Research Article Open Access
Gastrointestinal Adverse Effects due to Use of Non-Steroidal Anti- Inflammatory Drugs (NSAIDs) in Non-Traumatic Painful Musculoskeletal Disorders
Sunil Nikose1,*, Mridul Arora1, Pradeep Singh1, Devashree Nikose2, Swapnil V Gadge1 and Sohael Khan1
1Department of Orthopedic Surgery, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), Maharashtra, India
2NKP Salve Institute of Medical Sciences, Nagpur, Maharashtra, India
- *Corresponding Author:
- Dr. Sunil Nikose
Department of Orthopaedics
DMIMS, Sawangi (M)
Wardha-442001, Maharashtra, India
Tel: 917152287701
E-mail: sunilnikose@gmail.com
Received date: August 24, 2015 Accepted date: October 07, 2015, Published date: October 20, 2015
Citation: Nikose S, Arora M, Singh P, Nikose D, Gadge SV, et al. (2015) Gastrointestinal Adverse Effects due to Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Non-Traumatic Painful Musculoskeletal Disorders. J Gastrointest Dig Syst 5:348. doi: 10.4172/2161-069X.1000348
Copyright: © 2015 Nikose S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: The present study aims at investigation of the gastrointestinal adverse drug reactions (ADRs) with use of NSAIDs in patients of Non-Traumatic musculoskeletal painful disorders. Our study is unique in terms that it aims at investigations and evaluations of the gastrointestinal adverse drug reactions (ADRs) with use of NSAIDs in patients of non-traumatic musculoskeletal painful disorders, where most of the concurrent and concomitant risk factors like hepatobiliary system, renal system are mostly excluded, along with a dedicated follow up of large population included in our study. Non-Steroidal anti-inflammatory drugs (NSAIDs) such as Diclofenac sodium, Ibuprofen and others are effective for non-traumatic musculoskeletal painful disorders ranging from acute to chronic conditions. NSAIDs are probably most commonly used medications by medical fraternity worldwide for relief from acute, recurring or chronic pain conditions and are easily available in India in many forms as over-the-counter analgesic. Prolonged unsupervised use of these medications leads to disturbance in achieving pain relief and risk of gastrointestinal symptoms ranging from dyspepsia to acute and chronic gastrointestinal ulcer. The most frequent ADRs due to NSAIDs use was gastrointestinal effects leading to discontinuation of treatment. The other ADRs for which NSAIDs are blamed widely are alteration of renal or cardiac parameters in a small subset of patients. Renal and cardiac side effects are well documented and evaluated by clinicians prior to administration of NSAIDs, but the gastrointestinal adverse reactions are overlooked by medical fraternity while
Keywords
Non-steroidal anti-inflammatory drugs (NSAIDS); Gastrointestinal adverse drug reactions (ADRs); Non traumatic musculoskeletal painful disorders; Proton pump inhibitors (PPIs)
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) including selective Cyclooxygenase 2 (COX 2) inhibitors, have anti-inflammatory and analgesic and antipyretic properties. NSAIDs are more commonly used for the acute, recurring or chronic non traumatic musculoskeletal condition such as backache, arthritis, myalgia etc. [1,2]. NSAIDs are most frequently used drug by an orthopedic surgeon and are easily available as over-the-counter-analgesic in almost all over Indian subcontinent. Prolonged and/or unsupervised use leads to risk of adverse reactions pertaining to gastrointestinal system and the symptoms range from dyspepsia to catastrophic acute and chronic gastrointestinal ulcer. The most frequent ADRs due to NSAIDs use was gastrointestinal effects leading to discontinuation of treatment. The other reported ADRs are cardiac, psychological, dermatological and renal in a small subset of patients [3]. Renal and cardiac side effects are well documented and evaluated by clinicians prior to administration of NSAIDs, but the gastrointestinal adverse reactions are overlooked by medical fraternity while prescribing the NSAIDs, thinking that Proton Pump Inhibitors (PPIs) will take care of these gastrointestinal ADRs.
