerman” and then “Hormozgan” showed maximum Ne, I, and PL values, which implied their importance for future date palm breeding programs. However, it seems that “Kerman” should be considered more than others because of its male rootstocks and the male genotypes that could influence the quantity and quality of progenies [29]. According to the model-based clustering for the genetic structure of date palm individuals, seven genetically distinctive subpopulations were presented that were not formed in line with their geographical location. The most probable clustering of genotypes was observed at this K level and showed admixture structure among genotypes. Our result displayed studied populations with a common genetic background and shared common alleles among them. The most differentiated genotypes were observed in “Kerman” region, which showed fewer admixtures than the rest regions. Generally, the structure results showed shared ancestry between date palm genotypes and cultivars that were early introduced [30].
Figure 2: (A) Bilateral charts to access the optimal number of sub-populations in the studied date palm panel (K = 7). (B) Genetic relatedness of individuals from 70 date palm genotypes using STRUCTURE software. Numbers on the y-axis show the membership coefficient to sub-populations. The numbers on the x-axis show that the individual code belongs to date palm genotypes.
Conclusion
Genetic diversity is desirable for long-term crop improvement such as date palm and reduction of vulnerability in plants to important crop diseases. In the present study, the ISSR marker was handled to evaluate the genetic diversity of date palm germplasm and confirm the potential use of ISSR markers in date palm fingerprinting. The studied region “Kerman” manifested remarkable genetic variability compared with other regions. That is expectable because “Kerman” is the first producer of date palm in Iran and evolved most cultivated varieties and genotypes. The genetic structure of inspected germplasm depicted 7 subpopulations with mostly mixed genetic backgrounds implying a common genetic background and shared alleles between them. In this study, the clustering pattern of genotypes was independent of their geographical distances. In summary, the analysis of ISSR markers of the date palm genotypes documents the significant variation present in the in situ found in the regions and confirms the need to conserve this valuable resource.
References
- Malek JA (2010) Next generation DNA sequencing applied to the Date palm tree (Phoenix dactylifera). Acta Hortic 882: 249–252.
- Jaradat AA (2011) Biodiversity of date palm. Eolss Publishers, Oxford, UK: 31.
- Khanam S, Sham A, Bennetgen JL, Mohammed AMA (2012) Analysis of molecular marker-based characterization and genetic variation in date palm (Phoenix dactylifera L.). Austral J Crop Sci 6: 1236-1244.
- Bashah M (1996) Date variety in the Kingdom of Saudi Arabia. In: King Abdulaziz Univ guidance booklet palms and dates. King Abdul Aziz Univ Press, Riyadh, Saudi Arabia: 1225–1319.
- Cheruth AJ, Kurup SS, Subramaniam S (2015) Variations in hormones and antioxidant status in relation to flowering in early, mid, and late varieties of date palm (Phoenix dactylifera) of United Arab Emirates. Sci World J 2015: 846104.
- Aljaloud S, Colleran HL, Ibrahim SA (2020) Nutritional value of date fruits and potential 8 use in nutritional bars for athletes. Food Nutr Sci 11: 463–480.
- Mansouri A, Embarek G, Kokkalou E, Kefalas P (2005) Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem 89: 411–420.
- Al-Dous EK, George B, Al-Mahmoud ME, Al-Jaber MY, Wang H, et al. (2011) De novo genome sequencing and comparative genomics of date palm (Phoenix dactylifera). Nat Biotechnol 29: 521–527.
- Xu Y (2010) Molecular plant breeding. CABI, United Kingdom: 734.
- Kurup SS, Hedar YS, Al Dhaheri MA, El-Heawiety AY, Aly MAM, Alhadrami G (2009) Morpho-physiological evaluation and RAPD markers-assisted characterization of date palm (Phoenix dactylifera L.) varieties for salinity tolerance. J Food Agric Env 7: 503–507.
- Trifi M, Rhouma A, Marrakchi M (2000) Phylogenetic relationships in Tunisian date-palm (Phoenix dactylifera L.) germplasm collection using DNA amplification fingerprinting. Agronomie 20: 665–671.
