Case Report Open Access
Gastric Duplication Complicated by Malignant Transformation in Adults: Report of Three Cases
Yimiao Zhu1,2, Lihong LV1,3, Wensheng Pan1,2*, Pingping Ren4, Tiemei Han1 and Xiang Xu5
1Department of Gastroenterology, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, China
2Department of Gastroenterology, The Second Affiliated Hospital Binjiang Campus, School of Medicine, Zhejiang University, Hangzhou 310009, China
3Department of Gastroenterology, Xianju People’s Hospital, Zhejiang Province, 325000, China
4Department of Nephrology, The First Affiliated Hospital Binjiang Campus, School of Medicine, Zhejiang University, Hangzhou 310009, China
5Department of Pharmacy, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, China
- *Corresponding Author:
- Wensheng Pan, MD, PhD.
Professor of Gastroenterology, Consultant Gastroenterologist
Department of Gastroenterology, The Second Affiliated Hospital
Zhejiang University School of Medicine, 88# Jiefang Road
Hangzhou 310009, China
Tel: +86-(571)-8778-3527
Fax: +86-(571)-8778-3527
E-mail: wspan223@163.com
Received date: November 17, 2015 Accepted date: December 22, 2015 Published date: December 29, 2015
Citation: Zhu Y, Lihong LV, Pan W, Ren P, Han T, et al. (2015) Gastric Duplication Complicated by Malignant Transformation in Adults: Report of Three Cases . J Gastrointest Dig Syst 5:374. doi:10.4172/2161-069X.1000374
Copyright: © 2015 Zhu Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Gastric duplication anomaly is an uncommon congenital disease, mostly reported in children. Malignant transformation of the duplication lesion is extremely rare, with only 11 cases reported in the English literature. Here we report three cases of early cancer found in gastric duplications or the adjacent gastric wall. Out of these three cases, one is an early cancer arising from the duplication lesion, one from the gastric wall and another form both. To the best of our knowledge, early cancer arising from both the duplication cyst and the adjacent wall is first reported. Once detected gastric duplication, surgical resection is recommended because of its potential for malignant transformation.
Keywords
Gastric duplication; Malignant transformation; Adenocarcinoma; Early cancer; Digestive system abnormalities
Introduction
Gastrointestinal duplications are rare congenital malformations, which may occur anywhere in the digestive tract and are common in the small intestine, while the gastric involvement is relatively rare. These duplication lesions usually attach to the mesenteric border of the gastrointestinal tract, with well-developed coat of smooth muscle outside and epithelial lining inside [1,2]. Malignant transformation of duplication lesion in adults is extremely rare, with only 11 cases having been reported so far in the English literature [3-21]. We encountered three cases of gastric duplication from 2005 to 2006 of which two had early cancer arising from the gastric duplication and the third showed early cancer in the adjacent gastric wall.
Case Report
Case 1
A 62-year-old Chinese man was admitted to the hospital because of intermittent epigastric pain. The patient’s past medical history and family history were non-contributory. All the laboratory tests results, including tumor marker examination were within normal limits. An upper gastrointestinal endoscopy showed a hemisphere protruding lesion size about 4.0 × 3.5 cm located in the greater curvature of the stomach, with smooth mucosa surface (Figure 1). The biopsy showed chronic inflammation in the mucosae. Computed tomography revealed a 3.8 × 3.2 cm cystic lesion at greater curvature of the lower corpus, without signs of metastasis (Figure 1). Ultrasonography showed a well-defined mass in a heterogeneous low-echoic pattern arose from the submucosa of the gastric wall. Adenocarcinoma in duplication was found according to the intraoperative frozen section examination, thus a total gastrectomy was performed. The patient had an uneventful recovery.
