Genetic Diversity among a Collection of L. chinense Germplasm Analyzed with SSR Markers
Received: 06-Nov-2017 / Accepted Date: 12-Nov-2017 / Published Date: 18-Nov-2017
Abstract
Population is one of the most important units of evaluation in plant science. Genetic differentiation will be occurred via mutation, knock down or knock out. In this study, we used Liriodendron chinense germplasm. Genomic DNA was extracted and amplified with SSR-PCR to investigate diversity. Our results revealed that the genetic diversity of L. chinense is at high level in comparing with other woody plants. In addition, our results suggested that L. chinense can improve its genome by repair mechanisms under sever and variable conditions to keep its genetic diversity. This study detects genetic diversity of L. chinense using SSR marker information and distinguishes geographic populations. The aim of this study is designed to provide theoretical guidance for the effective germplasm protection of L. chinense.
Keywords: SSR; Liriodendronchinense; Geneticdiversity
Introduction
Population is the basic unit of evolution, and population genetic structure is one of the most fundamental characteristics of a species. Genetic differentiation among populations will be generated due to mutation, gene flow, selection and genetic drift. The study of genetic differentiation among populations and its influence factors is an important issue of population genetics and constitutes the core of conservation biology [1]. Habitat fragmentation and long-term artificial selection pressure have great impact on genetic change of geographical population of L. chinense . The results of provenance testing showed that the main traits of L. chinense have significant genetic differences among geographic populations and displays random geographic variation pattern that is even two adjacent populations could present huge differentiation. This study detects genetic diversity of L. chinense using SSR marker information and distinguishes geographic populations; the aim of this study is designed to provide theoretical guidance for the effective germplasm protection of L. chinense.
Materials and Methods
Experimental materials
Altogether, eighty genotypes/germplasm collected from twelve provenances of L. chinense were taken as plant materials. All these trees were planted at Xiashu forest farm affiliated to Nanjing Forestry University in 1993. In late April, 2013, 2~3 fresh leaves of each plant have been collected, and put in the prepared sampling box with ice cube rapidly after they were stored in valve bag. And finally, they were brought back and kept in refrigerator under the condition of -70°C.
Experimental Methods
Extraction and purification of total DNA
In this experiment, the improved CTAB lysis-silica beads assay was used, in order to extract the genomic DNA of L. chinense (Table 1).
| Provenance | Population code | Sample size |
|---|---|---|
| Wuyi mountain of Jiangxi province | WYS | 8 |
| Yellow mountain of Anhui province | HS | 9 |
| Suining of Hunan province | HSN | 7 |
| Sangzhi of Hunan Province | SZ | 9 |
| Sinan of Guizhou province | GSN | 3 |
| West of hubei province | EX | 8 |
| Fuyang of Zhejiang province | FY | 5 |
| Youyang of Sichuan province | YY | 8 |
| Xuyong of Sichuan province | XY | 6 |
| Xianning of hubei province | XN | 5 |
| Songyang of Zhejiang province | SY | 7 |
| Lushan of Jiangxi province | LS | 5 |
Table 1: Code and sample size for the 12 provenances of L. chinense.
Add 2 mL CTAB to 10 mL centrifuge tube extract and 60 μL mercaptoethanol, in 65°C water bath pot pre-heating.
The sample was placed in the ice water bath to make good rapid cooling to room temperature, add volume (2 mL) such as chloroform, isoamyl alcohol (24:1), thoroughly incorporated under -4°C after 12000 RPM centrifuge for 10 min, take that 1 mL in 1.5 mL centrifugal supernatant fluid pipe, in -20°C refrigerator save for later use.
Remove the desired DNA containing 1.5 mL centrifuge tube, and placed at room temperature so that the crude extract DNA melting into liquid. Add 100 μL suspension of silica beads (1.2 g silica beads powder was dissolved in 10 mL of sterile water), the shock with a vortex shaker for about 15 s, after sufficiently mixing, at room temperature for 10 min, then the shock of about 5 s, it mix again and then centrifuged at 8000 rpm 15 s, the supernatant obtained silica beads- nucleic acid complexes.
Add 1 mL into the compound lotion, then put in the ball grinding apparatus in the frequency of 30 Hz was 30 s, make it thoroughly incorporated and 8000 rpm centrifugal 15 s, the supernatant was discarded.
Repeat step (5).
Add 1 mL 70% ethanol into the compound, and then puts the ball mill instrument in turmoil in 30 Hz frequency for 1 min, make it’s thoroughly incorporated and 8000 rpm centrifugal 15 s, abandon the supernatant (repeat).
Add 1 mL ethanol to the complex of anhydrous, then put in ball mill instrument turmoil in 30 Hz frequency for 1 min, make its thoroughly incorporated, 8000 rpm centrifugal 15 s again, abandon the clear liquid, and then open the lid centrifuge tube inversion on absorbent paper about 5 min, finally the composite was dried in vacuum for about 10 min.
