Review Article Open Access
Gastrointestinal Dysfunction in Chronic Liver Disease
Dep Huynh1,2, and Nam Q Nguyen1,2,*
1Department of Gastroenterology and Hepatology, Royal Adelaide Hospital, North Terrace, Adelaide, 5000, South Australia, Australia
2Discipline of Medicine, University of Adelaide, North Terrace, Adelaide, 5000, South Australia, Australia
- Corresponding Author:
- Nam Q Nguyen
Department of Gastroenterology
Royal Adelaide Hospital, North Terrace
Adelaide, 5000 South Australia, Australia
Tel: 61-88222-5207
Fax: 61-88222-5885
E-mail: quoc.nguyen@health.sa.gov.au
Received Date: May 28, 2014; Accepted Date: February 15, 2015 Published Date: February 25, 2015
Citation: Huynh D, Nguyen NQ (2015) Gastrointestinal Dysfunction in Chronic Liver Disease. J Gastrointest Dig Syst 5:257. doi:10.4172/2161-069X.1000257
Copyright: © 2015 Huynh D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Gastrointestinal dysfunction frequently occurs in liver cirrhosis and increases with disease severity. The major abnormalities are altered gastrointestinal motility, disrupted gut barrier function with increased intestinal permeability and malabsorption. Not only does the presence of these gut abnormalities impair oral intake and lead to malnutrition, they can play a central role in the pathogenesis of many of the complications of liver cirrhosis as well as liver disease progression. The aim of this paper is review the details of gastrointestinal dysfunction in chronic liver disease and the associated impact on the clinical outcomes.
Keywords
Liver cirrhosis; Gastrointestinal motility; Intestinal absorption; Intestinal permeability
Introduction
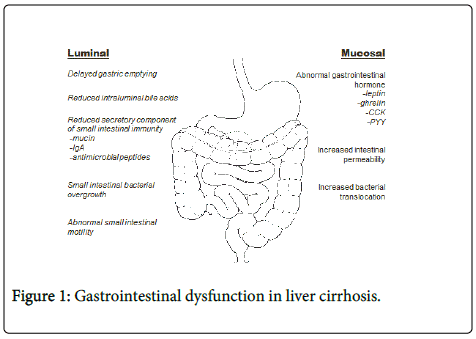
Various abnormalities in gastrointestinal structure and function have been described in patients with liver cirrhosis, including altered gastrointestinal motility, intestinal permeability and absorption. While these changes may not be as clinically overt as other common complications of chronic liver disease, they not only influence nutritional status, but can also contribute to clinical complications such as hepatic encephalopathy and spontaneous bacterial peritonitis. Disruption of the gut barrier function has also been implicated in the pathogenesis of liver fibrosis in alcoholic and non-alcoholic fatty liver disease. This review aims to highlight the abnormalities in gastrointestinal function that are commonly observed in patients with chronic liver disease and their impact on the overall outcomes in these patients (Figure 1).
Gastric emptying and gastric accommodation in cirrhosis
Patients with cirrhosis frequently experience gastrointestinal symptoms [1,2]. Abnormal gastric motor function may contribute to symptom generation in these patients. Whilst delayed gastric emptying has been reported in patients with cirrhosis and has been associated with post-prandial fullness and bloating [3-8], this is not a consistent finding and both normal [8-11] and rapid gastric emptying [12,13] have also been observed in these patients. This inconsistency in the rate of gastric emptying in these patients is most likely related to the different methodologies used to determine gastric emptying [14-17], as well as the differences in disease aetiology and severity of the patients at the time of assessment. Overall, the reported prevalence of gastroparesis varies from 24% to 95% [6,18].