Mechanism of action
All NSAIDs inhibit COX, an enzyme that converts arachidonic acid to prostaglandins, thereby mediating pain, inflammation, and fever. In the process, prostaglandin H2 is converted to five primary prostaglandins, including thromboxane A2 (which stimulates platelet aggregation and blood clot formation) in platelets and prostacyclin (a vasodilator that inhibits platelet aggregation) in the endothelium [4]. Two COX isoenzymes (COX-1 and COX-2) are commonly recognized. In general, COX-1 is constitutively expressed and is involved in gastro protection from stomach acid and in thromboxane formation by platelets. COX-2 is inducible by inflammatory mediators in a wide range of tissues and has been associated with inflammation; however, it may also be constitutively expressed, where it contributes to renal physiology, reproductive function, bone resorption, and neurotransmission [4].
NSAIDs, though are beneficial are also associated with a number of side effects. The two main adverse reactions, associated with NSAIDs relate to gastrointestinal effects and renal adverse effects. These effects are mainly dose-dependent, depends on duration of drug administered, and in many cases severe enough to pose the risk of ulceration and perforation leading to severe upper gastrointestinal bleeding, and occasionally death, limiting the use of NSAID therapy. The main drawback of NSAIDs is that they can cause ulcers and other problems in the esophagus, stomach, or small intestine. Common gastrointestinal side effects include - nausea, vomiting, dyspepsia, peptic ulcers, perforations of the upper gastrointestinal tract, and gastrointestinal bleeding [3,5].
Pathogenesis of NSAID-induced intestinal toxicity
NSAID-induced damage to the gastrointestinal tract results from three sources of exposure: (1) The local effects before the actual absorption of the medications and due to direct exposure to epithelium after oral administration, (2) systemic effects of drug after absorption, and (3) Recurrent effects on epithelium of gastrointestinal tract due to enterohepatic recirculation of the medications. The relative damage inflicted from each type of access remains largely unknown [6].
Treatment of NSAID-induced enteropathy
NSAIDs results in development of gastrointestinal side-effects. Presently various modalities have been employed to reduce or prevent the occurrence of ADRs and subsequently this damage to gastrointestinal system. The most effective means of reducing GI toxicity has been to withdraw the offending NSAIDs. This approach is sometimes not feasible and suitable for patients dependent on NSAIDs for relief from pain responding only to NSAIDs. Other approach is the concomitant use of gastro protective substances to circumvent epithelial damage. Review of literature suggests that Sucralfate is a cytoprotective agent and provides gastro protective effect in upper GI tract and in Gastro Intestinal Reflux Disorder (GERD) as well as in stress ulcer. Sucralfate is chemically a combination of disaccharide sugar sucrose, sulphate and aluminum and binds to mucosa which effectively creates a barrier. This barrier impairs diffusion of hydrochloric acid in the gastrointestinal tract, this prevention of degradation by acid [7]. Misoprostol, a prostaglandin analogue is used to treat NSAIDs induced gastric ulcers and can be concomitantly used in patients who are at high risk of development of NSAIDs induced ulcers. It acts on parietal cells and inhibits gastric acid secretion [8,9]. Non-Steroidal Anti-Inflammatory Drugs (NSAID’s) increase risk of dyspepsia and peptic ulcers, including life threatening Gastrointestinal (GI) bleeding. This increased risk is reduced (but not eliminated) by prescription of Proton Pump Inhibitor medications (PPI’s). Proton Pump Inhibitors (PPIs) are credited with potent reduction of gastric acid production during long term and most widely used in gastrointestinal ADRs either in preventive mode or therapeutic mode. However, the effectiveness has not been demonstrated in every case [10]. The H2 receptor antagonists (H2 RA) blocks the action of histamine on parietal cells of stomach and reduces acid production in stomach. Presently there use have been surpassed by more effective and potent PPIs.
Methods and Materials
We prospectively reviewed and evaluated 2897 patients on NSAID and who were treated in the orthopedic facility for non-traumatic musculoskeletal disorders between 2007 and 2012. Demographic profile of all the patient were recorded that included age, gender, addictions, and other co morbid medical disorders. Type of NSAIDs, indications and duration of treatment were noted. Adverse drug reactions (ADRs) related to gastrointestinal effects were recorded and categorized in three grades according to symptomatology and its treatment. We have included all the patients with age 20 and above.