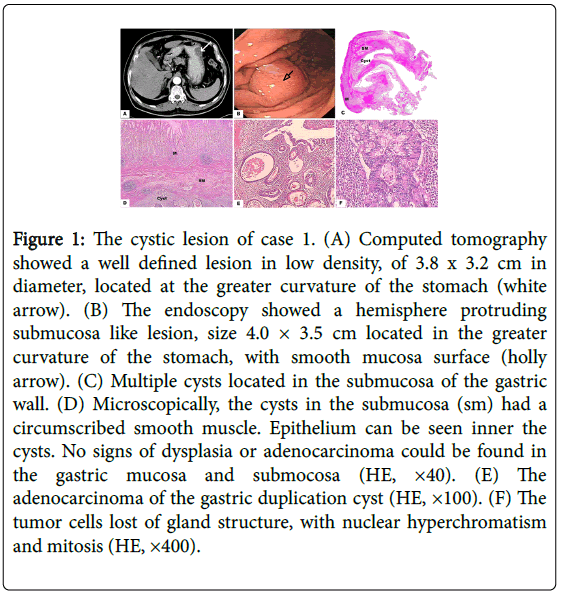
Figure 1: The cystic lesion of case 1. (A) Computed tomography showed a well defined lesion in low density, of 3.8 x 3.2 cm in diameter, located at the greater curvature of the stomach (white arrow). (B) The endoscopy showed a hemisphere protruding submucosa like lesion, size 4.0 × 3.5 cm located in the greater curvature of the stomach, with smooth mucosa surface (holly arrow). (C) Multiple cysts located in the submucosa of the gastric wall. (D) Microscopically, the cysts in the submucosa (sm) had a circumscribed smooth muscle. Epithelium can be seen inner the cysts. No signs of dysplasia or adenocarcinoma could be found in the gastric mucosa and submocosa (HE, ×40). (E) The adenocarcinoma of the gastric duplication cyst (HE, ×100). (F) The tumor cells lost of gland structure, with nuclear hyperchromatism and mitosis (HE, ×400).
The macroscopic finding showed a multilocular lesion of size 4.0 × 3.5 cm, with mucus in it, attached to the greater curvature of the lower part of the gastric body. There was no communication between the cystic lesion and gastric lumen.
Microscopically, multiple cysts in the submucosa had a well circumscribed smooth muscle layer that, shared with the stomach. The gastric glands inner lining were comprised of mitoses and hyperchromatism cells in an irregular structure, which indicated a well differentiated adenocarcinoma in the cystic lesion. The adenocarcinoma was localized in the submucosa of the cyst wall without muscularis mucosae invaded. Inflammation and helicobacter pylori were detected. No signs of dysplasia or adenocarcinoma change were found in the adjacent gastric wall (Figure 1).
Case 2
A 43-year-old Chinese man was admitted to the hospital because of intermittent abdominal pain and melena for 6 months. The patient’s past medical history and family history were not remarkable. The fecal occult blood was positive and the other laboratory tests were within normal limits. The upper gastrointestinal endoscopy showed a 3.0 × 3.0 cm protruding lesion from the angular incisure to the antrum with erosive mucosa and stenosis (Figure 2). The biopsy showed chronic inflammation in the mucosae and moderate to high grade dysplasia of the epithelium with Hp detected. Computed tomography revealed a 3.0 × 2.8 cm multilocular cystic lesion at the greater curvature of the gastric antrum without lymphadenopathy in the abdomen (Figure 2). Ultrasonography showed focally thickened mucosa and submucosa in the gastric antrum. No adenocarcinoma in duplication was found according to the intraoperative frozen section examination. The surgeon performed a laparotomy and dissection the cyst. And the patient recovered uneventfully.
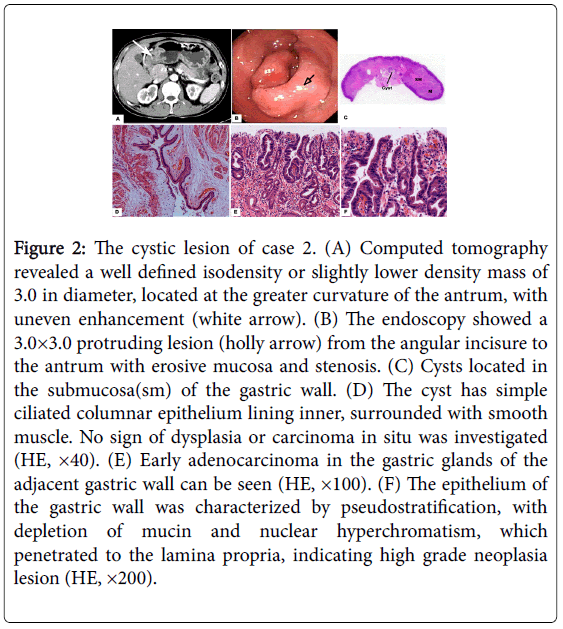
Figure 2: The cystic lesion of case 2. (A) Computed tomography revealed a well defined isodensity or slightly lower density mass of 3.0 in diameter, located at the greater curvature of the antrum, with uneven enhancement (white arrow). (B) The endoscopy showed a 3.0×3.0 protruding lesion (holly arrow) from the angular incisure to the antrum with erosive mucosa and stenosis. (C) Cysts located in the submucosa(sm) of the gastric wall. (D) The cyst has simple ciliated columnar epithelium lining inner, surrounded with smooth muscle. No sign of dysplasia or carcinoma in situ was investigated (HE, ×40). (E) Early adenocarcinoma in the gastric glands of the adjacent gastric wall can be seen (HE, ×100). (F) The epithelium of the gastric wall was characterized by pseudostratification, with depletion of mucin and nuclear hyperchromatism, which penetrated to the lamina propria, indicating high grade neoplasia lesion (HE, ×200).