After adding 100 μL 1× TE buffer full Vortex, put the pan in a water bath at 56°C water bath for 10 min, and then remove it after the shock mix 12000 rpm centrifugal 5 min, the supernatant.
Repeat steps (9).
The supernatant was obtained by mixing two; 12000 rpm centrifugal 10 min, the supernatant solution is the desired DNA.
Using ultraviolet spectrophotometer to detect the DNA of the proposed concentration and purity, save in -20°C refrigerator.
SSR - PCR amplification reaction
10 μL system of SSR-PCR amplification reaction (Table 2) and Touch-down PCR amplification reaction process (Table 3) were used in this experiment.
| Ingredients | Final addition |
|---|---|
| ddH2O | 6 μL |
| 10x Buffer | 1 μL |
| 25mu-MgCl2 | 1.25 μL |
| 10mM-dNTP | 0.2 μL |
| F-Primer | 0.25 μL |
| R-Primer | 0.25 μL |
| r-Taq | 0.05 μL |
| Template DNA | 1 μL |
Table 2: System of SSR-PCR amplification, dNTP and Mg2+ to oscillation blender before use.
| Cycle | Temperature | Time | ||
|---|---|---|---|---|
| Pre-denaturation | 94°C | 4 min | ||
| Touch-down PCR | 15 cycles | 94°C | 15 s | |
| 60°C | 15 s | (Δ°=-0.7) | ||
| 72°C | 30 s | |||
| Ordinary PCR | 15 cycles | 94°C | 15 s | |
| 49.5°C | 15 s | |||
| 72°C | 30 s | |||
| Extension | 72°C | 20 min | ||
| Conservation | 4°C | ∞ |
Table 3: Procedure of PCR amplification.
After the end of the PCR amplification reaction, adding loading buffer (0.25% bromophenol blue, 15% Ficoll) after 7 μL, fully Shakers, on 4°C refrigerator spare.
Polyacrylamide gel electrophoresis of PCR products
8% polyacrylamide denaturing gel electrophoresis was applied to separate amplification products.
Polyacrylamide gel electrophoresis process
Preparation of the glass electrophoresis tank: Each electrophoresis tank includes two glass plates, one on both sides of the glass with the ear, a smooth glass. The glass plate with ear down to soak in the antisuicide agent about 30 min; in a positive light panel drops 3-4 drops of silicified agent, with a sponge to dab the plate surface in one direction and make it even painted the entire board face, standing 10 min, then put in the hood to dry (about 30 min).
The sealing glue: Will bring ear glass panel with ear side and light stick in the rubber coated with a face of silicide agent box, flat in the electrophoresis tank, tighten, rubber frame clamp. Use heat to melt the agar gel glass plate and rubber frame exposure to block, in case of leak adhesive should be a little bit at the bottom of the overflow groove advisable, let stand for 15 min.
The glue: Measure around 40 mL 8% acryl amide gel, adding 270 μL 10% APS and
55 μL TEMED, gently shake mix quickly with a large dropper Glue, Glue after the completion of the need to check whether there is a bubble, the bubble should be used if the blade the bubble pick; when inserted comb should be level and close slowly down the glass insert, comb head cannot have bubbles. Insert the comb should be completed within 15 min timely follow-up observations, if found leaking glue, glue should be filled promptly. Then put it aside until the glue solidified, pour 0.5x TBE buffer.
The holes: After the bubble will comb through parallel pull it out with a pipette or dropper blown clean gelatinous substance inside the hole, loading process if the hole has been found in the debris blockage, and then blow it clean 200 μL of then continue loading.
Sample: With a range of suitable pipette Add 1 μL amplification good DNA and added Marker in a suitable position for subsequent interpretation strip.
Running glue: After the addition was complete, timely plug wires, open electrophoresis, with a constant voltage electrophoresis about 1 hour, when the bromophenol blue 1-2 cm away from the glass at the bottom, stopping electrophoresis, demolition slot.
Pry slide: Slightly open in the middle of inserting two glass edge parallel with scissors, do not need to insert too deep, slowly separated the two pieces of glass. Will be coated with light panel is placed on the side of silicide agent, recycling AGAR gel will comb after wash clean, dry, so that next time; Put kindergarten will have ears with glue glass disc, flush with ddH2O, preparing silver stain.
Silver staining:
(A) Put the tray placed on a shaker, shake it slightly, adding fixative into the tray, not over the glass surface can be fixed 10 min.
(B) Drained fixative, washed twice with ddH2O added AgNO3 solution (0.15%), not over the glass surface can, silver staining 6-7 min.
(C) Drained AgNO3 solution, washed twice with ddH2O, the AgNO3 wash, pour developing solution, liquid glass cannot over until showing up with a clear strip.
(D) Remove the glass; paste the appropriate label after photographs.
