Genetic Diversity and Population Structure of Boro Rice Landraces of Bangladesh
Received: 08-Jun-2022 / Manuscript No. rroa-22-66141 / Editor assigned: 10-Jun-2022 / PreQC No. rroa-22-66141 / Reviewed: 15-Jun-2022 / QC No. rroa-22-66141 / Revised: 18-Jun-2022 / Manuscript No. rroa-22-66141 / Published Date: 30-Jun-2022 DOI: 10.4172/2375-4338.1000305
Abstract
Molecular characterization, evaluation of genetic diversity and assessment of relationship by simple sequence repeat (SSR) markers of 48 Bangladeshi Boro rice landraces was performed. Among the total 58 SSR markers, 55 markers has been distributing over 12 rice chromosomes showed clear polymorphic band patterns, and they were selected for genetic relationship assessment. A total of 228 alleles were detected with an average of 4.15 alleles per locus. The average values of gene diversity and polymorphic information content (PIC) were 0.39 and 0.36, respectively. Primer RM206 had the highest PIC value (0.78) and the highest number of alleles (10).Therefore, RM206 was detected for the highest level of polymorphism and RM206 is supposed to be the best marker for characterizing the 48 Boro rice landraces. The genetic distance-based results in the unrooted neighbor-joining (NJ) tree revealed four (4) major clusters (I,II,III and IV)and a model-based population structure analysis generated two clusters (A and B). Both neighbors joining tree analysis and the population structure analysis method showed the tested landraces as highly diverse in structure. The two and three dimensional graphical views of Principal Coordinate Analysis (PCoA) revealed that the landraces Mi-Pajang, Gopal Beshi, Borail, Madhabsail, Boro (sunga) and Jala Boro were found far away and distributed around the centroid of the cluster. This rice collection and information gained in this study will be useful for future rice breeding program.
Keywords
Rice; Genetic diversity; Molecular characterization; Simple sequence repeat
Introduction
Rice has shaped the culture, diets and economic of thousands of millions of peoples. For more than half of the humanity “rice is life”. Bangladesh has abundant diversified rice landraces from time immemorial, since rice plays an important role in the livelihood, cultures and socio-economic aspects of the people, and is also the main cereal food in Bangladesh [1]. It has been cultivated in Asia since ancient times and for generations farmers have maintained thousands of different landraces [2]. Now, 90% of world rice is produced in Asia on an area of almost 150 million hectares. Rice accounts for 50% of agricultural income in Asia and supplies almost 80% of the region’s nutrition. In Bangladesh rice engages more than 70% of the rural population and is central to agriculture and the national economy [3].Due to great significance and intimate association of rice in food security and local ways of life and culture, Asian farmers have selected and maintained a vast array of over thousands of years. Scientists estimate that more than 1,40,000 rice varieties have been developed/selected/isolated in Asia [4]. More than 1,32,000 rice accessions and wild relatives can be found in the world’s largest Genebank for rice at IRRI (International Rice Research Institute) located in the Philippines (https://www.irri. org/international-rice-genebank). Until now, Bangladesh Rice Research Institute (BRRI) has collected and preserved more than 8,000 varieties/ landraces/cultivars/advanced lines/wild types from indigenous and exotic sources [4]. After establishment of BRRI, characterization or DNA fingerprinting has been done only for a small number of local landraces. Many countries in the world have characterized their indigenous different crop landraces at both molecular and phenotypic level. This has been done for keeping their crop identity and for searching new genes for further crop improvement. But information about the genetic diversity of local landraces as well as Boro rice is very limited. The needs for varietal improvement for such situations are very important.
Molecular markers are the powerful tools to detect genetic variation and genetic relationship within and among species. DNA markers are used for unmasking new genes and for the improvement of crop varieties [5]. The use of DNA markers has been suggested for precise and reliable characterization and discrimination of rice genotypes [6]. For genetic variability assessment, DNA markers are extensively used because they are not affected by environmental factors. Microsatellites (SSRs) are the marker of choice because of their advantages over other markers. These markers are polymorphic, abundant in eukaryotic organisms, and well distributed throughout the genome [7,8]. The SSRs are most suitable for rice because of their reproducibility, multiallelic nature, hypervariablility, codominant inheritance, relative abundance, and genome-wide coverage [9]. In addition, SSRs often have flanking regions that are highly conserved in related species, which allows the use of the same primer pairs in related genomes [10,11]. The SSR markers are particularly suitable for evaluating genetic diversity and relationships among plant species, populations, or individuals [12, 13]; studying rice landraces for either conservation or utilization [14]; marker-assisted selection breeding [15,16]; cultivar identification; and hybrid purity analysis and gene mapping studies [17,18,19,20]. Selection of parents based on genetic divergence using SSR markers has been successfully utilized in multiple crop species [21, 6].
Assessment of genetic diversity is very important in plant breeding or in biotechnology, if selection is the basis of improvement. For the assessment or analysis of genetic diversity, molecular markers are superior to morphological, pedigree, heterosis and biochemical data [22]. Genetic diversity is generally measured by genetic distance or genetic similarity, which imply that there are either differences or similarities at the genetic level of the plant [23]. Molecular marker based Genetic Diversity Analysis (MMGDA) also has potential for assessing changes in genetic diversity over time and space [24]. As the variation among the genotypes comes from the variations in DNA sequence, therefore, variations in DNA sequence are the basis of genetic diversity analysis [25]. Though, rice genome sequence is a valuable [26], most researchers are trying to identify particular segment of DNA or gene in a definite chromosome [25]. Molecular markers are the molecules that can trace a required gene in observed genotypes [27].
Information on the genetic diversity within and among closely related crop varieties is essential for a rational use of genetic resources and is of fundamental interest to plant breeders. It contributes to monitor landraces and can also be used to predict potential genetic gains [28]. Information regarding genetic variability at the molecular level could be used to help identify and develop genetically unique landraces that complements existing cultivars [29]. The objectives of this research were to assess the genetic variation and diversity of 48 Boro rice landraces, determine the genetic relationship among these landraces for breeding purposes and characterize these boro rice landraces.
Materials and Methods
Rice materials
In total, 48 Boro rice landrces of Bangladesh were studied (Table 1). Five gram seed from each of the entry was germinated and then sown in seedbed for growth and subsequent DNA extraction.