The rate of gastric emptying in chronic liver disease appears to be influenced by age and disease severity based on Childs-Pugh Score [3], albumin, bilirubin, prothrombin time and platelet count [19,20]. Aetiology of liver disease may also impact on the rate of gastric emptying, with increased gastrointestinal transit in non-cirrhotic portal hypertension disease and slower transit in alcoholic liver disease [21,22]. The mechanisms underlying the abnormal gastric emptying and intestinal transit in patients with chronic liver disease remain unclear. Autonomic dysfunction is common in liver cirrhosis, irrespective of alcoholic or non-alcoholic aetiology [23-25], and may contribute to the abnormal GI transit. In patients with liver cirrhosis, the presence of autonomic dysfunction is a risk factor for delayed gastric emptying [3,26]. As in healthy and diabetic subjects [9,26], postprandial hyperglycaemia and hyperinsulinaemia have also been found to be associated with reduced gut motility and delayed gastric emptying in cirrhosis [27]. This is particularly relevant as glucose intolerance and diabetes are common in patients with cirrhosis [28-30], characterized by the marked, sustained post-prandial hyperglycaemia following carbohydrate ingestion with increased peripheral plasma insulin levels [31-33]. Although the levels of several gut hormones that are involved in the regulation of gastrointestinal motility are deranged in chronic liver disease, their contribution to gastrointestinal dysmotility remains uncertain. The attenuated rise of post-prandial ghrelin, an orexigenic peptide hormone produced by the stomach and proximal small bowel that also has a gastroprokinetic effect [34,35], appears most relevant as it correlated positively with gastric emptying time and weight loss in patients with liver cirrhosis [36,37]. Both plasma cholecystokinin (CCK) and peptide YY (PYY) are potent satiation peptides that slow gastric emptying [27,38,39]. Whilst they have been shown to be elevated in cirrhosis [40-42], their roles in gastric emptying, energy intake and nutritional status have not been formally evaluated with antagonists. Although elevated serum secretin levels have been correlated with dysrhythmic electrogastrography waveform in liver cirrhosis, it remains unclear how secretin mediates gastric dysmotility in these patients [43]. The presence of ascites is another potential contributor to gastroparesis in these patients [44,45]. While satiety and caloric intake are improved following large volume paracentesis, these symptomatic improvements were not directly related to accelerated gastric emptying [46]. Instead, impaired gastric accommodation appears to be more relevant [43,47], and using single photon emission computed tomography (SPECT), the presence of ascites in patients with cirrhosis is associated with decreased accommodation, which improves after large volume paracentesis [48].
Intestinal dysmotility in cirrhosis
Data relating to the small intestinal transit in patients with liver disease are conflicting. Using radio-opaque markers to calculate small bowel residence time and lactulose hydrogen breath test to determine oro-caecal transit, small intestinal transit has shown to be prolonged in patients with liver cirrhosis [20,49,50]. In contrast, the transit time of cirrhotic subjects was similar to healthy subjects when magnet based Motility Tracking System [51] and scintigraphic [52] techniques were used. Despite these conflicting findings, manometric studies have consistently demonstrated a number of motor disturbances in the small intestine of these patients, including absence of inter-digestive migratory motor complex (MMC), prolonged duration of MMC cycles and changes in the pattern of contractions with multiple clustered contractions [53-55]. As with gastric emptying, there is a relationship between liver disease severity and small intestinal dysmotility [56], and more importantly, these motor abnormalities normalize after liver transplantation [57].
Small intestinal bacterial overgrowth
Small intestinal bacterial overgrowth (SIBO) is common in patients with cirrhosis and the prevalence appears to vary according to the evaluating techniques. Whilst prevalence of SIBO can be as high as 48-73% based on cultures of jejunal aspirate [5-7,21,58,59], it is much lower (30% to 38%) when glucose hydrogen breath test is used [60-62]. Although glucose hydrogen breath test has been shown to be unreliable in patients with cirrhosis[63], jejunal aspiration requires upper endoscopy, sedation and is invasive [64]. These weaknesses limit the routine clinical application of jejunal aspiration in the investigation of SIBO.
Overall, the risk factors for SIBO in chronic liver disease are advanced disease severity, presence of ascites and hyperbilirubinemia [65-67]. The aetiology of SIBO in these patients remains unclear. As bile acids have a secondary function of inhibiting the growth of bacteria in the small intestine [68,69], the reduced bile acid secretion in cirrhotic patient is thought to contribute to pathogenesis of SIBO [70-72]. Additionally, reduction in the conversion of primary bile acids to secondary bile acids is associated with an increase in the prevalence of more pathogenic bacteria such as Enterobacteriaceae [73] which are also more efficient at bacterial translocation from the gastrointestinal lumen [74]. Gastric acid is also important for decontamination of the stomach and proximal small bowel [38,75]. Acid suppressive therapy and spontaneous fasting hypochlorhydria have both been shown to be strong predictors for SIBO in patients with cirrhosis [76].
Whilst intestinal dysmotility has been associated with SIBO in non-cirrhotic patients and experimental models of cirrhosis [77-79], the data relating the impact of small bowel motor abnormalities on SIBO are more controversial in patients with cirrhosis. Chesta et al. [22] found no difference in the motility pattern in cirrhotic patients with or without SIBO, and treatment of bacterial overgrowth with tetracycline did not significantly alter small intestinal motility apart from reducing the time in phase 2 that was occupied by multiple-clustered contractions. In contrast, a more recent study found that long term treatment with alternating norfloxacin and neomycin was associated with decreased bacterial overgrowth, reduced oro-caecal transit time and improved cyclic activity of MMC on intestinal manometry [80]. This suggests that SIBO itself may contribute to small intestinal dysmotility, therefore creating a vicious cycle that promotes further bacterial overgrowth.