Electrophoresis and silver stain process and solution of reagent used
8% denaturing polyacrylamide preparation: Weigh 420 g of urea, 2 g methylene, 78 g acryl amide, into 1000 mL beaker, adding a small amount of can and 100 mL 5x TBE, the stirring with a glass rod uniformly dissolved by heating in a microwave oven (dissolved stir every 1 min process after removing dissolved), wait until completely dissolved after can volume to 1 L, with filter paper after 2 times in 4°C refrigerator.
TBE electrophoresis liquor (5x) of the preparation: Weigh 54 g Tris base, 27 g of boric acid, amount of 20 mL 0.5 mol / EDTA (pH=8.0), into a 1000 mL beaker with ddH2O volume to 1 L. When electrophoresis is used 1x working solution, 1: 4 mixed with water, stored at room temperature.
Preparation of anti-silicidation agent: amount of 500 mL ddH2O, with glacial acetic acid to pH adjusted to 3.5, followed by addition of 3 mL Bind-silica, a magnetic stirring apparatus with hot mix.
Preparation of filling glue: the amount of about 40 mL 8% acryl amide gel, adding 270 μL 10% APS and 55 μL TEMED, gently shake to mix. (10% APS: ammonium per sulfate was added to 1 g 10 mL autoclaved water).
Preparation fixative: amount of each 200 mL and 10 mL of pure ethanol, acetic acid, into the 2 L flask with ddH2O volume, stored at room temperature after Akira uniform.
The preparation AgNO3: The 6 mL AgNO3 stock solution was diluted 100-fold with ddH2O, stored. (AgNO3 stock solution preparation: Weigh 7.5 g AgNO3 join 50 mL ddH2O, backlit save after mixing).
The preparation of the developer: Weigh 30 g NaOH, respectively 20 mL 0.756% amount of sodium tetraborate and 20 mL of formaldehyde with ddH2O volume to 2 L, put in 4°C refrigerators.
Primer selection
Formerly, 200 pairs of EST-SSR primers had been developed from L. chinense in our lab. In order to screen polymorphism SSR markers, four DNA samples of L. chinense and L. tulipifera were chosen randomly to conduct SSR-PCR reaction amplification, 8% polyacrylamide denaturing gel electrophoresis were used to separate amplification products. Screening criteria of polymorphism primers followed in this experiment are as follows: amplification products should have distinct banding pattern on electropherogram of 8% polyacrylamide denaturing gel electrophoresis, various allelic variations should exist among samples, and the eliminated loci of primer should only have 1~2 bp differences. After preliminary screening, the primers which have no amplification products or undesirable polymorphisms of amplification products should be eliminated, moreover, the primers which have clear amplification bands and obvious polymorphism were selected. Finally, 22 SSR primers with obvious banding pattern, good repeatability and high stability were screened and used for this experiment.
Data management and statistical analysis
Band interpretation: SSR is co-dominant marker; the band with consistent electrophoretic mobility in the amplification product of the same primer has homology. The band data should be numbered as increasing sequences and the length of band with A, B, C, D, E…… for instance, the lowest is A, and the top most is D or E or others, the result of band reading is AB, BC, etc., besides, if one sample only has one band, the result of band reading is AA, BB, and so on.
Data analysis
Analysis of genetic diversity: The genetic parameter of populations can be obtained by using POPGENE 1.32 [2], including average number of alleles (A), effective number of allele (Ne), [3], observed heterozygosity (Ho), expected heterozygosity (He), [4], Shannon diversity index (I), [5], Nei diversity index [6] and genetic distance (D).
Analysis of genetic relationship: Clustering analysis is conducted by utilizing software NTSYS-pc 2.10e [7], and the clustering figure should be established with the method of UMPGA.
Results
Analysis of overall genetic diversity of L. chinense
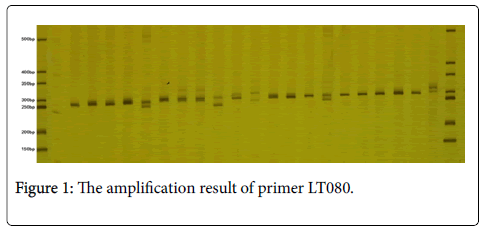
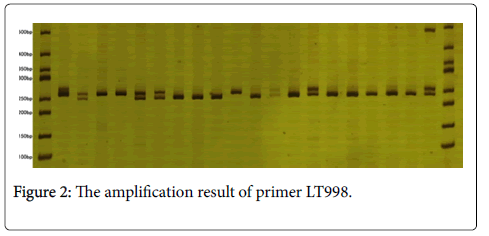
In the tested 80 individuals of 12 L. chinense provenances, 125 alleles are detected totally through 26 pairs SSR primers. The number of alleles (A) on each locus changes from four to six according to observation, the average number of alleles is 4.8077; and the effective number of allele (Ne) changes from 1.9539 to 5.1388, and the average effective number of allele is 3.1584. Meanwhile, the electrophoretic results of partial amplification products generated by primers (LT080 and LT998) are shown in Figures 1 and 2 respectively.