The patient who had previous gastrointestinal adverse conditions, abnormal kidney and liver function tests were excluded from our study. The points noted were the NSAIDs drug use, age and sex of patients, duration and dosage of medications, use of concurrent medications, alcohol, smoking and/or tobacco use, need for admission due to gastrointestinal ADRs for intervention or management. All the patients received gastro protective Proton Pump Inhibitors (PPIs) in appropriate dosage along with prescription of NSAIDs. Those patients who did not receive PPIs were not included in our study. GI Adverse events for NSAIDs were classified into 3 grades. Grade I ADRs included Dyspepsia, Nausea, Gastric irritation, GERD, Epigastric discomfort VAS <6. Grade II ADRs included Vomiting, Epigastric pain VAS >6, diarrhoea, Colitis. Grade III ADRs included acute or chronic gastrointestinal bleed, Melena, Hematemesis, Gastric ulcer and perforation.
Results
Total 2897 patients were analyzed in the study. The male preponderance 1477 (50.98%) were noted (Tables 1 and 2). All the patients were divided into various age groups and maximum patients were in 40-50 years age group i.e. 768 and the lowest were in more than 70 years age group i.e. 151 (Table 1). The patients were given different types of oral NSAIDs according to the duration and intensity of pain and choice given to patients after counseling regarding the efficacy and probable adverse effects (Tab. Diclofenac, Tab. Ibuprofen, Tab. Aceclofenac, Tab. Etoricoxib, Tab. Indomethacin and Tab. Nimesulide) and out of this most patients were given Tab. Diclofenac sodium i.e. 719 and the least no. of patients got oral Indomethacin (Table 3).
| Age distribution (years) | 20-30 | 30-40 | 40-50 | 50-60 | 60-70 | >70 | Total |
|---|---|---|---|---|---|---|---|
| No. of Patients | 226 (7.80%) | 424 (14.63%) | 768 (26.51%) | 682 (23.54%) | 646 (22.29%) | 151 (5.21%) | 2897 (100%) |
Table 1: Age wise distribution of patients.
| Gender | No of patients |
|---|---|
| Male | 1477 (50.98%) |
| Female | 1420 (49.02%) |
| Total | 2897 (100%) |
Table 2: Gender wise distribution.
| Type of NSAID | No. of patients (%) |
|---|---|
| Diclofenac Sodium | 1152 (39.76%) |
| Ibuprofen | 1117 (38.55%) |
| Aceclofenac Sodium | 364 (12.56%) |
| Etoricoxib | 149 (5.14%) |
| Indomethacin | 47 (1.62%) |
| Nimesulide | 68 (2.34%) |
| Total | 2897 (100%) |
Table 3: Types of NSAIDs used in our study.
Adverse effects for NSAIDs were classified into 3 grades. Grade I ADRs included Dyspepsia, Nausea, Gastric irritation, GERD, Epigastric discomfort VAS <6. Grade II ADRs included Vomiting, Epigastric pain VAS >6, diarrhea and colitis. Grade III ADRs included gastrointestinal bleed, malena, hematemesis, gastric ulcer and perforation. 987 (34.06%) patients had Grade I adverse gastrointestinal effects, which were relieved either by discontinuation of NSAIDs or concomitant use of Sucralfate in addition to PPIs already prescribed. 348 (22.05%) patients had Grade II gastrointestinal adverse effects and this was relieved by further addition of medications like Misoprostol in addition to the treatment already administered for Grade I ADRs. 277 (9.56%) patients had Grade III adverse effects that required medications, admission and Gastro-duodenoscopy and surgical intervention (Tables 4, 5 and Figure 1).
| GI Side Effect | No. of patients (%) |
|---|---|
| None | 1285 (44.35%) |
| Grade I Adverse Effects | 987 (34.06%) |
| Grade II Adverse Effects | 348 (12.01%) |
| Grade III Adverse Effects | 277 (9.5%) |
| Total | 2897 (100%) |
Table 4: Gastrointestinal side effects.