| Sl. No | Landraces | Acc. no. | Location of collection | Sl. No | Landraces | Acc. no. | Location of collection |
|---|---|---|---|---|---|---|---|
| G 1 | Mi-Pajang | 149 | Tangail | G 25 | Boro Dhan | 1808 | Kishorganj |
| G 2 | Dholi Boro | 180 | Tangail | G 26 | Boro Jagli | 1809 | Kishorganj |
| G 3 | Kumri Boro | 257 | Mymensingh | G 27 | Jagli | 1810 | Kishorganj |
| G 4 | Bairagi Sail | 261 | Mymensingh | G 28 | Deshi Boro | 1815 | Kishorganj |
| G 5 | Tepi Khorch | 931 | Sylhet | G 29 | Boro Dhan | 1816 | Kishorganj |
| G 6 | Pankaich | 937 | Sylhet | G 30 | Boro (Sunga) | 1861 | Dinajpur |
| G 7 | Boro Deshi | 938 | Sylhet | G 31 | Jala Boro | 1866 | Dinajpur |
| G 8 | Gopal Beshi | 939 | Sylhet | G 32 | Kali Boro 2/2 | 2189 | Gazipur |
| G 9 | Borail | 940 | Sylhet | G 33 | Kali Boro 4/1 | 2190 | Gazipur |
| G 10 | Boro 6/2 | 2206 | Gazipur | G 34 | Kali Boro 26 | 2191 | Gazipur |
| G 11 | Kali Boro | 1049 | Khulna | G 35 | Kali Boro 41/1 | 2192 | Gazipur |
| G 12 | Sonar Geye | 1050 | Khulna | G 36 | Kali Boro 48/1 | 2193 | Gazipur |
| G 13 | Joya Boro | 1051 | Khulna | G 37 | Kali Boro 80/3 | 2194 | Gazipur |
| G 14 | Amboro2 (Golden) | 1473 | Dhaka | G 38 | Kali Boro 80/5 | 2195 | Gazipur |
| G 15 | Batti Boro | 1477 | Dhaka | G 39 | Kali Boro 109/4 | 2196 | Gazipur |
| G 16 | Madhabsail | 1651 | Dhaka | G 40 | Kali Boro 138/2 | 2197 | Gazipur |
| G 17 | Jagli | 1704 | Faridpur | G 41 | Kali Boro 139/2 | 2198 | Gazipur |
| G 18 | Jagli | 1705 | Faridpur | G 42 | Kali Boro 200 | 2199 | Gazipur |
| G 19 | Local Boro | 1753 | Khulna | G 43 | Kali Boro 208 | 2200 | Gazipur |
| G 20 | Saita | 1794 | Kishorganj | G 44 | Kali Boro 259 | 2201 | Gazipur |
| G 21 | Dud Saita | 1795 | Kishorganj | G 45 | Kali Boro 266 | 2202 | Gazipur |
| G 22 | Bogra Boro | 1804 | Kishorganj | G 46 | Kali Boro 576 | 2203 | Gazipur |
| G 23 | Deshi Boro | 1805 | Kishorganj | G 47 | Kali Boro 600 | 2204 | Gazipur |
| G 24 | Jagli (DeshiBoro) | 1806 | Kishorganj | G 48 | Kali Boro 704 | 2205 | Gazipur |
Table 1: List of 48 Boro rice landraces.
SSR markers
A total of 55 SSR markers (Table 2) were found polymorphic and used for molecular characterization and diversity analysis.
Genomic DNA extraction and amplification
Total genomic DNA was extracted from young leaves of threeweek- old plants following the quick DNA extraction protocol of Ferdous et al. [30]. PCR analysis was performed in 10μl reaction sample containing 3μl of DNA template, 4.5 μl of GoTaq G2 Green Master Mix (Promega, USA), 1.5 μl of Nuclease-Free Water, 0.5 μl each of 10 μM forward and reverse primers using a GeneAtlas G (Astec, Japan) 96-well thermal cycler. The mixture was overlaid with 10 μl of mineral oil to prevent evaporation. After initial denaturation for five minutes at 94°C, each cycle comprised 30 sec denaturation at 95°C, 30 sec annealing at 55°C, and 25 sec extension with a final extension for 5 min at 72°C at the end of 32 cycles. The PCR products were analyzed by electrophoresis on 8% polyacrylamide gel with a 1 Kb plus DNA ladder (Thermo Scientific, USA) using mini vertica lpolyacrylamide gels for high throughput manual genotyping (CBS Scientific Co. Inc., CA, USA). 2.5 μl of amplification products were resolved by running gel in 0.5X TBE buffer for 1.5-2.5 hrs depending upon the allele size at around 100volts and 500 mA current. The gels were stained in 5 μl SYBR Safe DNA gel stain (10,000X concentration in DMSO, USA) with 200 ml 0.5X TBE buffer for 15 min and exposed to UV light using a molecular imager gel documentation unit (XR System, Uvitec Cambridge, France) for visualization. Microsatellite or simple sequence repeat (SSR) markers were used for molecular analysis [31,32]. We used fifty-five well-distributed SSRs for the diversity analysis; position (cM), repeat motifs, and chromosomal positions for the SSR markers can be found in the rice genome database (Gramene Portals, 2017). Most of these markers were obtained from a panel of fifty standard SSR markers, which has been proposed by CGIAR (Consultative Group for International Agricultural Research), for rice diversity analysis [33,34].
Data analysis
The band-size for each of the markers was scored using the AlphaEaseFC 4.0 software. Using PowerMarker version 3.25 [35], summary statistics included the following: the number of alleles, the major allele size and its frequency, gene diversity, and the polymorphism information content (PIC) value. For the unrooted phylogenic tree, the genetic distance was calculated using MEGA 6 based on Nei’s unbiased pairwise [36,37]. Binary form for allele frequency was prepared using PowerMarker software and used for dendrogram construction by NTSYS-pc software [38]. The unweighted pair grouping method using arithmetic average (UPGMA) was used to determine a similarity matrix following the Dice coefficient with the SAHN subprogram. Population STRUCTURE for landraces was determined using STRUCTURE, (version 2.3.4) [39, 40]. The number of clusters (K) investigated, in this study, ranged from one to fifteen, with five replications for analysis of each K value. The model following admixture and correlated allele frequency with a 5,000 burn period and a run length of 50,000 were used for conducting model-based structure analysis. Output of analysis was collected using the STRUCTURE harvester [41] and identified 4 as the best K value based on the LnP(D) and Evanno’s ΔK [42]. Principal components analysis (PCA) analysis was conducted also using the NTSYS-pc software.
Results and Discussion
SSR marker-based diversity and molecular characterization
All the 48Boro rice landraces distributed overall 12 chromosomes of rice were genotyped with 55 simple sequence repeat (SSR) markers. All 55 markers exhibiting polymorphism.
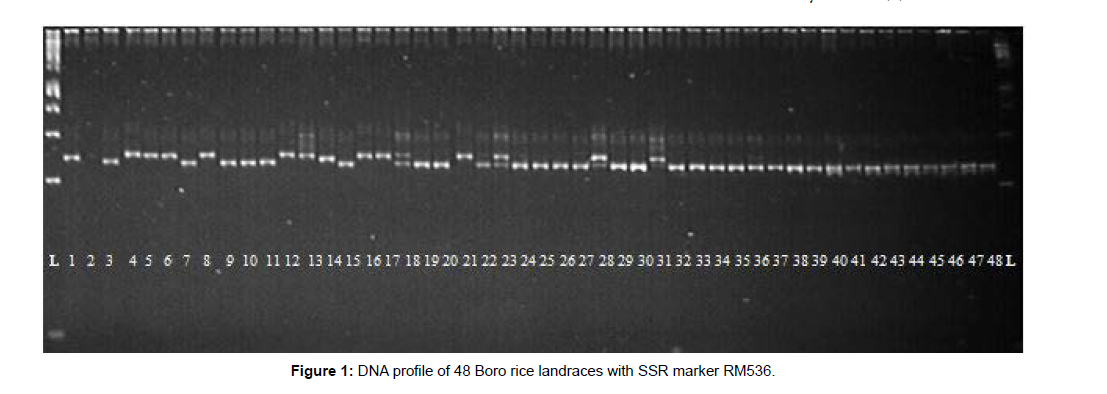
A total of 228 alleles were identified at 55 SSR markers over 48 Boro landraces (Table 2). RM484 (296bp) produced the maximum amplicon size and RM289 (88bp) was the minimum. In the case of RM472 (286- 306bp), a maximum range of band sizes was found and succeeded by RM484 (289-296bp) and RM591 (247-279 bp), respectively. The average number of alleles per locus was 4.15, ranged from 2 (RM133, RM145, RM212, RM316, RM320, RM338, RM411, RM452, RM455 and RM484) to 10 (RM206). The polymorphism information content (PIC) for the SSR loci ranged from 0.04 (RM455) to 0.78 (RM206) with an average of 0.36. Marker RM206 had the highest PIC value (0.78) and the highest number of alleles (10). SSRs which have higher PIC values have a higher number of alleles. Lower PIC value shows that the landraces under study are closely allied, whereas the higher value of PIC stipulates the higher array of materials which is the utmost need for parents selection for hybridization as well as for the new improved variety development, accordingly. Therefore, RM206 was detected as the highest level of polymorphism and RM206 is supposed to be the best marker for characterizing the 48 Boro rice landraces. The gene diversity ranged from 0.04 to 0.81, with an average of 0.39.The frequency of the most common allele at each locus ranged from 29.17% (RM206) to 97.92% (RM455). On average, 73.83% of the 48 rice landraces shared a common major allele at any given locus. The DNA profiles of 48 Boro rice landraces with RM536 are shown in (Figure 1).