Gastrointestinal permeability in cirrhosis
In the normal gut, the integrity of the intestinal epithelial barrier prevents diffusion of bacteria and endotoxins across the epithelium. In patients with cirrhosis, there are changes in epithelial barrier function that lead to an increased in intestinal permeability, as assessed by urinary recovery of orally administered test markers [25,81,82]. Available studies suggest that the changes in the structure and function of the enterocyte tight junctions may be responsible for the elevated permeability [83-85]. In vitro studies indicated that alcohol and its metabolite acetaldehyde can inhibit the expression of tight junction protein, resulting in increased permeability [83,86] Additionally, alcohol can impair microtubule cytoskeleton in intestinal epithelial cells by inducing nitric oxide overproduction and oxidation/nitration of cytoskeletal proteins [87]. A recent study has demonstrated that the changes to tight junctions occur in liver cirrhosis of mixed aetiology [88], and are not limited to alcoholic liver disease. More importantly, the reduction in tight junction proteins appears to relate to the disease severity, and is more pronounced in decompensated patients as compared to compensated patients [3].
Consequences of gastrointestinal dysfunction in liver cirrhosis
The combination of gastrointestinal dysmotility, SIBO, and abnormal intestinal epithelial barrier function have been proposed as major risk factors for the development of malnutrition as well as complications and progression of liver cirrhosis.
Malnutrition is common in patients with liver cirrhosis and the underlying mechanisms are not fully understood. Reduced oral intake is thought to be an important contributor of malnutrition and is mediated by a number of factors. It has been proposed that the impaired gastric emptying and intestinal motility are responsible for the adverse gastrointestinal symptoms such as poor appetite, early satiety and bloating [49]. Alterations in appetite regulating gut hormones also may also contribute to the reduced oral intake, including increased fasting leptin [47], attenuated rise in post-prandial ghrelin [47], elevated fasting PYY [89] and CCK [90]. Malabsorption may also contribute to the malnutrition in liver cirrhosis. In experimental models of cirrhosis, intestinal absorption of carbohydrate, fat and protein have been shown to be significantly reduced [16,17,77]. Although fat malabsorption has been reported to occur in up to two-thirds of patients with cirrhosis [59], the absorption of carbohydrate does not appear to be reduced in these patients, using combined sugar probes (D-Xylose/3-O-methyl-D-glucose and rhamnose/3-OMG) [13,91]. The data relating the intestinal absorption of protein in patients with chronic liver disease are lacking. The proposed mechanisms that contribute to fat malabsorption are: decreased bile acid secretion, small bowel bacterial overgrowth, pancreatic exocrine insufficiency in alcoholic liver disease, and use of medications that lead to malabsorption such as neomycin and lactulose [92,93]. While gastric mixing is not essential for carbohydrate digestion as it is with lipid and protein digestion, delayed gastric emptying determines the rate at which nutrient is delivered to the small intestine and has the potential to reduce the rate and extent of carbohydrate absorption [19,31]. The disruption to small intestinal motility after AAA repair has recently been shown to impair fat but not carbohydrate absorption [94]. The contribution of abnormal gastrointestinal motor function to malabsorption has not been evaluated in liver cirrhosis.
The impaired intestinal epithelial barrier function in cirrhotic patients carries a number of important clinical implications that are known to increase both morbidity and mortality. These include ascites [78,87], spontaneous bacterial peritonitis [95], hepatic encephalopathy [78], infections in hospitalized cirrhotic patients with gastrointestinal haemorrhage [53], and liver disease progression [15]. Bacterial translocation is a key step in the pathogenesis of many of these complications, and is defined by the migration of viable microorganisms and/or bacterial products (lipopolysaccharides, peptidoglycans, muramyl-dipeptides, bacterial DNA) from the intestinal lumen to mesenteric lymph nodes and/or other extra-intestinal sites [8]. SIBO, increased permeability of the intestinal mucosa and impaired intestinal immunity, including the secretory component (immunoglobulin A, mucin and defensins) contribute to pathological bacterial translocation in liver cirrhosis [96]. Translocation of endotoxins, particularly Lipopolysaccharides (LPS), contribute to the pathogenesis of liver fibrosis through the activation of immune cells, particularly Kupffer cells, leading to an increase in the production of pro-inflammatory cytokines and nitric oxide. This in turn, results in chronic active inflammation and fibrosis in viral hepatitis, alcoholic liver disease and non-alcoholic liver disease [15,97,98]. Furthermore, increased plasma endotoxin levels positively correlate with the severity of liver dysfunction [23,33], whereas inactivation of Kupffer cells and deficiency of lipopolysaccharide-binding–protein are associated with less liver injury and fibrosis [1,40]. Nitric oxide overproduction further increases intestinal permeability and bacterial translocation[26], and has also been implicated in exacerbating the hyperdynamic circulatory derangements seen in liver cirrhosis [99,100].