| Type of NSAID* GI Side Effect | ||||||
|---|---|---|---|---|---|---|
| Count | ||||||
| GI Side Effect | Total (%) | |||||
| No GI effect | Grade I Side Effect | Grade II Side Effect | Grade III Side Effect | |||
| Type of NSAID | Diclofenac (100%) | 454 (39.40%) | 432 (37.5%) | 177 (15.36%) | 89 (7.72%) | 1152 (39.76%) |
| Ibuprofen (100%) | 473 (42.34%) | 365 (32.67%) | 131 (11.72%) | 148 (13.24%) | 1117 (38.55%) | |
| Aceclofenac (100%) | 243 (66.75% | 74 (20.32%) | 14 (3.84%) | 33 (9.06%) | 364 (12.56)% | |
| Etoricoxib (100%) | 57 (18.12%) | 76 (51.0%) | 15 (10.06%) | 1 (0.67%) | 149 (5.14%) | |
| Indomethacin (100%) | 15 (31.91%) | 23 (48.93%) | 6 (12.76%) | 3 (6.38%) | 47 (1.62%) | |
| Nimesulide (100%) | 43 (63.23%) | 17 (25%) | 5 (7.35%) | 3 (4.41%) | 68 (2.34%) | |
| Total (100%) | 1285 (44.35%) | 987 (34.06%) | 348 (12.01%) | 277 (9.56%) | 2897 (100%) | |
Table 5: Co-relation of gender with GI side effects.
The gastrointestinal adverse effects were more in females and this was statistically significant (p<05) by chi square test (Table 6). Some patients had addictions like alcoholism, smoking and tobacco chewing, of which there were 435 smokers with male preponderance (Males-319 (11.01%), Females-116 (4.00%)) and this was statistically significant (Table 7). There was also male preponderance in tobacco chewing (Male: 319 (11.01%), Female: 159 (5.48%)) and this was also statistically significant by chi square test (Table 7). 319 (11.01%) males had alcohol addiction and 87 (3.00%) of the female population had alcohol addiction and this was also found to be statistically significant i.e. p<0.5 (Table 7). NSAIDs were given to the patients till the pain was relieved or till report of any adverse reactions requiring therapeutic intervention.
| GI Side Effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Grade I Side Effect | Grade II Side Effect | Grade III Side Effect | Total | ||||||
| Gender | Female | 469 (16.18%) | 602 (20.78%) | 232 (8.00%) | 117 (4.03%) | 1420 (49.02%) | ||||
| Male | 816 (28.16%) | 385 (13.28%) | 116 (4.01%) | 160 (5.52%) | 1477 (50.98%) | |||||
| Total | 1285 (44.35%) | 987 (34.06%) | 348 (12.01%) | 277 (9.56%) | 2897 (100%) | |||||
| Chi-Square Tests | ||||||||||
| Value | Df | Asymp. Sig. (2-sided) 0.004 |
||||||||
| Pearson Chi-Square | 13.468a | 3 | ||||||||
Table 6: Co-relation of gender with gastrointestinal side effects.
| Non Smoker | Smoker | Non Tobacco Chewer | Tobacco Chewer | No Alcohol | Alcohol | ||
|---|---|---|---|---|---|---|---|
| Gender | Female (100%) | 1304 (91.83%) | 116 (8.16%) | 1261 (88.80%) | 159 (11.19%) | 1333 (93.87%) | 87 (6.12%) |
| Male (100%) | 1158 (78.40%) | 319 (21.59%) | 1158 (78.40%) | 319 (21.59%) | 1158 (78.40) | 319 (21.59%) | |
| Total (100%) | 2462 (84.98%) | 435 (15.01%) | 2419 (83.50%) | 478 (16.49%) | 2491 (85.98%) | 406 (14.02%) | |
| Pearson Chi-Square | Asymp. Sig. (2-sided) 0.008 |
Asymp. Sig. (2-sided) 0.049 |
Asymp. Sig. (2-sided) 0.002 |
||||
Table 7: Co-relation of addictions (Smoking, tobacco chewing and Alcohol).
