Generalized Giant Cell Arteritis Discovered on Positron Emission Tomography
Received: 03-Jan-2018 / Accepted Date: 22-Jan-2018 / Published Date: 29-Jan-2018 DOI: 10.4172/2167-7964.1000288
Abstract
An eighty two year old man with a remote history of metastatic melanoma status post chemotherapy presented with fatigue, generalized weakness, low grade fevers, abdominal pain and weight loss. Positron emission tomography (PET) scan was obtained, which revealed diffuse and symmetric increased F-18 Fluorodeoxyglucose (FDG) uptake throughout the large arterial vasculature in the neck, chest, abdomen, pelvis and lower extremities. CT angiogram showed subtle wall thickening involving the bilateral vertebral, bilateral axillary, left subclavian, abdominal aorta, bilateral common iliac, internal iliac, common femoral, superficial femoral, popliteal and anterior tibial arteries, consistent with large vessel vasculitis. Subsequent left temporal artery biopsy revealed multiple giant cells with associated lymphoid cells infiltrating all levels of the artery, but most focused in the media and adventitia, consistent with giant cell arteritis (GCA). The patient was started on prednisone, resulting in prompt improvement in his constitutional symptoms and normalization of his sedimentation rate.
Keywords: Giant cell arteritis; Positron emission tomography (PET)
Introduction
Giant cell arteritis, a granulomatous vasculitis of large and mediumsized arteries, is the most common form of vasculitis in patients older than 50 years of age. The estimated annual incidence of giant cell arteritis is 15-33 per 100,000 people, with those of Northern European ancestry particularly affected [1]. It has a predilection for the branches of the proximal aorta and in particular tends to affect the extracranial branches of the carotid artery [2]. Imaging with Positron emission tomography (PET) is not routinely obtained in patients with GCA and therefore clinical utility of this imaging modality, especially in patients without focal symptoms is unknown. Given lack of knowledge in these aspects, we present a case of generalized GCA discovered on a PET study.
Case
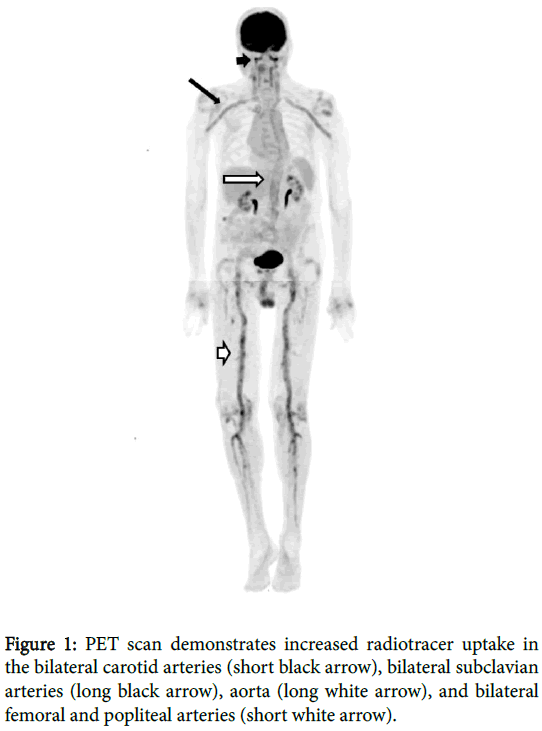
An 82 year old male presented with fatigue, generalized weakness, low grade fevers, abdominal pain and weight loss. The patient reported no history of typical GCA symptoms such as headache, jaw pain or claudication. Past medical history included metastatic melanoma diagnosed and treated more than 10 years ago. There was no history of treatment with immunomodulators for his melanoma. Erythrocyte sedimentation rate (ESR) was markedly elevated to 116 mL/h (normal range 0 to 22 mL/h). To evaluate for possible metastatic melanoma, PET scan was obtained, which incidentally revealed diffuse and symmetric increased F-18 Fluoroeoxyglucose (FDG) uptake throughout the large arterial vasculature in the neck, chest, abdomen, pelvis and lower extremities (Figure 1).