In humans, fasting for 4 days is associated with a decrease in intestinal epithelial proliferation and increases apoptosis [101], leading to mucosal atrophy, reduced villus height and crypt depth, impaired intestinal permeability and reduced absorptive surface area [102]. In addition, luminal nutrient deprivation has also been associated with reduced disaccharidase activity including maltase, sucrase and lactase [103-105], further impairing intestinal absorption. More importantly, refeeding has been shown to associate with repair of mucosal atrophy and reduced permeability [11]. In liver cirrhosis, malnutrition increases intestinal permeability [106-108], and facilitates bacterial translocation [109]. Thus, given that reduced oral intake and “relative starvation” are common in these patients, optimization of oral intake or enteral nutritional support may potentially improve gut barrier function, as well as prevent the related complications.
Conclusion
Gastrointestinal dysfunction frequently occurs in liver cirrhosis and increases with disease severity. It has a central role in the pathogenesis of many of the complications of liver cirrhosis. Abnormal gastrointestinal motility in liver cirrhosis contributes to frequent gastrointestinal symptoms and poor oral intake; it is also associated with quantitative and qualitative changes in the small intestinal microbiota. Loss of mucosal integrity together with SIBO facilitates the translocation of bacteria and endotoxin, which have been linked to the pathogenesis of hepatic encephalopathy and the infectious complications of cirrhosis including spontaneous bacterial peritonitis. Bacterial translocation also leads to cascades of event that trigger the release of pro-inflammatory cytokines that can potentially promote the progression of underlying liver disease. Thus, in order to improve oral intake, nutrient absorption and potentially reduce bacterial translocation, it is important to identify and manage the presence of gastrointestinal dysfunction early.
References
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG (1994) Inactivation of Kupffer cells prevents early alcohol-induced liver injury.Hepatology 20: 453-460.
- Aqel BA, Scolapio JS, Dickson RC, Burton DD, Bouras EP (2005) Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 3:1095-1100
- Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, et al. (2012) Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. European journal of clinical investigation 42: 439-446.
- Balan KK, Grime S, Sutton R, Critchley M, Jenkins SA (1996) Abnormalities of gastric emptying in portal hypertension.Am J Gastroenterol 91: 530-534.
- Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, et al. (2000) Diagnosis of small intestinal bacterial overgrowth in patients with cirrhosis of the liver: poor performance of the glucose breath hydrogen test.J Hepatol 33: 382-386.
- Bauer TM, Steinbruckner B, Brinkmann FE, Ditzen AK, Schwacha H, et al. (2001) Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol 96:2962-2967
- Bauer TM, Schwacha H, Steinbruckner B, Brinkmann FE, Ditzen AK, et al. (2002) Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol 97:2364-2370 .
- Berg RD, Garlington AW (1979) Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infection and immunity 23: 403-411.
- Björnsson ES, Urbanavicius V, Eliasson B, Attvall S, Smith U, et al. (1994) Effects of hyperglycemia on interdigestive gastrointestinal motility in humans.Scand J Gastroenterol 29: 1096-1104.
- Bode C, Kolepke R, Schafer K, Bode JC (1993) Breath hydrogen excretion in patients with alcoholic liver disease--evidence of small intestinal bacterial overgrowth. Zeitschrift fur Gastroenterologie 31:3-7.
- Boza JJ, Möennoz D, Vuichoud J, Jarret AR, Gaudard-de-Weck D, et al. (1999) Food deprivation and refeeding influence growth, nutrient retention and functional recovery of rats.J Nutr 129: 1340-1346.
- Braganca AC, Alvares-da-Silva MR (2010) Prevalence of diabetes mellitus and impaired glucose tolerance in patients with decompensated cirrhosis being evaluated for liver transplantation: the utility of oral glucose tolerance test. Arquivos de gastroenterologia 47: 22-27.
