Case Report Open Access
Generalized Skin Rashe After Oral Administration of Ayurvedic Drugs: An Unintened Drug Reaction
| Manjunath Ajanal1* and Prasad BS2 | |
| 1Research Associate, KLEU Shri BMK Ayurved Mahavidhyalaya, Belgaum, Karnataka, India | |
| 2Principal, KLEU Shri BMK Ayurved Mahavidhyalaya, Belgaum, Karnataka, India | |
| Corresponding Author : | Manjunath Ajanal Research Associate KLEUb Shri BMK Ayurved Mahavidhyalaya, Belgaum Karnataka – 590003, India Tel: +09036616510 E-mail: manju.ajanal@gmail.com |
| Received July 01, 2013; Accepted July 29, 2013; Published August 04, 2013 | |
| Citation: Ajanal M, Prasad BS (2013) Generalized Skin Rashe After Oral Administration of Ayurvedic Drugs: An Unintened Drug Reaction. J Homeop Ayurv Med 2:128. doi: 10.4172/2167-1206.1000128 | |
| Copyright: © 2013 Ajanal M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
We report a case of skin rashes that occurred in a 20-year-old South Indian female of Pittakaphala prakruthi
(constitution) after beginning therapy with Aragwadadi kashaya (poly-herbal formulation) and syp talekt (poly-herbal patent formulation) for the treatment of reccurent incidence of abscess. Rash disappeared after stopping the suspected drug and treatment with Vibhitaki kashaya (decoction of Terminilia bellarica) and Shatadauta gruta (washed ghee). Possible and Probable (Score 6) were the causality according to WHO-UMC and Naranjo’s ADR Probability Scale and grouped under type-B reaction. To our knowledge, this is the first case of skin rashes induced by Aragwadadi kashaya and syp talekt. This report highlights the need of implementation of Pharmacovigilance centre in hospital level and additional research in the field of skin toxicity of Aragwadadi kashaya and syp talekt.
| Keywords |
| Skin rashes; Aragwadadi kashaya; Talekt; Abscess; Ayurveda |
| Introduction |
| Rashes are most common cutaneous ADRs to any type of drugs and appear within first week of the drug therapy [1]. Overall incidence of allergic drug reactions is variously reported as being between 2 to 25% [2,3]. Common causes of skin rashes are infective, drug, sensitive skin, and some of known cutaneous eruption by Penicillin, Phenytoin, Sulphonamide, Sulphonylurea, Thiazide, antituberculous drugs, antimalarials, penicillamine, ampicillin, amoxicillin etc [4]. “Ayurveda” is an ancient system of healthcare that is native to India. There are references in Ayurvedic literature on way in which drug induced consequences. Such as,manifestation of skin diseases onexternal administration of juice of Ballathaka (Semecarpus anacardium) [5], by suppressing urge of Charddi (vomiting) [6], Vamana mithyayoga (inadequate administration of emesis) [7], Dashanga lepa (poly herbal formulation) induced skin rashes [8] and thyroiditis followed by ginger consumption [9]. |
| Recurrent mucocutaneous infection is a common clinical problem. The most common causesareimmune deficiency, chronic skin disease, Diabetes Mellitus (DM) and inflammatory bowel disease (IBD)[10]. |
| Aragwadadi Kashaya(ARK) is well known herbecious decoction of Ayurveda used to treat various skin ailments. It contains Cassia fistula Linn, Holarrhenaantidysenterica Wall, Stereospermumsuaveolens DC, SwertiachirataBuch.Ham, AzadirachtaindicaA.juss, Tinosporacordifolia (Willd).Miers, Marsdeniatenacissima Wight and Arn, SolanumxanthocarpumSchrad&Wendl, Cissampelospareira Linn, Andrographis paniculata Wall.ex Nees, Barleriaprionitis Linn, TrichosanthesdioicaRoxb, Pongamiapinnata (Linn) Merr, Holopteleaintegrifolia Planch, Alstoniascholaris R.B, Plumbagozeylanica Linn, ElettariacardamomumMaton, RandiadumetorumLamkZizyphusjujuba Mill as ingredients and it is thereupeticaly indicated in Emesis, Morbidity due to Poisonous substance, Disorders due to vitiation of Kaphadosha, Urinary disorders, Non-healing ulcer and skin diseases [11]. Syp Talekt was another patent medication from Himalaya drug company and each 5 ml of syrup contains extract of 18 mg of (Cassiafistula Linn and Curcumalonga Linn, 16mg of AzadirachtaindicaA.juss) and Tinosporacordifolia (Willd.) and 15.5mg of Triphala[Terminaliachebula Retz., Terminaliabellirica (Gaertn) Roxb, and Phyllanthusemblica Linn)], Emblicaribes Burm.f., Ecliptaalba (L.) Hassk, SwertiachirayitaKarsten and chemicals namely Methyleparabin sodium propyle and Sodium benzoate were preservatives and company has claimedas “Effective management on skin disorders”. |
| These formulations are found to be safe and dermatological manifestations are extremely rare. This article discusses a case of skin rash in apatient on oral administration of ARK and Syp Talekt. |
| Case Report |
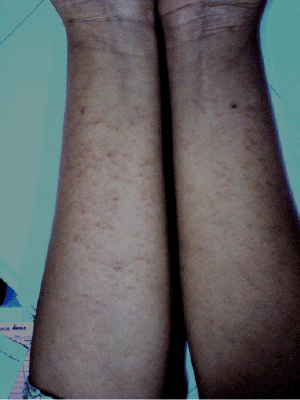