| Sl. No. | Marker Name | Chro. No. |
Forward primer (5′–3′) | Reverse primer (5′–3′) | Annealing Temp. |
|---|---|---|---|---|---|
| 1 | RM1 | 1 | GCGAAAACACAATGCAAAAA | GCGTTGGTTGGACCTGAC | 55 |
| 2 | RM5 | 1 | TGCAACTTCTAGCTGCTCGA | GCATCCGATCTTGATGGG | 55 |
| 3 | RM212 | 1 | CCACTTTCAGCTACTACCAG | CACCCATTTG | 55 |
| 4 | RM472 | 1 | CCATGGCCTGAGAGAGAGAG | AGCTAAATGGCCATACGGTG | 55 |
| 5 | RM283 | 1 | GTCTACATGTACCCTTGTTGGG | CGGCATGAGAGTCTGTGATG | 55 |
| 6 | RM495 | 2 | AATCCAAGGTGCAGAGATGG | CAACGATGACGAACACAACC | 55 |
| 7 | RM307 | 2 | GTACTACCGACCTACCGTTCAC | CTGCTATGCATGAACTGCTC | 55 |
| 8 | RM145 | 2 | CCGGTAGGCGCCCTGCAGTTTC | CAAGGACCCCATCCTCGGCGTC | 56 |
| 9 | RM208 | 2 | TCTGCAAGCCTTGTCTGATG | TAAGTCGATCATTGTGTGGACC | 55 |
| 10 | RM213 | 2 | ATCTGTTTGCAGGGGACAAG | AGGTCTAGACGATGTCGTGA | 55 |
| 11 | RM262 | 2 | CATTCCGTCTCGGCTCAACT | CAGAGCAAGGTGGCTTGC | 55 |
| 12 | RM263 | 2 | GCGCTGGTGGAAAATGAG | GGCATCCCTCTTTGATTCCTC | 55 |
| 13 | RM452 | 2 | CTGATCGAGAGCGTTAAGGG | GGGATCAAACCACGTTTCTG | 55 |
| 14 | RM207 | 2 | CCATTCGTGAGAAGATCTGA | CACCTCATCCTCGTAACGCC | 55 |
| 15 | RM411 | 3 | ACACCAACTCTTGCCTGCAT | TGAAGCAAAAACATGGCTAGG | 55 |
| 16 | RM520 | 3 | AGGAGCAAGAAAAGTTCCCC | GCCAATGTGTGACGCAATAG | 55 |
| 17 | RM3646 | 3 | ACTAGAGCACCCTCGCTGAG | CTCAGCCACCCCATCAAC | 55 |
| 18 | RM338 | 3 | CACAGGAGCAGGAGAAGAGC | GGCAAACCGATCACTCAGTC | 55 |
| 19 | RM16 | 3 | CGCTAGGGCAGCATCTAAA | AACACAGCAGGTACGCGC | 55 |
| 20 | RM252 | 4 | TTCGCTGACGTGATAGGTTG | ATGACTTGATCCCGAGAACG | 55 |
| 21 | RM273 | 4 | GAAGCCGTCGTGAAGTTACC | GTTTCCTACCTGATCGCGAC | 55 |
| 22 | RM303 | 4 | GCATGGCCAAATATTAAAGG | GGTTGGAAATAGAAGTTCGGT | 55 |
| 23 | RM334 | 5 | GTTCAGTGTTCAGTGCCACC | GACTTTGATCTTTGGTGGACG | 55 |
| 24 | RM26 | 5 | GAGTCGACGAGCGGCAGA | CTGCGAGCGACGGTAACA | 55 |
| 25 | RM289 | 5 | TTCCATGGCACACAAGCC | CTGTGCACGAACTTCCAAAG | 55 |
| 26 | RM413 | 5 | GGCGATTCTTGGATGAAGAG | TCCCCACCAATCTTGTCTTC | 55 |
| 27 | RM190 | 6 | CTTTGTCTATCTCAAGACAC | TTGCAGATGTTCTTCCTGATG | 55 |
| 28 | RM170 | 6 | TCGCGCTTCTTCCTCGTCGACG | CCCGCTTGCAGAGGAAGCAGCC | 55 |
| 29 | RM125 | 6 | ATCAGCAGCCATGGCAGCGACC | AGGGGATCATGTGCCGAAGGCC | 55 |
| 30 | RM133 | 6 | TTGGATTGTTTTGCTGGCTCGC | GGAACACGGGGTCGGAAGCGAC | 61 |
| 31 | RM253 | 6 | TCCTTCAAGAGTGCAAAACC | GCATTGTCATGTCGAAGCC | 55 |
| 32 | RM510 | 7 | AACCGGATTAGTTTCTCGCC | TGAGGACGACGAGCAGATTC | 55 |
| 33 | RM320 | 7 | CAACGTGATCGAGGATAGATC | GGATTTGCTTACCACAGCTC | 55 |
| 34 | RM455 | 7 | AACAACCCACCACCTGTCTC | AGAAGGAAAAGGGCTCGATC | 55 |
| 35 | RM223 | 8 | GAGTGAGCTTGGGCTGAAAC | GAAGGCAAGTCTTGGCACTG | 55 |
| 36 | RM342 | 8 | CCATCCTCCTACTTCAATGAAG | ACTATGCAGTGGTGTCACCC | 55 |
| 37 | RM515 | 8 | TAGGACGACCAAAGGGTGAG | TGGCCTGCTCTCTCTCTCTC | 55 |
| 38 | RM447 | 8 | CCCTTGTGCTGTCTCCTCTC | ACGGGCTTCTTCTCCTTCTC | 55 |
| 39 | RM201 | 9 | CTCGTTTATTACCTACAGTACC | CTACCTCCTTTCTAGACCGATA | 55 |
| 40 | RM205 | 9 | CTGGTTCTGTATGGGAGCAG | CTGGCCCTTCACGTTTCAGTG | 55 |
| 41 | RM316 | 9 | CTAGTTGGGCATACGATGGC | ACGCTTATATGTTACGTCAAC | 55 |
| 42 | RM228 | 10 | CTGGCCATTAGTCCTTGG | GCTTGCGGCTCTGCTTAC | 55 |
| 43 | RM304 | 10 | TCAAACCGGCACATATAAGAC | GATAGGGAGCTGAAGGAGATG | 55 |
| 44 | RM591 | 10 | CTAGCTAGCTGGCACCAGTG | TGGAGTCCGTGTTGTAGTCG | 55 |
| 45 | RM484 | 10 | TCTCCCTCCTCACCATTGTC | TGCTGCCCTCTCTCTCTCTC | 55 |
| 46 | RM536 | 11 | TCTCTCCTCTTGTTTGGCTC | ACACACCAACACGACCACAC | 55 |
| 47 | RM202 | 11 | CAGATTGGAGATGAAGTCCTCC | CCAGCAAGCATGTCAATGTA | 55 |
| 48 | RM206 | 11 | CCCATGCGTTTAACTATTCT | CGTTCCATCGATCCGTATGG | 55 |
| 49 | RM209 | 11 | ATATGAGTTGCTGTCGTGCG | CAACTTGCATCCTCCCCTCC | 55 |
| 50 | RM144 | 11 | TGCCCTGGCGCAAATTTGATCC | GCTAGAGGAGATCAGATGGTAGGCATG | 55 |
| 51 | RM224 | 11 | ATCGATCGATCTTCACGAGG | TGCTATAAAAGGCATTCGGG | 55 |
| 52 | RM19 | 12 | CAAAAACAGAGCAGATGAC | CTCAAGATGGACGCCAAGA | 55 |
| 53 | RM277 | 12 | CGGTCAAATCATCACCTGAC | CAAGGCTTGCAAGGGAAG | 55 |
| 54 | RM1337 | 12 | GTGCAATGCTGAGGAGTATC | CTGAGAATCTGGAGTGCTTG | 55 |
| 55 | RM12 | 12 | TGCCCTGTTATTTTCTTCTCTC | GGTGATCCTTTCCCATTTCA | 55 |
Table 2: List of 55 SSR markers.