- Budillon G, Parrilli G, Pacella M, Cuomo R, Menzies IS (1985) Investigation of intestine and liver function in cirrhosis using combined sugar oral loads.J Hepatol 1: 513-524.
- Buzzelli G, Chiarantini E, Cotrozzi G, Relli P, Matassi L, et al. (1988) Estimate of prevalence of glucose intolerance in chronic liver disease. Degree of agreement among some diagnostic criteria.Liver 8: 354-359.
- Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, et al. (2010) Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage.Dig Liver Dis 42: 200-204.
- Castilla-Cortazar I, Prieto J, Urdaneta E, Pascual M, Nuñez M, et al. (1997) Impaired intestinal sugar transport in cirrhotic rats: correction by low doses of insulin-like growth factor I.Gastroenterology 113: 1180-1187.
- Castilla-Cortázar I, Picardi A, Tosar A, Ainzúa J, Urdaneta E, et al. (1999) Effect of insulin-like growth factor I on in vivo intestinal absorption of D-galactose in cirrhotic rats.Am J Physiol 276: G37-42.
- Chang CS, Chen GH, Lien HC, Yeh HZ (1998) Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis.Hepatology 28: 1187-1190.
- Chapman MJ, Fraser RJ, Matthews G, Russo A, Bellon M, et al. (2009) Glucose absorption and gastric emptying in critical illness.Crit Care 13: R140.
- Chesta J, Lillo R, Defilippi C, Jouanee E, Massone MA, et al. (1991) [Patients with liver cirrhosis: mouth-cecum transit time and gastric emptying of solid foods].Rev Med Chil 119: 1248-1253.
- Chesta J, Silva M, Thompson L, del Canto E, Defilippi C (1991) [Bacterial overgrowth in small intestine in patients with liver cirrhosis].Rev Med Chil 119: 626-632.
- Chesta J, Defilippi C, Defilippi C (1993) Abnormalities in proximal small bowel motility in patients with cirrhosis.Hepatology 17: 828-832.
- Choi Y, Jeon WK, Hwang SJ, Kim BI, Sohn CI, et al. (2011) The role of the gut barrier function in the pathophysiology of viral liver cirrhosis.Hepatogastroenterology 58: 1244-1247.
- Dumitrascu DL, Barnert J, Wienbeck M (1997) Gastric emptying in liver cirrhosis. The effect of the type of meal.Eur J Gastroenterol Hepatol 9: 1073-1080.
- Ersöz G, Aydin A, Erdem S, Yüksel D, Akarca U, et al. (1999) Intestinal permeability in liver cirrhosis.Eur J Gastroenterol Hepatol 11: 409-412.
- Forsythe RM, Xu DZ, Lu Q, Deitch EA (2002) Lipopolysaccharide-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock 17: 180-184.
- Fried M, Erlacher U, Schwizer W, Lochner C, Koerfer J, et al. (1991) Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology 101: 503-511.
- Galati JS, Monsour HP, Dyer CH, Seagren S, Quigley EMM (1995) A survey or the frequency of gastrointestinal complaints in patients with chronic liver disease. Gastroenterology 108:A1068.
- Galati JS, Holdeman KP, Bottjen PL, Quigley EM (1997) Gastric emptying and orocecal transit in portal hypertension and end-stage chronic liver disease. Liver transplantation and surgery: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 3: 34-38.
- Gentile S, Marmo R, Peduto A, Montella F, Coltorti M (1994) Autonomic neuropathy in liver cirrhosis: relationship with alcoholic aetiology and severity of the disease.Ital J Gastroenterol 26: 53-58.
- Gonlachanvit S, Hsu CW, Boden GH, Knight LC, Maurer AH, et al. (2003) Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig Dis Sci 48:488-497.
- Grassi M, Lazzari S, Palmisano P, Nocchi S, Fontana M, Raffa S, Antonelli M (1994) [Evaluation of exocrine pancreatic insufficiency in cirrhotic patients,using the fecal chymotrypsin test]. La Clinica terapeutica 144:501-509.
- Grinko I, Geerts A, Wisse E (1995) Experimental biliary fibrosis correlates with increased numbers of fat-storing and Kupffer cells, and portal endotoxemia.J Hepatol 23: 449-458.
- Gumurdulu Y, Yapar Z, Canataroglu A, Serin E, Gumurdulu D, et al. (2003) Gastric emptying time and the effect of cisapride in cirrhotic patients with autonomic neuropathy.J Clin Gastroenterol 36: 175-178.