- Al-Khalifah NS, Askari E (2003) Molecular phylogeny of date palm (Phoenix dactylifera L.) cultivars from Saudi Arabia by DNA fingerprinting. Theor Appl Genet 107: 1266–1270.
- El-Assar AM, Krueger RR, Devanad PS, Chao CT (2005) Genetic analysis of Egyptian date (Phoenix dactylifera L.) accessions using AFLP markers. Genet Resour Crop Evol 52: 601–607.
- Adawy SS, Hussein EHA, El-Khishin D, Saker MM, Mohamed AA, et al. (2004) Genotyping Egyptian date palm cultivars using RAPD, ISSR, AFLP markers and estimation of genetic stability among tissue culture derived plants. Arab J Biotech 8: 99-114.
- Williams JGK, Rubelik AR, Livak KJ, Rafalski A, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18: 6531–6535.
- Gupta M, Chyi YS, Severson JR, Owen JL (1994) Amplification of DNA markers from evolutionarily diverse genomes using single primers of simple-sequence repeats. Theor Appl Genet 89: 998–1006.
- Bornet BC, Muller FP, Branchard M (2002) Highly informative nature of inter simple sequence repeat (ISSR) sequences amplified using tri- and tetra-nucleotide primers from DNA of cauliflower (Brassica oleracea var. ‘botrytis’ L.). Genome 45: 890–896.
- Zehdi S, Trifi M, Ould MSA, Rhouma A, Marrakchi M (2004) Molecular characterization of Tunisian date palm germplasm using ISSR markers. J Genet Breed 56: 77–83.
- Karim K, Chokri B, Amel S, Wafa H, Richid H, et al. (2010) Genetic diversity of Tunisian date palm germplasm using ISSR markers. Int J Bot 6: 182–186.
- Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprint by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20: 176–183.
- Peakall R, Smouse PE (2006) GENEALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6: 288–295.
- Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945–959.
- Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611.
- El hadrami I, El hadrami A (2009) Breeding date palm. In: Jain SM, Priyadarshan PM (eds) Breeding plantation tree crops: tropical species. Springer, New York, USA: 191-216.
- Hussein EHA, Adawy SS, Ismail SEM, El-Itriby HA (2004) Molecular characterization of some Egyptian date palm germplasm using RAPD and ISSR markers. Arab J Biotech 8: 83-98.
- Serrote CML, Reiniger LRS, Silva KB, Rabaiolli SMS, Stefanel CM (2020) Determining the polymorphism information content of a molecular marker. Gene 726: 144-175.
- Meszaros K, Karsai I, Kuti C, Banyai J, Lang L, et al. (2007) Efficiency of different marker systems for genotype fingerprinting and for genetic diversity studies in barley (Hordeum vulgare L.). South Afric J Bot 73: 43-48.
- Gan ST, Teo CJ, Manirasa S, Wong WC, Wong CK (2021) Assessment of genetic diversity and population structure of oil palm (Elaeis guineensis Jacq.) field genebank: A step towards molecular-assisted germplasm conservation. PLoS ONE 16: e0255418.
- Rivera D, Alcaraz F, Rivera-Obón D, Obón C (2022) Phenotypic diversity in wild and cultivated date palm (Phoenix, Arecaceae): Quantitative analysis using information theory. Horticulturae 8: 287.
- Ahmed W, Feyissa T, Tesfaye K, Farrakh S (2021) Genetic diversity and population structure of date palms (Phoenix dactylifera L.) in Ethiopia using microsatellite markers. J Genet Eng Biotechnol 19: 64.
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Citation: Khorshidi F, Khoshroo SMR, Toolir JF, Damankeshan B (2022) Genetic Diversity and Population Structure of Date Palm (Phoenix dactylifera L.) Germplasm from Iran using ISSR Assay. J Plant Genet Breed 6: 125.
Copyright: © 2022 Khorshidi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2109
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1761
- PDF downloads: 348