| A 20-year-old South Indian female of Pittakaphala prakruthi (constitution), who had a resident of Belgaum, Karnataka since birth, was consulted forabscess in right thighthat had developed 3-months back together with fever and myalgia. On adminitration of ARK3tsf with water and syp Talekt 3tsf with water, she has presented widespread skin rashes involving the abdomen, thighs, and upper arms (Figures 1 and 2). No mucosal involvement was noted. The remainder of the physical examination was normal with no signs of conjunctivitis. |
| Her history includes regular incidence of abscess once in 6 to 9 months. A month back she was on Serratio peptidase with Diclofenac sodium (15:200 mg) with dose of 1tb b.i.dfor 7-days and patient was not known for allergy to any food, drugs or chemical preservative. Previous day of the event patient was on ARK 3 tsf with water before food thrice daily. On the next day along with previous drug syp Talekt was administered with dose of 3tsf with water before food in thrice daily. |
| After 3rd dose of above medication when she woke from bed on the following dayshe experienced itching, redness with rashes. Subsequently syp-talekt was discontinued following worsening of event that included burning sensation and itching. And later even stopped AR kashaya and advised Vibhitaki kashaya(decoction of Terminilia bellarica) for washing followed by application of Shatadautagruta (washed ghee) (AFI) and oral medications of Vibhitakikashaya (decoction of Terminilia bellarica) 3tsf with warm water before food and ChandanasavaAFI 3tsf with equal amount of water after food three time in a day, subsequently reduced the skin iching and rashes. |
| Patient written consent was taken for documenting and publication of this case. |
| Discussion |
| We hereby presented a case of skin rash after consuming ARK and syp-talekt. There was a temporal relationship and positive dechallenge which points towards the association between the suspected formulation and the event. No such reaction was noticed by any other patient on taking syp-talekt and ARK of the same batch. This point’s towards the susceptibility of the patient towards the reaction. |
| Causality assessment was done by WHO-UMC Causality Assessment Scale and Naranjo’s ADR Probability Scale the score of which are Possible and Probable (Score 6) with Syp-talekt and ARK administration respectively and severity was moderate. Dermatological reaction to ARK was not known, infact the present formulation is known for its vast thereupetic applications on skin disorders [10] hence,in present event cause is difficult to trace, but it can be attributed to Parabine (chemical preservative). |
| Parabine is a well known preservative used in many formulation it has known to cause skin irritation and contact dermatitis in selective paraben allergy individuals [12,13] hence, we believe present drug event could be hypersensitivity to the preservative even though patient history does’t include known allergy to preservatives. |
| Learning points |
| This may be the good example for unpredictable or idiosyncratic reaction because it is very hard to predict cause and effect relation in modern pharmacology or Ayurvedic pharmacologyas in Dashanga lepa induced skin rashes [8] so it may be grouped under type-B type of adverse drug reaction. |
| Such unpredictable adverse reactions are not necessarily due to errors or negligence. It is difficult to predict host susceptibility to such response and thus it becomes very important to document, evaluate and report such reactions so that it recurrence can be prevented in future. |
| The detection of unknown and unexpected relationship between drug exposure and adverse events is one of the major challenges of pharmacovigilance. It is likely for an unexpected adverse reaction to go unnoticed until a very substantial number of patients have been adversely affected. Early detection of unknown and unexpected adverse reactions depends upon proper identification of signals and voluntary reporting system. Identifying and reporting such events plays an important role in preventing its recurrence. |
| Developing a practice of identifying ADR and its reporting will play an important role in successful implementation of National pharmacovigilance program for Ayurveda Siddha and Unani (ASU) drugs. |
| This case has been reported to National pharmacovigilance centre for ASU drugs, Jamanagar, India on 08.01.2011. |
References
|
Figures at a glance
 |
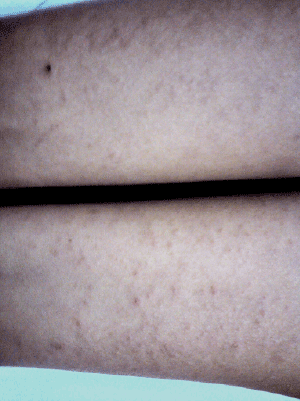 |
| Figure 1 | Figure 2 |
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 20904
- [From(publication date):
September-2013 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 16210
- PDF downloads : 4694