The parameters of diversity on different loci have great difference, among which, the maximal Shannon diversity index (I) is 1.6897, the minimum is 0.8485, and the average value is 1.2618; Nei diversity index (He) ranges from 0.4913 to 0.8107, and the average value is 0.6750. Observed heterozygosity (Ho) ranges from 0.0263 to 0.4605, and the average value of Ho is 0.2790. From Table 4, it can be found that 12 provenances of L. chinense maintain high level of genetic diversity.
| Locus | A | Ne | I | Ho | He |
|---|---|---|---|---|---|
| LT002 | 6 | 3.2471 | 1.4062 | 0.3125 | 0.6964 |
| LT015 | 6 | 3.8328 | 1.5233 | 0.3158 | 0.744 |
| LT022 | 4 | 3.171 | 1.2659 | 0.0263 | 0.6892 |
| LT026 | 4 | 3.1445 | 1.2437 | 0.0649 | 0.6864 |
| LT049 | 5 | 3.1548 | 1.26 | 0.1282 | 0.6874 |
| LT056 | 4 | 2.5563 | 1.0526 | 0.2949 | 0.6127 |
| LT062 | 6 | 2.8523 | 1.2729 | 0.3333 | 0.6536 |
| LT071 | 5 | 4.3069 | 1.5261 | 0.3625 | 0.7726 |
| LT080 | 5 | 3.4773 | 1.3424 | 0.225 | 0.7169 |
| LT091 | 6 | 3.2667 | 1.3721 | 0.2911 | 0.6983 |
| LT102 | 4 | 3.3975 | 1.2959 | 0.137 | 0.7105 |
| LT121 | 6 | 3.6488 | 1.3949 | 0.45 | 0.7305 |
| LT124 | 4 | 3.2701 | 1.2824 | 0.1899 | 0.6986 |
| LT151 | 5 | 2.4577 | 1.1354 | 0.3077 | 0.5969 |
| LT199 | 6 | 3.2379 | 1.2973 | 0.4557 | 0.6956 |
| LT233 | 5 | 3.2072 | 1.3105 | 0.1875 | 0.6925 |
| LT274 | 6 | 5.1388 | 1.6897 | 0.4605 | 0.8107 |
| LT297 | 5 | 2.9781 | 1.2222 | 0.45 | 0.6684 |
| LT298 | 5 | 3.3891 | 1.3486 | 0.4557 | 0.7094 |
| LT316 | 4 | 2.5352 | 1.0787 | 0.1875 | 0.6094 |
| LT371 | 4 | 1.9539 | 0.8485 | 0.325 | 0.4913 |
| LT414 | 4 | 2.4201 | 1.0493 | 0.3125 | 0.5905 |
| LT446 | 4 | 2.2327 | 0.9206 | 0.3247 | 0.5557 |
| LT488 | 4 | 2.7587 | 1.16 | 0.2133 | 0.6418 |
| LT921 | 4 | 3.3623 | 1.2849 | 0.2436 | 0.7071 |
| LT978 | 4 | 3.1212 | 1.2238 | 0.2 | 0.6839 |
| Mean | 4.81 | 3.1584 | 1.2618 | 0.279 | 0.675 |
Table 4: Genetic diversity of 26 SSR loci within 12 provenances of L. chinense. An average number of alleles; Ne Effective number of alleles; I Shannon’s Information index; Ho observed heterozygosity; He: average expected heterozygosity.
Analysis of genetic diversity of each L. chinense provenance
Among 12 provenances of L. chinense , the effective number of allele (Ne) of FY is highest (2.3466), while the effective number of allele of GSN is lowest (1.6032). The variation range of Nei diversity index ranges from 0.2906 (GSN) to 0.5307 (SZ). The maximal Shannon diversity index (I) is 0.9080 (SZ), while the minimum is 0.4473 (GSN) (Table 5).