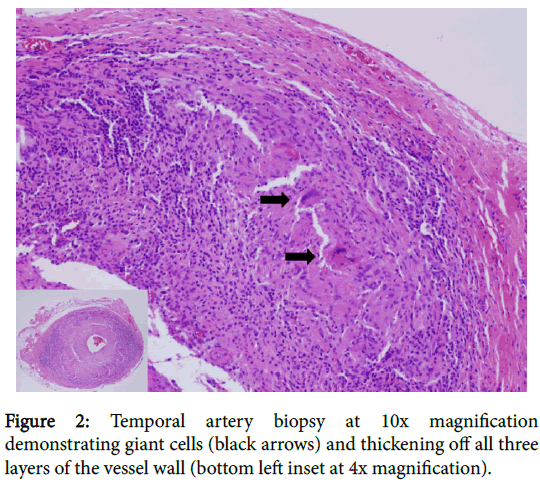
Subsequent CT angiogram demonstrated large vessel arterial wall thickening involving the bilateral vertebral, axillary, common iliac, internal iliac, common femoral, superficial femoral, popliteal and anterior tibial arteries, and also the left subclavian artery, and abdominal aorta. The constellation of findings was consistent with large vessel vasculitis. Subsequent left temporal artery biopsy revealed multiple giant cells with associated lymphoid cells infiltrating all levels of the artery, but most focused in the media and adventitia, consistent with giant cell arteritis (GCA) (Figure 2). Therefore, the patient was started on prednisone, resulting in prompt improvement in his constitutional symptoms and normalization of his sedimentation rate.
Discussion
GCA typically affects large and medium sized arteries. Typical symptoms include headache and visual disturbances [2]. Rarely, they may affect predominantly lower extremity arteries, in which case the patients are typically women who develop rapidly progressive lower extremity claudication with absent cranial symptoms [3].
In contrast to these presentations, our patient who demonstrated incidental generalized disease on the PET scan did not report symptoms of lower extremity claudication or typical cranial symptoms. In fact the PET scan was performed to evaluate for melanoma metastases but eventually lead to the discovery of extensive vasculitis. The subsequent CTA which showed arterial disease in multiple territories more commonly attributed to atherosclerotic changes in patients this age, but likely related to at least in part vasculitis in our patient. Upon review of the current literature, PET scan appears to be an emerging and promising imaging technique for early detection and subsequent monitoring of treatment success of large-vessel inflammation in giant cell arteritis. Whole-body imaging with FDGPET uptake by large vessels can be effective in assessing the activity and extent of giant cell arteritis and of Takayasu’s arteritis [4,5].
Grayson et al. investigated the clinical value of PET in large vessel vasculitis demonstrating 85% sensitivity and 83% specificity for active large vessel vasculitis [6]. Interestingly, more than 50% patients demonstrated persistent activity even while in clinical remission. When this activity is high, the patients are more likely to have a future clinical relapse at a median follow-up of 15 months. GCA has been recently reported as a complication of immune checkpoint inhibitor therapy for melanoma [7]. However, our patient did not receive immune checkpoint inhibitor therapy for his melanoma and in addition, the disease and treatment occurred more than 10 years prior to the current presentation of GCA.
Conclusion
Our case illustrates the utility of PET scan for diagnosing and evaluating the extent of large-vessel vasculitis, even with lack of focal symptoms in the involved vessels. The current literature supports increasing utility of this modality as an adjunct in diagnosing and monitoring this disease.
References
- Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE (2004) Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Res 51: 264-268.
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B (2016) Polymyalgia rheumatica and giant cell arteritis: A systematic review. Jama 315: 2442-2458.
- Kermani TA, Matteson EL, Hunder GG, Warrington KJ (2009) Symptomatic lower extremity vasculitis in giant cell arteritis: A case series. J Rheumatol 36: 2277-2283.
- Prieto-González S, Depetris M, GarcÃa-MartÃnez A, Espigol-Frigole G, Tavera-Bahillo I, et al. (2014) Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: A prospective, case-control study. Ann Rheum Dis 73: 1388-1392.
- Walter MA (2006) [(18) F] fluorodeoxyglucose PET in large vessel vasculitis. Radiol Clin North Am 45: 735-744.
- Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, et al. (2017) Positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol.
- Goldstein BL, Gedmintas L, Todd DJ (2014) Drug-Associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of CTLA-4. Arthritis Rheumatol 66: 768-769.
Citation: Habbu A, Hatti K, Lancaster L, Hendricks N, Grosh W, et al. (2018) Generalized Giant Cell Arteritis Discovered on Positron Emission Tomography. OMICS J Radiol 7: 288. DOI: 10.4172/2167-7964.1000288
Copyright: ©2018 Habbu A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4432
- [From(publication date): 0-2018 - Apr 19, 2025]
- Breakdown by view type
- HTML page views: 3616
- PDF downloads: 816