- Hamilton I, Worsley BW, Cobden I, Cooke EM, Shoesmith JG, et al. (1982) Simultaneous culture of saliva and jejunal aspirate in the investigation of small bowel bacterial overgrowth.Gut 23: 847-853.
- Hebbard GS, Sun WM, Dent J, Horowitz M (1996) Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. European journal of gastroenterology & hepatology 8: 211-217.
- Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, et al. (1999) Gut mucosal atrophy after a short enteral fasting period in critically ill patients.J Crit Care 14: 73-77.
- Heyman MB; Committee on Nutrition (2006) Lactose intolerance in infants, children, and adolescents.Pediatrics 118: 1279-1286.
- Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, et al. (2006) Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor.Proc Natl Acad Sci U S A 103: 3920-3925.
- Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, et al. (2006) LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. American journal of physiology Gastrointestinal and liver physiology 290:G1318-1328.
- Ishizu H, Shiomi S, Kawamura E, Iwata Y, Nishiguchi S, et al. (2002) Gastric emptying in patients with chronic liver diseases.Ann Nucl Med 16: 177-182.
- Isobe H, Sakai H, Satoh M, Sakamoto S, Nawata H (1994) Delayed gastric emptying in patients with liver cirrhosis.Dig Dis Sci 39: 983-987.
- Izbéki F, Kiss I, Wittmann T, Várkonyi TT, Légrády P, et al. (2002) Impaired accommodation of proximal stomach in patients with alcoholic liver cirrhosis.Scand J Gastroenterol 37: 1403-1410.
- Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, et al. (2013) Modulation of the fecal bile acid profile by gut microbiota in cirrhosis.J Hepatol 58: 949-955.
- Kakiyama G, Muto A, Takei H, Nittono H, Murai T, et al. (2014) A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS.J Lipid Res 55: 978-990.
- Kalaitzakis E, Simren M, Olsson R, Henfridsson P, Hugosson I, et al. (2006) Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scandinavian journal of gastroenterology 41:1464-1472 .
- Kalaitzakis E, Bosaeus I, Ohman L, Björnsson E (2007) Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: correlations with energy intake and resting energy expenditure.Am J Clin Nutr 85: 808-815.
- Kalaitzakis E, Bjornsson E (2008) Hepatic encephalopathy in patients with liver cirrhosis: is there a role of malnutrition?World J Gastroenterol 14: 3438-3439.
- Kalaitzakis E, Sadik R, Holst JJ, Ohman L, Bjornsson E (2009) Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 7:346-352.
- Kanayama S, Himeno S, Kurokawa M, Shinomura Y, Kuroshima T, et al. (1985) Marked prolongation in disappearance half-time of plasma cholecystokinin-octapeptide in patients with hepatic cirrhosis.Am J Gastroenterol 80: 557-560.
- Kanayama S, Himeno S, Higashimoto Y, Yamasaki Y, Kitani T, et al. (1987) Plasma cholecystokinin-octapeptide like immunoreactivity in patients with hepatic cirrhosis.Life Sci 41: 1915-1920.
- Karlsen S, Fynne L, Grønbæk H, Krogh K (2012) Small intestinal transit in patients with liver cirrhosis and portal hypertension: a descriptive study.BMC Gastroenterol 12: 176.
- Kim BI, Kim HJ, Park JH, Park DI, Cho YK, et al. (2011) Increased intestinal permeability as a predictor of bacterial infections in patients with decompensated liver cirrhosis and hemorrhage.J Gastroenterol Hepatol 26: 550-557.
- Kingston ME, Ali MA, Atiyeh M, Donnelly RJ (1984) Diabetes mellitus in chronic active hepatitis and cirrhosis.Gastroenterology 87: 688-694.
- Lakshmi CP, Ghoshal UC, Kumar S, Goel A, Misra A, et al. (2010) Frequency and factors associated with small intestinal bacterial overgrowth in patients with cirrhosis of the liver and extra hepatic portal venous obstruction.Dig Dis Sci 55: 1142-1148.
- Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, et al. (2006) Ghrelin stimulates gastric emptying and hunger in normal-weight humans.J Clin Endocrinol Metab 91: 3296-3302.
- Levine GM, Deren JJ, Steiger E, Zinno R (1974) Role of oral intake in maintenance of gut mass and disaccharide activity.Gastroenterology 67: 975-982.
- Lindgren S, Lilja B, Verbaan H, Sundkvist G (1996) Alcohol abuse exaggerates autonomic dysfunction in chronic liver disease.Scand J Gastroenterol 31: 1120-1124.