| Population code | A | Ne | I | Ho | He | F | Nei |
|---|---|---|---|---|---|---|---|
| WYS | 2.6538 | 2.0348 | 0.743 | 0.2985 | 0.4747 | 0.3712 | 0.4438 |
| HS | 2.7308 | 2.1077 | 0.783 | 0.2564 | 0.5038 | 0.4911 | 0.4747 |
| EX | 3.0385 | 2.2258 | 0.8639 | 0.3276 | 0.5417 | 0.3952 | 0.5068 |
| FY | 3.0385 | 2.3466 | 0.8956 | 0.325 | 0.5776 | 0.4373 | 0.5191 |
| XN | 2.3846 | 1.8837 | 0.6499 | 0.2923 | 0.4393 | 0.3346 | 0.3954 |
| SY | 2.8846 | 2.2623 | 0.8568 | 0.3626 | 0.5482 | 0.3386 | 0.509 |
| LS | 2.5769 | 2.025 | 0.7447 | 0.2942 | 0.5002 | 0.4118 | 0.45 |
| HSN | 2.6154 | 1.9847 | 0.7172 | 0.1758 | 0.4577 | 0.6159 | 0.425 |
| SZ | 3.1538 | 2.291 | 0.908 | 0.2423 | 0.5639 | 0.5703 | 0.5307 |
| YY | 2.8077 | 2.2083 | 0.8189 | 0.2429 | 0.5212 | 0.534 | 0.4869 |
| XY | 2.5 | 1.9334 | 0.6941 | 0.2885 | 0.4586 | 0.3709 | 0.4204 |
| GSN | 1.8077 | 1.6032 | 0.4473 | 0.2692 | 0.3487 | 0.228 | 0.2906 |
| Eastern | 2.7863 | 2.1291 | 0.7958 | 0.2861 | 0.5119 | 0.4411 | 0.4727 |
| Western | 2.3718 | 1.9147 | 0.6534 | 0.2669 | 0.4428 | 0.3972 | 0.3993 |
Table 5: The genetic diversity among 12 provenances of L. chinense. A: An average number of alleles; Ne: Effective number of alleles; I: Shannon’s Information index; Ho: observed heterozygosity; He: average expected heterozygosity; F: Fixation index; Nei: Nei’s expected heterozygosity
Generally, the level of genetic diversity of SZ is highest, on the contrary, that of GSN is lowest. Moreover, as for fixation index (F value) of 12 provenances, the overall value of F>0, among which, the fixation index of HSN is highest (F=0.6159), but the degree of deviation of NK from equilibrium is largest, however, the degree of deviation of GSN from equilibrium is smallest, although its fixation index is 0.2280.
On the basis of characteristics of geographical distribution of L. chinense, L. chinense provenances are divided into eastern groups and western groups. According to the test results of Shannon diversity index (I) and Nei expected heterozygosity (Nei), gene diversity of L. chinense of eastern groups is higher than that of west groups.
Fixation index (F) measures the degree of deviation between actual proportion of genotype and expected proportion of Hardy-Weinberg theory, and F<0 or F>0 means that heterozygosis is excessive or homozygosis is excessive respectively. When panmixia of individuals in populations cannot be realized, its actual proportion of genotype will deviate to some extent from Hardy-Weinberg equilibrium inevitably. As for the fixation index on each locus, their F>0, which indicate that L. chinense , on these 26 loci, deviate from Hardy-Weinberg equilibrium, and have insufficient heterozygote.
Genetic structure of twelve of L. chinense provenances
According to the degree of differentiation measured by F-statistics, the genetic differentiation coefficient of these 12 provenances is 0.3225, therefore, 32.25% genetic variation exist among provenances, and 67.75% genetic variation exist within provenance, which accounts for a greater part of the whole genetic variation, there out, genetic variation, or namely variation of genetic diversity, of these 12 L. chinense provenances mainly exist in populations.
Gene flow (Nm) refers to the flow of gene within population and among populations, which mainly reflects communication frequency of gene among most individuals within population and among populations, and is accomplished through migration of animal population, or blowing in the wind of plant pollen. According to Wringt, when gene flow (Nm)>1, gene flow can prevent genetic difference among populations effectively caused by genetic drift, and thus, the gene flow of these L. chinense provenances is 0.5252, which indicates that certain gene flow exists among them, but the level is relative low.
The genetic distance among twelve of L. chinense provenances
Genetic identity (I) and genetic distance (D) are parameters that applied to accurately assess the similarity among populations [8]. When the genetic identity (I) is 0, the two populations have no genetic relationship, on the contrary, when (I) is 1, these two populations are identical. The genetic identity (I) and genetic distance (D) of these 12 L. chinense provenances are shown in Table 6. The genetic identity (I) of these 12 provenances changes from 0.3781 to 0.8561, the average genetic identity is 0.5705, and the genetic distance (D) varies from 0.1554 to 0.9726, and the average genetic relationship is 0.5756, hence, it can be deduced that these 12 L. chinense provenances have great genetic variation, and remote genetic relationship. Among which, the maximal genetic identity (I) of EX and SY is 0.8561, which shows that the genetic relationship of these two populations is closer than other populations. However, the minimum genetic relationship of GSN and FY is 0.3781, which indicates that the genetic relationship of these two populations is closer than other populations.