In recent past, molecular characterization, genetic diversity and population structure of Bangladeshi rice landraces have been studied by using molecular (SSR) markers [1, 36, 34, 43].
From our present study, the genetic diversity is similar to earlier studies [29], they identified 4.18 alleles per locus and an average PIC value of 0.488 among 21 rice landraces (Table 3). Also, the average PIC value 0.44 was observed among 43 Thai and 57 IRRI landraces of rice by Chakhonkaen et al. [45]. But, Ahmed et al. [1] found 350 alleles from similarly named rice landraces using 45 SSR markers and several alleles per locus ranged from 3 to 14 with an average of 7.8 which was higher than our present study. On the other hand, a lower genetic diversity was disclosed among 50 Bangladeshi red rice varieties were collected from different agro-climatic regions of Bangladesh having 3.24 alleles per locus with mean PIC value of 0.32, also reported by Islam et al. [34]. Also, a marginally lower genetic diversity was disclosed among 28 restorer lines of hybrid rice with an average of 2.67 alleles per locus and an average PIC value of 0.29 [46].
| SL. No. | Marker | Chromosome No. |
Position (cM) |
Motif* | Allele No. |
Unique allele |
Size range (bp) |
Size (bp) | Frequency (%) | Gene diversity | PIC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RM1 | 1 | 29.7 | (GA)26 | 4 | - | 80-112 | 112 | 54.17 | 0.36 | 0.34 |
| 2 | RM5 | 1 | 94.9 | GA)14 | 4 | - | 111-127 | 111 | 43.75 | 0.63 | 0.56 |
| 3 | RM16 | 3 | 131.5 | (TCG)5(GA)16 | 4 | 1 | 184-240 | 184 | 85.42 | 0.26 | 0.25 |
| 4 | RM12 | 12 | 109.1 | (GA)21 | 6 | 2 | 150-215 | 150 | 72.92 | 0.45 | 0.43 |
| 5 | RM19 | 12 | 20.9 | (ATC)10 | 7 | 4 | 204-239 | 239 | 77.08 | 0.39 | 0.38 |
| 6 | RM26 | 5 | 118.8 | (GA)15 | 3 | - | 105-119 | 119 | 89.58 | 0.19 | 0.18 |
| 7 | RM133 | 6 | 0 | (CT)8 | 2 | - | 225-234 | 234 | 93.75 | 0.12 | 0.11 |
| 8 | RM144 | 11 | 123.2 | (ATT)11 | 5 | 1 | 220-247 | 247 | 54.17 | 0.63 | 0.58 |
| 9 | RM145 | 2 | 49.8 | - | 2 | - | 181-192 | 181 | 95.83 | 0.08 | 0.08 |
| 10 | RM170 | 6 | 2.2-7.4 | (CCT7) | 5 | - | 98-127 | 127 | 60.42 | 0.58 | 0.54 |
| 11 | RM190 | 6 | 7.4 | (CT)11 | 3 | - | 107-127 | 127 | 85.42 | 0.26 | 0.24 |
| 12 | RM201 | 9 | 81.2 | (CT)17 | 5 | 1 | 161-188 | 161 | 68.75 | 0.50 | 0.47 |
| 13 | RM202 | 11 | 54 | (CT)30 | 4 | - | 165-186 | 186 | 64.58 | 0.54 | 0.50 |
| 14 | RM205 | 9 | 114.7 | (CT)25 | 5 | 1 | 117-156 | 117 | 64.58 | 0.54 | 0.51 |
| 15 | RM206 | 11 | 102.9 | (CT)21 | 10 | 4 | 135-198 | 135 | 29.17 | 0.81 | 0.78 |
| 16 | RM207 | 2 | 191.2 | (CT)25 | 4 | 1 | 72-148 | 148 | 79.17 | 0.36 | 0.33 |
| 17 | RM208 | 2 | 186.4 | (CT)17 | 3 | - | 170-187 | 170 | 72.92 | 0.43 | 0.38 |
| 18 | RM209 | 11 | 73.9 | (CT)18 | 7 | 2 | 125-170 | 125 | 60.42 | 0.60 | 0.58 |
| 19 | RM212 | 1 | 148.7 | (CT)24 | 2 | - | 125-132 | 132 | 87.50 | 0.22 | 0.19 |
| 20 | RM213 | 2 | 186.4 | (CT)17 | 3 | - | 143-166 | 143 | 85.42 | 0.26 | 0.24 |
| 21 | RM223 | 8 | 80.5 | (CT)25 | 4 | 1 | 144-172 | 172 | 72.92 | 0.44 | 0.40 |
| 22 | RM224 | 11 | 120.1 | (AAG)8(AG)13 | 6 | 2 | 138-172 | 172 | 56.25 | 0.60 | 0.55 |
| 23 | RM228 | 10 | 130.3 | (CA)6(GA)36 | 4 | - | 108-145 | 145 | 77.08 | 0.38 | 0.36 |
| 24 | RM252 | 4 | 99 | (CT)19 | 5 | 1 | 206-230 | 206 | 75.00 | 0.42 | 0.39 |
| 25 | RM253 | 6 | 37 | (GA)25 | 4 | 1 | 125-160 | 160 | 72.92 | 0.44 | 0.41 |
| 26 | RM262 | 2 | 103.3 | (CT)16 | 5 | 2 | 145-167 | 145 | 70.83 | 0.47 | 0.44 |
| 27 | RM263 | 2 | 127.5 | (CT)34 | 4 | - | 192-217 | 199 | 85.42 | 0.26 | 0.25 |