| Clinical Features | Treatment | |
|---|---|---|
| Grade I | Dyspepsia, Nausea, Gastric irritation, GERD, Epigastric discomfort VAS <6 | Withdrawal of offending NSAIDs + Sucralfate |
| Grade II | Vomiting, Epigastric pain VAS >6, diarrhea, Colitis | Required added medication (Misoprostol) to treat Gastro intestinal adverse effect |
| Grade III | Gastrointestinal bleeding, Melena, Hematemesis, Gastric ulcer and perforation | Required admission and Intervention in form of Gastroduodenoscopy, Surgical intervention |
Table 8: Grading and treatment of gastro-intestinal adverse effects.
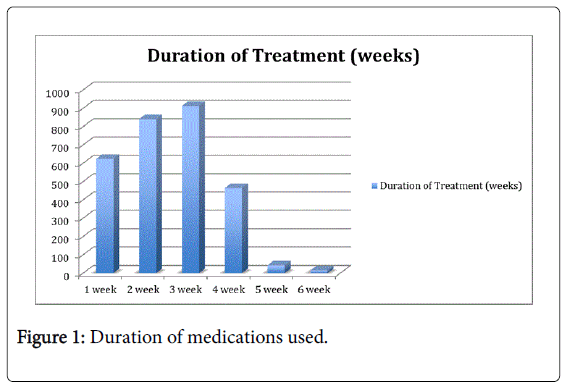
The maximum duration of the NSAIDs administered was 6 weeks and least being 1 week. In our study 912 (31.48%) patients were administered NSAIDs for 3 weeks being the maximum group of patients and the least group of patients was 1 weeks i.e. 15 (0.51%) patients.
The patients who were addicted to alcohol and those reported to be having gastrointestinal ADRs were de-addicted from alcohol is a significant development and contribution of this study.
Those de-addicted were the patients of chronic pain nature, in which sufficient time was available for counselling regarding the ill effects of alcohol also patients were counselled regarding adverse reactions due to concurrent use of alcohol along with NSAIDs. The smoking/tobacco de-addiction was also attempted, but ours being rural region of central India it was continued on another platform.
Discussion
There are tw o isoforms of COX, COX-1 and COX-2, which have different functions [11]. COX-1 is constitutively expressed and is responsible for the normal physiological protection of gastric mucosa. It is responsible for the synthesis of prostaglandins, which protects the stomach lining from the secreted acid, maintains blood flow in gastric mucosa, and produces bicarbonate [11,12]. We used non selective COX inhibitor (Diclofenac, Aceclofenac, Ibuprofen, and Indomethacin), selective COX-2 inhibitor (Etoricoxib) and preferential cox inhibitor (Nimesulide). We observed in our study that non selective COX inhibitors are most commonly associated with gastric side effects and our percentage of gastrointestinal side effects were higher than reported in the literature.
In a randomized controlled trial small-bowel injury was seen in 71% of NSAID users compared with 10% of controls (P<0.001). However, injury was mild (few or no erosions, absence of large erosions/ulcers) in 10% NSAID users compared with 2 controls. 12% NSAID users had major (>4 erosions or large ulcers/ulcers) damage compared with none in the control group. There were no complications or problems with the capsule endoscopy procedure [12]. We had 44.35% of patients who did not have any GI related adverse effects. 34.06% had Grade I GI effects (Table 4). In our study female gender were more prone to have gastric side effect than male despite being less addicted to tobacco, smoking or alcohol (Tables 6-8). However, grade III GI problem were more commonly and frequently observed in male patients in our study. This could be due to the fact that Indian men are more addicted to tobacco, smoking and alcohol. However, the combined correlation between smoking, alcohol addiction, tobacco could not be established in our study.
Adverse events related to gastrointestinal system for NSAIDs use has been classified into 3 categories: 1) “nuisance” symptoms, such as heartburn, nausea, dyspepsia, and abdominal pain; 2) mucosal lesions; and 3) serious GI complications, such as bleeding and perforation [13]. We divided our patients based on clinical history criteria and the treatment of those side effects. Classification of these patients in grading helped us in managing the side effects in a protocoled manner. However 1.3% of our patients required adjuvant treatment for GI side effects, which is statistically not significant.