| POP ID | XY | LS | GSN | XN | HS | YY | SZ | WYS | EX | SY | HSN | FY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XY | **** | 0.657 | 0.634 | 0.633 | 0.571 | 0.566 | 0.532 | 0.513 | 0.461 | 0.467 | 0.4723 | 0.469 |
| LS | 0.42 | **** | 0.576 | 0.654 | 0.622 | 0.632 | 0.541 | 0.538 | 0.523 | 0.494 | 0.4897 | 0.542 |
| GSN | 0.456 | 0.551 | **** | 0.749 | 0.539 | 0.581 | 0.544 | 0.509 | 0.397 | 0.4 | 0.3979 | 0.378 |
| XN | 0.457 | 0.425 | 0.289 | **** | 0.546 | 0.612 | 0.481 | 0.493 | 0.427 | 0.45 | 0.4622 | 0.438 |
| HS | 0.56 | 0.475 | 0.618 | 0.605 | **** | 0.674 | 0.65 | 0.586 | 0.574 | 0.566 | 0.5315 | 0.557 |
| YY | 0.569 | 0.46 | 0.542 | 0.491 | 0.395 | **** | 0.737 | 0.654 | 0.643 | 0.638 | 0.5774 | 0.628 |
| SZ | 0.631 | 0.615 | 0.608 | 0.733 | 0.43 | 0.305 | **** | 0.787 | 0.583 | 0.593 | 0.6093 | 0.583 |
| WYS | 0.668 | 0.62 | 0.675 | 0.707 | 0.535 | 0.424 | 0.239 | **** | 0.569 | 0.577 | 0.5726 | 0.5 |
| EX | 0.775 | 0.648 | 0.925 | 0.852 | 0.555 | 0.442 | 0.54 | 0.565 | **** | 0.856 | 0.656 | 0.684 |
| SY | 0.762 | 0.706 | 0.917 | 0.799 | 0.569 | 0.449 | 0.524 | 0.55 | 0.155 | **** | 0.7346 | 0.666 |
| HSN | 0.75 | 0.714 | 0.922 | 0.772 | 0.632 | 0.549 | 0.495 | 0.558 | 0.422 | 0.308 | **** | 0.678 |
| FY | 0.758 | 0.613 | 0.973 | 0.825 | 0.586 | 0.465 | 0.539 | 0.693 | 0.38 | 0.406 | 0.3892 | **** |
Table 6: Nei's original measures of genetic identity and genetic distance of Liriodendron chinense in 12 provenances. Nei's genetic identity (above diagonal) and genetic distance (below diagonal).
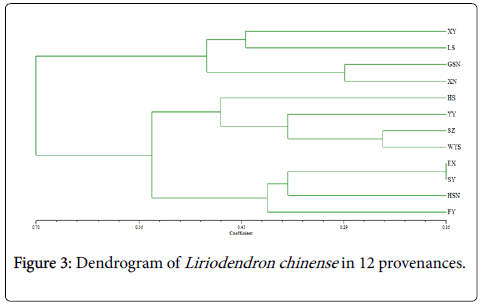
In order to dissect the genetic relationship among 12 L. chinense provenances, the genetic clustering groups should be conducted with the method of UMPGA. As shown in Figure 3, these two populations can be divided into two categories: among which, XY, LS, GSN, and XN belong to one category and the rest eight provenances belong to the other category. Meanwhile, each category can be further divided into two small subcategories: the first small subcategory of the first category includes two populations, namely, XY and LS, and the second small subcategory also consists of two populations, that is GSN and XN; what’s more, the first small subcategory of the second category includes HS, YY, SZ and WYS, the second small subcategory consists of EX, SY, HSN and FY. From Figure 3, it can be found that genetic relationship among adjacent populations is closer than that among remote populations, however, there is no obvious boundary between eastern groups and western groups, which is inconsistent with the result that there is great genetic differentiation among eastern groups and west ones obtained by allozyme analysis [9].
Discussion
Analysis of genetic diversity of L. chinense sarg
At present, effective number of allele (Ne) and heterozygosity (H), as genetic diversity index, are widely applied. Meanwhile, heterozygosity (H) can also reflect richness and degree of uniformity of allele among populations. According to the results of genetic diversity of 125 alleles, it can be found that the genetic diversity of L. chinense is at high level (He=0.6750) compared with other woody plants. For instance, diversity index of Populus tremuloides is 0.46 [10].
Picea abies (0.7890) [11], Castanopsis fargesii Franch (0.654), Quercus suber (0.648) [12], Pseudotsuga menziesii (0.6730) [13], Eucalyptus globulus (0.8200) [14], Quercus variabilis Bl (0.8095) [15], Picea asperata Mast (0.7070) [16] and Corylus heterophylla Fisch (0.5532). The above results show that although L. chinense is under "endangered" living environment, it owns good maintenance mechanism of genetic diversity, and thus its residual species can survive [17,18].
In 12 L. chinense provenances (Table 1), the polymorphism of each provenance has great differences. Its minimal expected heterozygosity is 0.2917, which is higher than that of perennial woody plants (Nei=0.18) and that of perennial woody variations with animal pollination (Nei=0.21) [19]. Perhaps, the reason of this result is that L. chinense has high degree of outcrossing [20] and outcrossing may increase heterozygosity level of filial generation. Although fragmented distribution and human interference increase the chance of inbreeding, the number of individuals generated by self-generates is very few, and the survival rate is also very low [21]. Meanwhile, low level genetic variation of L. chinense is prone to be influenced by habitat destruction, as the potentials of adaptation to environmental changes of the species rely on the level of genetic diversity [22].