| 28 | RM273 | 4 | 94.4 | (GA)11 | 3 | - | 127-222 | 222 | 89.58 | 0.19 | 0.18 |
| 29 | RM277 | 12 | 57.2 | (GA)11 | 3 | 1 | 124-138 | 124 | 89.58 | 0.19 | 0.18 |
| 30 | RM283 | 1 | 31.4 | (GA)18 | 3 | - | 159-174 | 159 | 66.67 | 0.49 | 0.43 |
| 31 | RM289 | 5 | 56.7 | G11(GA)16 | 5 | - | 88-146 | 88 | 39.58 | 0.70 | 0.65 |
| 32 | RM304 | 10 | 73 | (GT)2(AT)10GT)33 | 4 | - | 157-174 | 157 | 45.83 | 0.63 | 0.57 |
| 33 | RM316 | 9 | 1.8 | (GT)8(TG)9(TTTG)4(TG)4 | 2 | - | 205-212 | 212 | 89.58 | 0.19 | 0.17 |
| 34 | RM320 | 7 | 62.4 | (AT)11GTAT (GT)13 |
2 | - | 225-233 | 233 | 95.83 | 0.08 | 0.08 |
| 35 | RM338 | 3 | 108.4 | (CTT)6 | 2 | - | 191-198 | 191 | 95.83 | 0.08 | 0.08 |
| 36 | RM342 | 8 | 78.4 | (CAT)12 | 5 | 3 | 130-169 | 130 | 81.25 | 0.32 | 0.30 |
| 37 | RM411 | 3 | 127.9 | (GTT)7 | 2 | - | 108-115 | 108 | 85.42 | 0.25 | 0.22 |
| 38 | RM413 | 5 | 26.7 | (AG)11 | 4 | - | 170-198 | 170 | 58.33 | 0.59 | 0.54 |
| 39 | RM447 | 8 | 124.6 | (CTT)8 | 5 | 2 | 106-145 | 106 | 83.33 | 0.30 | 0.28 |
| 40 | RM452 | 2 | 58.4 | (GTC)9 | 2 | - | 197-206 | 206 | 79.17 | 0.33 | 0.28 |
| 41 | RM455 | 7 | 65.7 | (TTCT)5 | 2 | 1 | 125-131 | 131 | 97.92 | 0.04 | 0.04 |
| 42 | RM484 | 10 | 97.3 | (AT)9 | 2 | - | 289-296 | 296 | 91.67 | 0.15 | 0.14 |
| 43 | RM495 | 2 | 2.8 | (CTG)7 | 3 | - | 159-173 | 159 | 83.33 | 0.29 | 0.26 |
| 44 | RM515 | 8 | 80.5 | (GA)11 | 6 | 1 | 211-252 | 211 | 72.92 | 0.45 | 0.43 |
| 45 | RM520 | 3 | 191.6 | (AG)10 | 3 | - | 244-264 | 244 | 70.83 | 0.45 | 0.40 |
| 46 | RM536 | 11 | 55.1 | (CT)16 | 3 | - | 236-249 | 249 | 72.92 | 0.41 | 0.36 |
| 47 | RM591 | 10 | 118.3 | (AC)10 | 5 | 1 | 247-279 | 247 | 62.50 | 0.56 | 0.53 |
| 48 | RM1337 | 12 | 57.2 | (GA)11 | 5 | - | 194-225 | 194 | 75.00 | 0.42 | 0.39 |
| 49 | RM3646 | 3 | 6.32 | (GA)14 | 3 | - | 145-158 | 145 | 85.42 | 0.26 | 0.24 |
| 50 | RM303 | 4 | 116.9 | [AC(AT)210]9 (GT)7(ATGT)6] |
8 | 1 | 107-198 | 198 | 54.17 | 0.67 | 0.64 |
| 51 | RM307 | 4 | 191.2 | (CT)25 | 7 | 2 | 125-264 | 125 | 77.08 | 0.40 | 0.38 |
| 52 | RM334 | 5 | 141.8 | (CTT)48 | 7 | 1 | 156-218 | 156 | 45.83 | 0.72 | 0.68 |
| 53 | RM472 | 1 | 171.6 | (GA)21 | 3 | - | 286-306 | 286 | 58.33 | 0.57 | 0.50 |
| 54 | RM125 | 7 | 24.8 | (GCT)8 | 4 | 1 | 127-152 | 127 | 85.42 | 0.26 | 0.25 |
| 55 | RM510 | 6 | 20.8 | (GA)15 | 3 | 1 | 113-127 | 127 | 91.67 | 0.16 | 0.15 |
| Max. | 10.00 | 296 | 97.92 | 0.81 | 0.78 | ||||||
| Min. | 2.00 | 88 | 29.17 | 0.04 | 0.04 | ||||||
| Total | 228.00 | 39 | 4060.42 | 21.35 | 19.82 | ||||||
| Mean | 4.15 | 73.83 | 0.39 | 0.36 |
Table 3: Number of alleles, allele size range, frequency, gene diversity and polymorphism information content (PIC) among 48 Boro rice landraces against 55 microsatellite markers.
Legend: 1. Mi-Pajang, 2. Dholi Boro, 3. Kumri Boro, 4. Bairagi Sail 5. Tepi Khorch 6. Pankaich, 7. BoroDeshi, 8. Gopal Beshi, 9. Borail, 10. Boro 6/2, 11. Kali Boro, 12. Sonar Geye, 13. Joya Boro, 14. Amboro2 (Golden), 15. Batti Boro, 16. Madhabsail, 17. Jagli, 18. Jagli, 19. Local Boro, 20. Saita, 21.Dud Saita, 22. Bogra Boro, 23. Deshi Boro, 24. Jagli (DeshiBoro), 25. Boro Dhan, 26.Boro Jagli, 27. Jagli, 28. Deshi Boro, 29. BoroDhan, 30.Boro (Sunga), 31. Jala Boro, 32. Kali Boro 2/2, 33. Kali Boro 4/1, 34. Kali Boro 26, 35. Kali Boro 41/1, 36. Kali Boro 48/1, 37. Kali Boro 80/3, 38. Kali Boro 80/5, 39. Kali Boro 109/4, 40. Kali Boro 138/2, 41. Kali Boro 139/2, 42. Kali Boro 200, 43. Kali Boro 208, 44. Kali Boro 259, 45. Kali Boro 266, 46. Kali Boro 576, 47. Kali Boro 600, 48. Kali Boro 704, L= 1Kb plus Ladder.