- Linscheer WG (1970) Malabsorption in cirrhosis.Am J Clin Nutr 23: 488-492.
- Llovet JM, Bartoli R, March F, Planas R, Vinado B, et al. (1998) Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. J Hepatol 28:307-313.
- Madrid AM, Brahm J, Buckel E, Silva G, Defilippi C (1997) Orthotopic liver transplantation improves small bowel motility disorders in cirrhotic patients.Am J Gastroenterol 92: 1044-1045.
- Madrid AM, Cumsille F, Defilippi C (1997) Altered small bowel motility in patients with liver cirrhosis depends on severity of liver disease.Dig Dis Sci 42: 738-742.
- Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C (2001) Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function.Am J Gastroenterol 96: 1251-1255.
- Madsen JL, Brinch K, Hansen EF, Fuglsang S (2000) Gastrointestinal motor function in patients with portal hypertension.Scand J Gastroenterol 35: 490-493.
- Merli M, Caschera M, Piat C, Pinto G, Diofebi M, et al. (1992) The effect of lactulose and lactitol administration on fecal fat excretion in patients with liver cirrhosis. Journal of clinical gastroenterology 15:125-127.
- Miettinen TA (1972) Lipid absorption, bile acids, and cholesterol metabolism in patients with chronic liver disease.Gut 13: 682-689.
- Montes-de-Oca M, Blanco MJ, Marquez M, Soto MJ, Fernandez-Gutierrez C, et al.JA (2011) Haemodynamic derangement in human immunodeficiency virus-infected patients with hepatitis C virus-related cirrhosis: the role of bacterial translocation. Liver international: official journal of the International Association for the Study of the Liver 31: 850-858.
- Morencos FC, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F (1995) Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci 40:1252-1256.
- Nguyen NQ, Fraser RJ, Chapman M, Bryant LK, Wishart J, Holloway RH, Horowitz M (2006) Fasting and nutrient-stimulated plasma peptide-YY levels are elevated in critical illness and associated with feed intolerance: an observational, controlled study. Crit Care 10:R175.
- Nguyen NQ, Besanko LK, Burgstad CM, Burnett J, Stanley B, et al. (2011) Relationship between altered small intestinal motility and absorption after abdominal aortic aneurysm repair. Intensive Care Med 37:610-618.
- Nielsen MF, Caumo A, Aagaard NK, Chandramouli V, Schumann WC, et al.(2005) Contribution of defects in glucose uptake to carbohydrate intolerance in liver cirrhosis: assessment during physiological glucose and insulin concentrations. American journal of physiology Gastrointestinal and liver physiology 288: G1135-1143.
- Norman K, Pirlich M, Schulzke JD, Smoliner C, Lochs H, et al. (2012) Increased intestinal permeability in malnourished patients with liver cirrhosis.Eur J Clin Nutr 66: 1116-1119.
- Paloheimo LI, Clemmesen O, Dalhoff K, Rehfeld JF (1997) Plasma cholecystokinin and its precursors in hepatic cirrhosis.J Hepatol 27: 299-305.
- Pande C, Kumar A, Sarin SK (2009) Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease.Aliment Pharmacol Ther 29: 1273-1281.
- Papa A, Tursi A, Cammarota G, Certo M, Cuoco L, et al. (1998) Effect of moderate and heavy alcohol consumption on intestinal transit time.Panminerva Med 40: 183-185.
- Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, et al. (2000) Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis.Hepatology 31: 858-863.
- Pascual M, Castilla-Cortazar I, Urdaneta E, Quiroga J, Garcia M, et al. (2000) Altered intestinal transport of amino acids in cirrhotic rats: the effect of insulin-like growth factor-I. American journal of physiology Gastrointestinal and liver physiology 279: G319-324.
- Pascual S, Such J, Esteban A, Zapater P, Casellas JA, et al. (2003) Intestinal permeability is increased in patients with advanced cirrhosis.Hepatogastroenterology 50: 1482-1486.
- Perseghin G, Mazzaferro V, Sereni LP, Regalia E, Benedini S et al. (2000) Contribution of reduced insulin sensitivity and secretion to the pathogenesis of hepatogenous diabetes: effect of liver transplantation. Hepatology 31:694-703.
- Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G (1994) Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis.Hepatology 19: 616-627.
- Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, et al. (2006) Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. American journal of physiology Regulatory, integrative and comparative physiology 290:R668-677.
- Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK et al.(2007) Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. American journal of physiology Endocrinology and metabolism 293:E743-753.
- Rao RK (2008) Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer.Methods Mol Biol 447: 171-183.