In comparisons of GI adverse effects among NSAIDs users, results from several studies support a risk of GI adverse effects with nonselective COX inhibitors [14-17]. In a meta-analysis of data from three retrospective case-control studies found that ibuprofen had the lowest odds ratio (OR) for development of GI bleeding versus diclofenac, naproxen, piroxicam, and indomethacin, but that the OR increases with dose level for each agent [17]. The severity of side effects was closely related with strength of dose of medication. The length of treatment also contributes to the GI adverse risk. However, gender, tobacco, smoking and alcohol has confounding impact on the severity of GI side effects.
Conclusion
NSAIDs such as ibuprofen, Diclofenac sodium are considered to be generally safe and effective for individuals seeking a treatment of symptoms of pain for relief. The pharmacologic basis of these interactions strongly suggests that low therapeutic dose, short-term use of these NSAIDs such as non-selective COX inhibitors should be used to treat the painful conditions. More caution is warranted in elderly patients and in patients with alcohol, tobacco or smoking history. Since female genders seems to be more prone to develop milder GI adverse effects, the treatment in this subset of patients should be kept at minimum dose for minimum possible days. Physicians and health care providers should be more instrumental in educating patients regarding using NSAIDs at the lowest effective dose for the shortest required duration which becomes a vital tool for balancing the efficacy and safety of the drug to be used. Also concurrent use of alcohol, tobacco, and smoking should be cautioned against as more prone to develop GI adverse effects. Among the elderly population, an individualized approach would help in pain management and avoidance of GI adverse effects. This strategy has also been recommended by the World Health Organization (WHO) in order to minimize drug-drug interactions and adverse drug reactions due to NSAIDs.
References
- (2009) Naprelan® (naproxen sodium) [package insert] San Diego, CA: Victory Pharma, Inc.
- (2007) Orudis® (ketoprofen) [package insert] Philadelphia, PA: Wyeth Pharmaceuticals Inc.
- Harirforoosh S, Asghar W, Jamali F (2013) Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci 16: 821-847.
- Rao P, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11: 81s-110s.
- Al-Saeed A1 (2011) Gastrointestinal and Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs. Oman Med J 26: 385-391.
- Neal M. Davies, Joseph Y, Skjodt SNM (2000) Detection and Prevention of NSAID-Induced Enteropathy. J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3: 137-155.
- Aabakken L, Larsen S, Osnes M (1989) Sucralfate for prevention of naproxen-induced mucosal lesions in the proximal and distal gastrointestinal tract. Scand J Rheumatol 18: 361-368.
- Bjarnason I, Smethurst P, Fenn CG, Lee CE, Menzies IS, et al. (1989) Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci 34: 407-411.
- Bjarnason I (1990) Experimental evidence of the benefit of misoprostol beyond the stomach in humans. J Rheumatol Suppl 20: 38-41.
- Guidelines in primary care.
- Gudis K, Sakamoto C (2005) The role of cyclooxygenase in gastric mucosal protection. Dig Dis Sci 50 Suppl 1: S16-23.
- Konturek SJ, Konturek PC, Pawlik T, Sliwowski Z, Ochmaski W, et al. (2004) Duodenal mucosal protection by bicarbonate secretion and its mechanisms. J Physiol Pharmacol 55 Suppl 2: 5-17.
- Bjorkman DJ (1996) Nonsteroidal anti-inflammatory drug-induced gastrointestinal injury. Am J Med 101: 25S-32S.
- Henry D, Lim LL, Garcia Rodriguez LA, Perez Gutthann S, Carson JL, et al. (1996) Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 312: 1563-1566.
- Henry D, McGettigan P (2003) Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. Int J Clin Pract Suppl : 43-49.
- Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, et al. (2002) Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol 54: 320-326.
- Richy F, Bruyere O, Ethgen O, Rabenda V, Bouvenot G, et al. (2004) Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 63: 759-766.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14759
- [From(publication date):
December-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13685
- PDF downloads : 1074