Unique Alleles
A total of 39 unique alleles were detected at 25 microsatellite loci. Nineteen (19) of the rice landraces had unique alleles for at least one microsatellite locus. Notably, seven (7) SSR markers—RM144 (chromosome 11, 220bp), RM205 (chromosome 9, 117bp), RM206 (chromosome 11, 152bp), RM277 (chromosome 12, 138bp), RM342 (chromosome 8, 152bp), RM515 (chromosome 8, 211bp) and RM307 (chromosome 4, 125bp) amplified specific alleles only in a slender grain rice landrace Jala Boro (Acc. No-1866). Similarly, six (6) SSR markers—RM19 (chromosome 12, 239bp), RM224 (chromosome 11, 138bp), RM262 (chromosome 2, 167bp), RM455 (chromosome 7, 125bp), RM125 (chromosome 7, 152bp) and RM510 (chromosome 6, 113bp) amplified specific alleles only in rice landrace Mi-Pajang (Acc. No-149). Also, Tepi Khorch (Acc. No-931) showed unique alleles in 4 markers and Bairagi Sail (Acc. No-261), Pankaich (Acc. No-937) and Joya Boro (Acc. No-1051) showed unique alleles in 3 markers (Table 4).
| SL. No. | Marker | Chromosome No. | Unique Allele (bp) | Landraces |
|---|---|---|---|---|
| 1 | RM16 | 3 | 184 | G 30 (Boro (sunga)) |
| 2 | RM12 | 12 | 183 | G 6(Pankaich) |
| 3 | RM12 | 12 | 208 | G 15(Batti Boro) |
| 4 | RM19 | 12 | 204 | G 9 (Borail) |
| 5 | RM19 | 12 | 216 | G 4 (Bairagi Sail) |
| 6 | RM19 | 12 | 227 | G 2 (Dholi Boro) |
| 7 | RM19 | 12 | 239 | G 1 (Mi-Pajang) |
| 8 | RM144 | 11 | 220 | G 31(Jala Boro) |
| 9 | RM201 | 9 | 188 | G 40 (Kali Boro 138/2) |
| 10 | RM205 | 9 | 117 | G 31 (Jala Boro) |
| 11 | RM206 | 11 | 152 | G 31 (Jala Boro) |
| 12 | RM206 | 11 | 171 | G 32 (Kali Boro 2/2) |
| 13 | RM206 | 11 | 192 | G 28 (Deshi Boro) |
| 14 | RM206 | 11 | 198 | G 7 (Boro Deshi) |
| 15 | RM207 | 2 | 148 | G 5 (Tepi Khorch) |
| 16 | RM209 | 11 | 134 | G 13 (Joya Boro) |
| 17 | RM209 | 11 | 176 | G 4 (Bairagi Sail) |
| 18 | RM223 | 8 | 172 | G 5 (Tepi Khorch) |
| 19 | RM224 | 11 | 138 | G 1 (Mi-Pajang) |
| 20 | RM224 | 11 | 143 | G 5 (Tepi Khorch) |
| 21 | RM252 | 4 | 230 | G 16 (Madhabsail) |
| 22 | RM253 | 6 | 160 | G 5 (Tepi Khorch) |
| 23 | RM262 | 2 | 161 | G 13 (Joya Boro) |
| 24 | RM262 | 2 | 167 | G 1 (Mi-Pajang) |
| 25 | RM277 | 12 | 138 | G 31 (Jala Boro) |
| 26 | RM342 | 8 | 146 | G 14 (Amboro 2 (golden)) |
| 27 | RM342 | 8 | 152 | G 31 (Jala Boro) |
| 28 | RM342 | 8 | 169 | G 6 (Pankaich) |
| 29 | RM447 | 8 | 121 | G 13 (Joya Boro) |
| 30 | RM447 | 8 | 145 | G 18 (Jagli) |
| 31 | RM455 | 7 | 125 | G 1 (Mi-Pajang) |
| 32 | RM515 | 8 | 211 | G 31 (Jala Boro) |
| 33 | RM591 | 10 | 266 | G 4 (Bairagi Sail) |
| 34 | RM303 | 4 | 107 | G 21 (Dud Saita) |
| 35 | RM307 | 4 | 125 | G 31 (Jala Boro) |
| 36 | RM307 | 4 | 147 | G 6 (Pankaich) |
| 37 | RM334 | 5 | 172 | G 12 (Sonar Geye) |
| 38 | RM125 | 7 | 152 | G 1 (Mi-Pajang) |
| 39 | RM510 | 6 | 113 | G 1 (Mi-Pajang) |
Table 4: List of identified unique alleles along with markers for 19 Boro rice landraces.
Unique alleles are precious as they June be effectively indicator of particular landraces and also for a breeding purpose. Generally, the higher number of unique alleles in landraces specifies as a sources of novel alleles. The unique alleles for molecular characterization were also detected by Siwach et al. [47], Das et al. [48] and Islam et al. [34]. Siwach et al. [47] identified seven SSR markers (RM1, RM21, RM38,RM170, RM210, RM226, and RM229) that showed unique alleles which could distinguish basmati rice varieties from non-basmati rice varieties. Also, Saini et al. [49] noticed 58 unique alleles among Basmati and non-Basmati rice varieties.
Genetic distance-based analysis
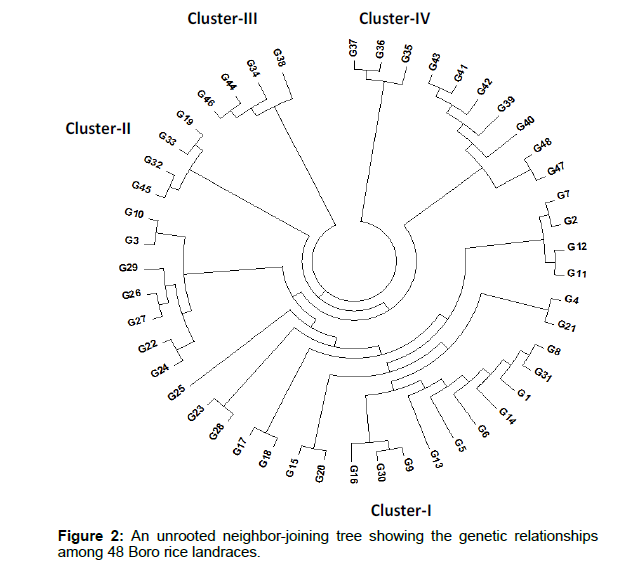
The genetic distance-based results seen in the unrooted neighborjoining (NJ) tree revealed that the 48 Boro rice landraces were grouped into four major clusters (Figure 2). The highest numbers of landraces (37) were found in cluster I followed by clusters II (4), III (4) and the lowest in cluster IV (3).
Genetic distance is useful for assessing the diversity between species and populations within a species. The highest number of germplasm (37) was found in cluster I. Again, cluster I was further divided into 2 sub clusters (A and B). Sub cluster-A, contains 30 landraces and Sub cluster-B consisted of 7 landraces. Cluster II contains 4 landraces (Local Boro, Kali Boro 2/2, Kali Boro 4/1 and Kali Boro266) and cluster III also contains 4 boro landraces (Kali Boro 26, Kali Boro 80/5, Kali Boro 259 and Kali Boro 576). The lowest number (3) of boro rice landraces was found in cluster IV (Kali Boro41/1, Kali Boro 48/1 and Kali Boro 80/3). In previous studies, Islam et al. [34] and Islam et al. [43] both found three (3) major clusters and few sub-clusters through the unrooted neighbor-joining (NJ) tree among 50 Bangladeshi red rice varieties and 113 aromatic rice landraces, respectively.