Based on the results of previous studies [23], the natural populations of L. chinense are divided into eastern groups and western ones, and the genetic diversity of the former is lower than that of the latter. However, our research results generally reveal that the genetic diversity of eastern groups is higher than that of western ones. It is mainly because the selected sample populations in previous studies are few, which cannot represent the overall situation of the eastern and western populations of L. chinense , while the selected populations in this paper cover all the distribution region of it, and represent the typical groups of the rest populations of L. chinense . Compared with western groups, eastern groups own higher genetic diversity, which is because most of western groups suffer significant bottleneck effect events. In fact, with the increase of the degree of fragmentation and human interference, genetic bottleneck effect will happen inevitably, and the level of genetic diversity of the species will decrease at last.
Genetic structure and gene flow of L. chinense sarg
Habitat fragmentation and the small group effect can reduce the level of gene flow among populations, and increase genetic differentiation [24], which can be detected through study the genetic structure of populations. Genetic differentiation coefficient (Fst) of each L. chinense provenance obtained through the analysis of population genetic structure in this study is 0.3225, namely, 32.25% of genetic variation exist among populations, and 67.75% of genetic variation exist within population, which is basically consistent with the result obtained with allozyme [9], RAPD marker [25], or SSR molecular marker [26]. Genetic variation among populations is much higher than critical value (10%) of outbreeding species, showing that high genetic variation exists among each L. chinense provenances.
Natural selection is the main evolutionary force of population differentiation, and gene flow is an important factor against selective action [27]. Low level gene flow will increase genetic variation and genetic differentiation among populations [24,28]. Generally, perennial woody plants maintain their level of gene flow through generating large number of pollen and seeds [29]. However, as for the gene flow of 12 L. chinense provenances obtained in this paper, it’s Nm=0.5252, which is the consequence of small gene flow resulted by small group and fragmentation of L. chinense [30]. The strength of gene flow has significant influence on genetic differentiation of populations, generally speaking, the species with fluent gene flow have low genetic differentiation among populations; on the contrary, the genetic differentiation among populations is high [31].
Genetic relationship of 12 L. chinense provenances
When Nm>1, gene flow would prevent genetic differentiation among populations caused by genetic drift. 32.25% of genetic variation of L. chinense exist among population, which shows that it has high level of genetic differentiation, and the gene flow among population is only 0.5252, meanwhile, it further reveals that the influence of small group effect and fragmentation on L. chinense provenances. Furthermore, with the gradual decrease of the level of gene flow among populations, the genetic differentiation will also increase, however, the genetic basis will narrow down gradually, which would reduce the counteraction of L. chinense on natural selection, and deepen endangered situation.
Protection and utilization of genetic resources of L. chinense sarg
The study of genetic diversity of L. chinense will contribute to the preparation of scientific strategies, so as to protect and manage the genetic resources of endangered species. Habitat fragmentation and human interference have seriously affected the survival and development of L. chinense sarg. Natural populations of L. chinense maintains high level of genetic diversity and genetic differentiation, therefore, comprehensive measures should be taken to inhibit extinction of the endangered species and in-situ conservation should be adopted to protect the remaining populations of L. chinense . Meanwhile, the most urgent measure is to curb deforestation, make every effort to preserve existing populations and individuals. Since small and isolated populations with low level genetic diversity are more vulnerable to the infringement of change of climate or habitat therefore, in the protective process of L. chinense , both in-situ conservation and ex-situ conservation should be taken simultaneously. In addition, gene banks should be founded by taking the germplasm all of the remaining natural populations as the basis, so as to maximize the preservation of genetic diversity L. chinense .
From the perspective of utilization, it is crucial to make sustainable improvements through maintaining the genetic diversity of species. Hence, in order to expand the genetic basis of breeding populations, seed orchards should be established by selecting elite germplasm of various provenances, which can produce improved varieties for commercial cultivation in the future. It will be a win-win situation, which not only achieve genetic gain, but also preserve genetic diversity.
Acknowledgment
We would like to thank all professors’ Huogen Li and Qiang Zhuge lab crews and students that helped us to carry out experiments and prepare manuscript.
References
- Yuping Z, Song G, Xiaodong W (2001) Molecular markers in systematic and evolutionary botany.
- Yeh FC, Yang RC, Boyle T (1999) POPGENE software package version 1.31 for population genetic analysis. University of Alberta.
- Kimura M, Crow J F (1964) The number of alleles that can be maintained in a finite population. Genetics 49: 725.
- Levene H (1949) On a matching problem arising in genetics. The Annals of Mathematical Statistics 20: 91-94.
- Lewontin RC (1972) The apportionment of human diversity. Evolutionary Biology. pp: 381-398.
- Nei M (1973) Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences 70: 3321-3323.