- Sadik R, Abrahamsson H, Björnsson E, Gunnarsdottir A, Stotzer PO (2003) Etiology of portal hypertension may influence gastrointestinal transit.Scand J Gastroenterol 38: 1039-1044.
- Sadik R, Abrahamsson H, Stotzer PO (2003) Gender differences in gut transit shown with a newly developed radiological procedure.Scand J Gastroenterol 38: 36-42.
- Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, et al. (2011) Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection.Gastroenterology 141: 1220-1230.
- Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, et al. (2010) Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed?Am J Gastroenterol 105: 323-327.
- Schoonjans R, Van Vlem B, Van Heddeghem N, Vandamme W, Vanholder R, et al. (2002) The 13C-octanoic acid breath test: validation of a new noninvasive method of measuring gastric emptying in rats.Neurogastroenterol Motil 14: 287-293.
- Schoonjans R, Van Vlem B, Vandamme W, Van Vlierberghe H, Van Heddeghem N, et al. (2002) Gastric emptying of solids in cirrhotic and peritoneal dialysis patients: influence of peritoneal volume load.Eur J Gastroenterol Hepatol 14: 395-398.
- Scolapio JS, Ukleja A, McGreevy K, Burnett OL, O'Brien PC (2002) Nutritional problems in end-stage liver disease: contribution of impaired gastric emptying and ascites.J Clin Gastroenterol 34: 89-93.
- Shindo K, Machida M, Fukumura M, Koide K, Yamazaki R (1998) Omeprazole induces altered bile acid metabolism.Gut 42: 266-271.
- Solga SF, Diehl AM (2003) Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics.J Hepatol 38: 681-687.
- Steffen EK, Berg RD, Deitch EA (1988) Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node.J Infect Dis 157: 1032-1038.
- Stotzer PO, Björnsson ES, Abrahamsson H (1996) Interdigestive and postprandial motility in small-intestinal bacterial overgrowth.Scand J Gastroenterol 31: 875-880.
- Sung JY, Shaffer EA, Costerton JW (1993) Antibacterial activity of bile salts against common biliary pathogens. Effects of hydrophobicity of the molecule and in the presence of phospholipids.Dig Dis Sci 38: 2104-2112.
- Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J (1998) Role of impaired gastric accommodation to a meal in functional dyspepsia.Gastroenterology 115: 1346-1352.
- Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, et al.(2008) Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcoholism, clinical and experimental research 32: 355-364.
- Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, et al.(2009) Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcoholism, clinical and experimental research 33:1220-1230.
- Thuluvath PJ, Triger DR (1989) Autonomic neuropathy and chronic liver disease.Q J Med 72: 737-747.
- Toh Y, Korenaga D, Maekawa S, Matsumata T, Muto Y, et al. (1997) Assessing the permeability of the gastrointestinal mucosa after oral administration of phenolsulfonphthalein.Hepatogastroenterology 44: 1147-1151.
- Tsai SC, Kao CH, Huang CK, Wang SJ, Chen GH (1996) Abnormal gastric emptying in patients with liver cirrhosis.Kaohsiung J Med Sci 12: 285-289.
- Usami A, Mizukami Y, Onji M (1998) Abnormal gastric motility in liver cirrhosis: roles of secretin. Dig Dis Sci 43:2392-2397.
- Valentini L, Schuetz T, Omar A, Gläser S, Kasim E, et al. (2011) Abnormal plasma peptide YY(3-36) levels in patients with liver cirrhosis.Nutrition 27: 880-884.
- Vantrappen G, Janssens J, Hellemans J, Ghoos Y (1977) The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. The Journal of clinical investigation 59:1158-1166.
- Verne GN, Soldevia-Pico C, Robinson ME, Spicer KM, Reuben A (2004) Autonomic dysfunction and gastroparesis in cirrhosis.J Clin Gastroenterol 38: 72-76.
- Vlahcevic ZR, Buhac I, Farrar JT, Bell CC Jr, Swell L(1971) Bile acid metabolism in patients with cirrhosis. I. Kinetic aspects of cholic acid metabolism.Gastroenterology 60: 491-498.
- Wiest R, Lawson M, Geuking M (2014) Pathological bacterial translocation in liver cirrhosis.J Hepatol 60: 197-209.
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, et al. (2001) Ghrelin enhances appetite and increases food intake in humans.J Clin Endocrinol Metab 86: 5992.
- Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, et al. (2004) Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci 49: 621-626.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 31060
- [From(publication date):
February-2015 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 26291
- PDF downloads : 4769