Population structure based on Model
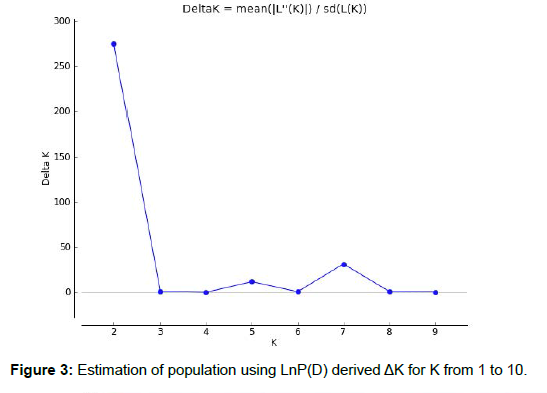
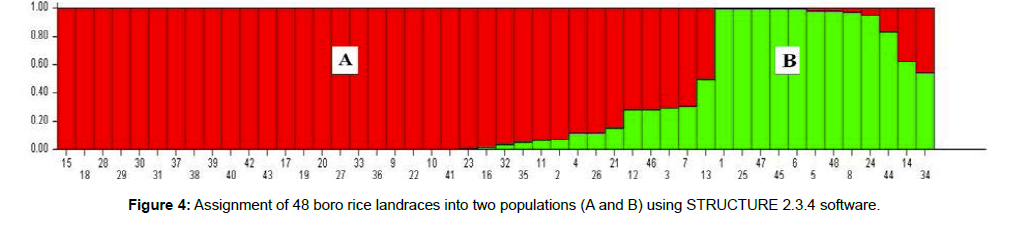
Model-based program STRUCTURE 2.3.4 was used to find out the genetic relationship among individual Boro rice landraces. By the admixture model, simulations were executed with K, range from 2 to 10 with 5 repetitions employing 48 landraces and the population structure analysis declared the log-likelihood value (ΔK) maximized to the highest value of at K= 2 (Figure 3), demonstrating a sharp peak expressing the classification of entire landraces into two specific subgroups, here denoted as Group A and Group B, respectively. To find out the number of pure and admixed individuals, populations were studied. The population Group A (red colour, Figure 4) and Group B (green colour, Figure 4) representing 75% (36) and 25% (12) of Boro landraces used in structure analysis, respectively. Overall, 11 (22.92%) admixed landraces were found at K = 2. It June be noted that Group A had 36 Boro rice landraces with 28 pure and 08 admixed landraces and Group B had 12 boro rice landraces with 09 pure and 03 admixed landraces. A similar population structure of two groups was observed in previous research by Pathaichindachote et al. [50] in a collection of 167 Thai and exotic rice accessions in Thailand. Islam et al. [43] have successfully classified three groups as indica aromatic rice landraces in Bangladesh and again, Islam et al. [34] observed four groups in a collection of 50 red rice landraces in Bangladesh, which were higher than our present study. The population structure analysis of different rice diversity panels has indicated different numbers of sub-groups, from 2 to 8 [48, 51, 52].
Population structure analysis from the SSR markers grouped all the landraces into 2 groups. Besides, four groups were also constructed using SSR markers data clustering analysis. So, the most dissimilar landraces observed from this study can be used in rice breeding program. Moreover, from this study, QTLs/genes mapping can be assembled by utilize the diverse landraces and highly polymorphic SSR markers that have been determined for different physicochemical quality characters.
Principal Coordinate Analysis
The Principal Coordinate Analysis (PCoA) displayed two and three dimensional graphical views of the spatial distribution of the landraces along the two principal axes (Figures 5 and 6). The landrace namely Mi- Pajang (G 1, Acc. 149), Gopal Beshi (G 8, Acc. 939), Borail (G 9, Acc. 940), Madhabsail (G 16, Acc. 1651), Boro (sunga) (G 30, Acc. 1861) and Jala Boro (G 31, Acc. 1866) were found far away from the centroid of the cluster and the rest of the genotypes were located more or less around the centroid. The present study showed that the landraces that were located far away from the centroid were more genetically diverse, while the genotypes that were sited close to the centroid designated more or less similar genetic background. Comparable results were also stated by other authors [4, 53, 54].
Conclusion
The present study was conducted to make DNA fingerprinting and to assess the genetic relationship among these landraces using SSR markers and microsatellite markers are considered appropriate for variety identification because of their ability to detect large numbers of distinct alleles frequently, exactly and efficiently. The study also confirmed the value of microsatellite loci for genetic diversity studies of rice landraces found in earlier studies. The diversity and the unique features of the Bangladeshi rice landraces collections examined in this study could be quite relevant to both domestic and global rice development.
Acknowledgments
The authors are grateful for the financial support provided by Project Implementation Unit-Bangladesh Agricultural Research Council-National Agricultural Technology Program-Phase II (PIUBARC- NATP-2) and technical support provided by Bangladesh Rice Research Institute (BRRI) through the Ministry of Agriculture,Bangladesh.
Conflict of Interest
The authors declare that they are no conflict of interest.
References
- Ahmed MSU, Khalequzzaman M, Bashar MK, Shamsuddin AKM (2016) Agro-Morphological, Physico-Chemical and Molecular Characterization of Rice Germplasm with Similar Names of Bangladesh. Rice Science 23: 211-8.
- Jackson, M T (1995) Protecting the heritage of rice biodiversity. Geo Journal 35: 267-274.
- Anonymous (2002) National Workshop on Rice Research and Extension-2002; Feeding the extra millions by 2025; Bangladesh Rice Research Institute, Gazipur-1,
- Siddique MA, Khalequzzaman M, Fatema K , Islam MZ, Islam MM and Chowdhury MAZ (2016a) Molecular Characterization and Genetic Diversity of Aman Rice (Oryza sativa L.) Landraces in Bangladesh. Bangladesh Rice J 20 (2): 1-11.
- Causse MA, Fulton TM, Cho YG, Ahn SN, Chunwongse J (1994) Saturated molecular map of the rice genome based on an interspecific backcross population. Genetics 138: 1251-1274.
- Karkousis A, Barr AR, Chalmers KJ, Ablett GA, Holton TA, et al. (2003) Potential of SSR markers for plant breeding and variety identification in Australian Barley germplasm. Aust. J. Agric. Res 54: 1,197-1,210.
- Tautz D (1989) Hypervariabflity of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res 17: 6,463–6,471.
- Morgante M, Olivieri A (1993) PCR-amplified microsatellites as markers in plant genetics. Plant J 3: 175-182.
- Powell W, Machray GC, Provan J (1996) Polymorphism revealed by simple sequence repeats. Trends Plant Sci 1: 215-222.
- Sosinski B, Gannavarapu M, Hager LD, Beck LE, King GJ, et al. (2000) Characterization of microsatellite markers in peach (Prunus persica L. Batsch). Theor Appl Genet 101: 421-428.
- Cipriani G, Lot G, Huang WG, Marrazzo MT, Peterlunger E et al. (1999) AC/GT and AG/CT microsatellite repeats in peach (Prunus persica L Batsch): isolation, characterization and cross-species amplification in Prunus. Theor Appl Genet 99: 65-72.
- Kostova A, Todorovska E, Christov N, Hristov K, Atanassov A (2006) Assessment of genetic variability induced by chemical mutagenesis in elite maize germplasm via SSR markers. J Crop Improv 16: 37-48.
- Tu M, Lu BR, Zhu Y, Wang Y (2007) Abundant within-varietal genetic diversity in rice germplasm from Yunnan province of China revealed by SSR fingerprints. Biochem Genet 45: 789-801.
- Sharma RC, Chaudhary NK, Ojha B, Yadav L, Pandey MP, Shrestha SM (2007) Variation in rice landraces adapted to the low lands and hills in Nepal. Plant Genet Res 5: 120-127.
- Perez-Sackett PT, Cianzio SR, Kara PC, Aviles M, Palmer RG (2011) QTL mapping of whitefly resistance in soybean. J Crop Improv 25: 134-150.
- Rani MG, Adilakshmi D (2011) Genetic analysis of blast resistance in rice with simple sequence repeats (SSR). J Crop Improv 25: 232-238.