- Rohlf F J (2002) NTSYS-pc: numerical taxonomy system ver. 2.1. Setauket, NY: Exeter Publishing Ltd.
- Nei M (1987)Â Molecular evolutionary genetics. Columbia university press.
- Zhu X, Ma J, Yao Q, Hao R, He S (1995) Allozyme verification on the population differentiation of Liriodendron chinense (Hemsl) Sarg. Journal of Plant Resources and Environment 4: 9-14.
- Dayanandan S, Rajora OP, Bawa KS (1998) Isolation and characterization of microsatellites in trembling aspen (Populus tremuloides). TAG Theoretical and Applied Genetics 96: 950-956.
- Bucci G, Vendramin GG (2000) Delineation of genetic zones in the European Norway spruce natural range: preliminary evidence. Molecular Ecology 9: 923-934.
- Hornero J, Gallego FJ, Martinez I, Toribio M (2001) Testing the Conservation of Quercus spp. Microsatellites in the Cork Oak, Q. suber L. silvae genetica 50: 162-167.
- Amarasinghe V, Carlson JE (2002) The development of microsatellite DNA markers for genetic analysis in Douglas-fir. Canadian Journal of Forest Research 32: 1904-1915.
- Jones RC, Steane DA, Potts BM, Vaillancourt RE (2002) Microsatellite and morphological analysis of Eucalyptus globulus populations. Canadian Journal of Forest Research 32: 59-66.
- Xu XL, Xu LA, Huan MR, Wang ZR  (2004) Genetic diversity of microsatellites (SSRs) of natural populations of Quercus variabilis. Yi chuan-Hereditas/Zhongguo yi chuan xue hui bian ji 26: 683-688.
- Wang Y, Zuo Y, Gao H (2006) Luminescence properties of nanocrystalline YVO 4: Eu 3+ under UV and VUV excitation. Materials research bulletin 41: 2147-2153.
- Given DR (1994) Principles and practices of plant conservation. Timber Press pp: 1-36.
- Hattemer HH (1995) Concepts and requirements in the conservation of forest genetic resources. Forest Genetics 2: 125-134.
- Hamrick JL (1989) Isozymes and the analysis of genetic structure in plant populations. Isozymes in plant biology 4: 87-105.
- Feng Y, Li H, Yang J, Wang X, Wang B, et al. (2010) Comparison of reproduction success of Liriodendron chinense Sarg and L. tulipifera Linn. Journal of Tropical and Subtropical Botany 18: 9-14.
- Junxiu Y, Huogen L, Liming B, Jisen S (2012) Inbreeding Depression in Liriodendron Revealed by Both Phenotypic and Genotypic Characteristics. Journal of Northeast Forestry University 7: 001.
- Zaouali Y, Chograni H, Trimech R, Boussaid M (2012) Genetic diversity and population structure among Rosmarinus officinalis L.(Lamiaceae) varieties: var. typicus Batt. and var. troglodytorum Maire. based on multiple traits. Industrial Crops and Products 38: 166-176.
- Zhu X, Ma J, Yao Q, Hao R, He S (1995) Allozyme verification on the population differentiation of Liriodendron chinense (Hemsl.) Sarg. Journal of Plant Resources and Environment 4: 9-14.
- Epperson B K, Allard R W (1989) Spatial autocorrelation analysis of the distribution of genotypes within populations of lodgepole pine. Genetics 121: 369-377.
- Luo G, Shi J, Yin T, Huang M, Wang M (2000) Comparison of genetic diversity between liriodendrom tulipifera linn. and liriodendron chinense (Hemsl.) sarg. By means of RAPD markers. Journal of Plant Resources and Environment 9: 9-13.
- Cheng L (2008) The genetic structure of natural populations in Liriodendron Chinense using SSR Markers. J of Nanjing Forestry University.
- Hamrick JL, Godt MW (1990) Allozyme diversity in plant species. Plant population genetics, breeding, and genetic resources pp: 43-63.
- Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science (Washington) 236: 787-792.
- Vranckx GUY, Jacquemyn H, Muys B, Honnay O (2012) Meta-analysis of susceptibility of woody plants to loss of genetic diversity through habitat fragmentation. Conservation Biology 26: 228-237.
- Semaan MT, Dodd RS (2008) Genetic variability and structure of the remnant natural populations of Cedrus libani (Pinaceae) of Lebanon. Tree genetics & genomes 4: 757-766.
- Rowe C (1998) COLLOQUIUM 9. In Proceedings of the Boston Area Colloquium in Ancient Philosophy 14: 239-259.
Citation: Yaghuti AA, Movahedi A, Mohammadi K, Zhuge Q, Li H (2018) Genetic Diversity among a Collection of L. chinense Germplasm Analyzed with SSR Markers. J Biochem Microb Toxicol 1: 102.
Copyright: © 2018 Yaghuti AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 6352
- [From(publication date): 0-2017 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 5493
- PDF downloads: 859