- Weising K, Winter P, Hüttel B, Kahl G (1997) Microsatellite markers for molecular breeding. J Crop Prod 1: 113-143.
- Altaf-Khan M, Qureshi SN, Saha S, Jenkins JN, Brubaker CL, Reddy OUK (2006) Usefulness of SSR derived from tetraploid gossypium spp. for analyses of diploid gossypium spp. J Crop Improv 16: 1-20.
- Rajendrakumar P, Biswal A, Sakthivel K, Madhav M, Neeraja C, Balachan, et al. (2009) Development and validation of class I SSR markers targeting (GATA)n repeat motifs in rice. Euphytica169: 263-271.
- Sarao NK, Vikal Y, Singh K, Joshi MA, Sharma RC (2010) SSR marker based DNA fingerprinting and cultivar identification of rice (Oryza sativa L.) in Punjab state of India. Plant GenetRes 8: 42-44.
- Xu W, Virmani S, Hernandez J, Sebastian L, Redoña E, Li Z (2002) Genetic diversity in the parental lines and heterosis of the tropical rice hybrids. Euphytica127: 139-148.
- Melchinger AE, Messmer MM, Lee M, Woodman WL, Lamkey KR (1991) Diversity and relationships among U.S. maize inbreds revealed by restriction fragment length polymorphisms. Crop Sci 31: 669.
- Weir BS (1990) Genetic Data Analysis: Methods for Discrete Population Genetic Data. Science 250-575
- Duvick DN (1984) Genetic diversity in major farm crops on the farm and in reserve. Econ Bot 38: 161-178.
- Semagn K, Bjørnstad Å, Ndjiondjop MN (2006) An overview of molecular marker methods for plants. Afr J Biotechnol 5: 2540-2568.
- IRGSP (2005) The map-based sequence of the rice genome. Nature 436: 793-800.
- Rashid MM, Imran S, Islam MA, Hassan L (2018) Genetic diversity analysis of rice landraces (Oryza sativa L.) for salt tolerance using SSR markers in Bangladesh. Fundamental and Applied Agriculture. 3 2: 460-466.
- Chakravarthi BK, Naravaneni R (2006) SSR marker based DNA fingerprinting and diversity study in rice (Oryza sativa. L). Afr J Biotechnol 5: 684-688.
- Rahman MM, Rasaul MG, Hossain MA, Iftekharuddaula KM, Hasegawa H (2012) Molecular Characterization and Genetic Diversity Analysis of Rice (Oryza sativa L.) Using SSR Markers. Journal of Crop Improvement 26: 244-257.
- Ferdous J, Hanafi MM, Rafiiand MY, Muhammad K (2012) A quick DNA extraction protocol: Without liquid nitrogen in ambient temperature. African Journal of Biotechnology 11 (27): 6956-6964.
- Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S and McCouch SR (2001) Computational and experimental analysis of micro-satellites in rice (Oryza sativa L.), frequency, length variation, transposon associations and genetic markerpotential. Genome Res 11: 1441-1452.
- McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang, et al.(2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9: 199-207.
- Roy S, Marndi B, Mawkhlieng B, Banerjee A, Yadav R, Misra A, et al. (2016) Genetic diversity and structure in hill rice (Oryza sativa L.) landraces from the North-Eastern Himalayas of India. BMC Genet 17 (1): 107.
- Islam MZ, Khalequzzaman M, Prince MFRK, Siddique MA, Rashid ESMH, Ahmed MSU, Pittendrigh BR, Ali MP (2018) Diversity and population structure of red rice germplasm in Bangladesh. Plos One 13 (5): 1-20.
- Liu K, Muse SV (2005) Power Marker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21 9: 21-28
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 27-39.
- Hall BG (2013) Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 30 : 122-135.
- Rohlf FJ (2002) NTSYS-pc. Numerical taxonomy and multivariance analysis system version 2.1. New York, USA: Exeter Software.
- Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155.
- Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164 (4): 1567±87. PMID: 129-3761
- Earl Da (2012) Structure Harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources 4 (2): 359-61.
- Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14 8: 26-110
- Islam MZ, Khalequzzaman M, Bashar MK, Ivy NA, Mian MAK, Pittendrigh BR, Haque MM, Ali MP (2018b) Variability Assessment of Aromatic Rice Germplasm by Pheno-Genomic traits and Population Structure Analysis. Scientific Reports 89-911.
- Siddique MA, Khalequzzaman M, Fatema K, Islam MZ, Baktiar MHK, Islam MM (2017) DNA Fingerprinting and Genetic Diversity in Aus Rice (Oryza sativa L.) Landraces of Bangladesh. Bangladesh Rice J 21 (1): 59-65.
- Chakhonkaen S, Pitnjam K, Saisuk W, Ukoskit K, Muangprom A (2012) Genetic structure of Thai rice and rice accessions obtained from the International Rice Research Institute. Rice 5:19.
- Islam MZ, Siddique MA, Akter N, Prince MFRK, Islam MR, Anisuzzaman M, Mian MAK (2019) Morpho-molecular Divergence of Restorer Lines for Hybrid Rice (Oryza sativa L.) Development.S Cereal Research Communications 47 (3): 531-540.
- Siwach P, Jain S, Saini N, Chowdhury VK, Jain RK ( 2004) Allelic diversity among basmati and non-basmati long-grain indica rice varieties using microsatellite markers. J Plant Biochem Biotechnol 13: 25-32.
- Das B, Sengupta S, Parida SK, Roy B, Ghosh M, Prasad M (2013) Genetic diversity and population structure of rice landraces from Eastern and North Eastern states of India. BMC Genetics 14-71.
- Saini N, Jain N, Jain S, et al. (2004) Assessment of genetic diversity within and among Basmati and non-Basmati rice varieties using AFLP, ISSR and SSR markers. Euphytica 140: 133-146.
- Pathaichindachote W, Panyawut N, Sikaewtung K, Patarapuwadol S, Muangprom A (2019) Genetic Diversity and Allelic Frequency of Selected Thai and Exotic Rice Germplasm Using SSR Markers. Rice Science 26 (6): 393-403.
- Garris AJ, Tai TH, Coburn J, Kresovich S, McCouch S (2005) Genetic structure and diversity in rice Oryza sativa L. Genetics 1631-1638.
- Huang M, Wu Y, Tao X (2015) Genetic diversity of main inbred Indica rice varieties applied in Guangdong Province as revealed by molecular marker. Rice Sci 22:1-8.
- Siddique MA, Khalequzzaman M, Islam MM, Fatema K, Latif MA (2016) Molecular characterization and genetic diversity in geographical indication (GI) rice (Oryza sativa L.) cultivars of Bangladesh. Braz. J. Bot 39: 631-640.
- Islam MZ, Khalequzzaman M, Bashar MK, Ivy NA, Haque MM, Mian MAK and Tomita M (2018) Agro-morphological characterization of Bangladeshi aromatic rice (Oryza sativa l.) germplasm based on qualitative traits. Bangladesh Rice J 22 (2): 41-54.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar,Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at,Google Scholar, Crossref
Citation: Khalequzzaman M, Islam MZ, Prince FRK, Rashid ESH, Siddique A (2022) Genetic Diversity and Population Structure of Boro Rice Landraces of Bangladesh. J Rice Res 10: 305. DOI: 10.4172/2375-4338.1000305
Copyright: © 2022 Khalequzzaman M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2561
- [From(publication date): 0-2022 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 2160
- PDF downloads: 401