At surgery, macroscopically, focally thickened gastric wall with multiple granular lesion on the surface were found in the antrum, 3.0 × 3.0 cm in dimension. Extensive smooth granular nodules can be seen inside.
Microscopically, multiple lesions with cystic structure located in the submucosa of the gastric wall, circumscribed with smooth muscle. In the cysts, mucus and Simple ciliated columnar epithelium can be detected without any dysplasia in the mucosae. In the adjacent gastric wall, high grade dysplasia originated from the epithelium, with penetrating to the lamina propria, and without muscularis mucosae invaded, indicated an early adenocarcinoma. Inflammation and helicobacter pylori were detected (Figure 2).
Case 3
A 72-year-old Chinese man was admitted to the hospital due to abdominal distention and intermittent regurgitation. The patient’s past medical and family history were non-contributory. Laboratory tests results were within normal limits. An upper gastrointestinal endoscopy revealed a 2.0×1.5 cm protruding lesion with ulceration located in the posterior wall of antrum (Figure 3). Biopsy specimens confirmed this lesion a moderate differentiated adenocarcinoma.
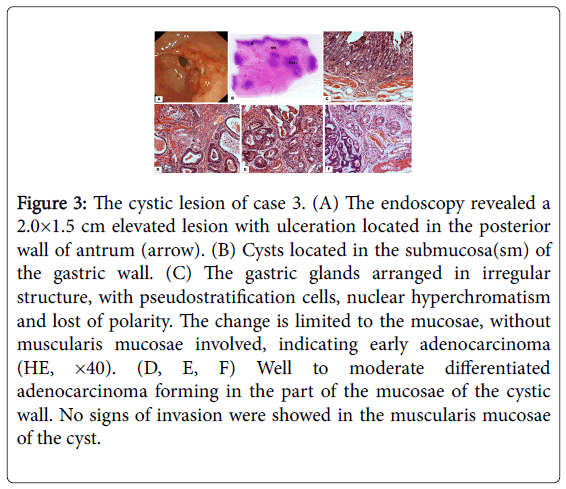
Figure 3: The cystic lesion of case 3. (A) The endoscopy revealed a 2.0×1.5 cm elevated lesion with ulceration located in the posterior wall of antrum (arrow). (B) Cysts located in the submucosa(sm) of the gastric wall. (C) The gastric glands arranged in irregular structure, with pseudostratification cells, nuclear hyperchromatism and lost of polarity. The change is limited to the mucosae, without muscularis mucosae involved, indicating early adenocarcinoma (HE, ×40). (D, E, F) Well to moderate differentiated adenocarcinoma forming in the part of the mucosae of the cystic wall. No signs of invasion were showed in the muscularis mucosae of the cyst.
A radical resection of gastric cancer was performed. The patient had a full postoperative recovery.
Gross feature of the specimen showed a 2.0 × 2.0 cm elevated lesion with ulceration in the posterior wall of antrum without serosa invaded.
Microscopically, the well differentiated adenocarcinoma invaded to the lamina propria of the gastric wall without muscularis mucosae involved. In the submucosa, multiple cystic lesions can be detected with well to moderate differentiated adenocarcinoma forming in the mucosae of the cystic wall. No signs of invasion were shown in the muscularis mucosae (Figure 3).
Discussion
Duplication of the alimentary tract is an infrequent congenital abnormality which is most common in children or infants, and occurs rarely in adults [1]. The etiology of duplication is controversial, but has been hypothesized to be due to split notochord etiology, abnormal recanalization during the bowel development and remaining diverticula [2]. That might be the reason that one third duplication cases are associated with other anomalies: esophageal atresia, diverticula, respiratory system abnormalities and vertebral abnormalities. The possible etiology could also explain the different mucosae lining the cysts: intestinal mucosa, gastric mucosa, pancreatic tissue and pseudostratified ciliated columnar epithelium. Gastric duplication accounts for 7%~8% of all gastrointestinal duplications [3] and is commonly located along the greater curvature or posterior wall of the stomach with tubular structures communicated with the stomach or with cystic ones which do not communicate with the stomach [4]. The common pathologic characteristics that are sued as criteria for the diagnosis of gastric duplication include: lesion is coated by smooth muscle, continued with the stomach, and inner lined with mucosae, which can be epithelium of any portion of the gastrointestinal tract. In addition, the lesion should be attached to the gastric wall [2]. In the present series of three cases, all these pathologic characteristics satisfied the criteria to confirm the diagnosis of gastric duplication.
Malignant transformation is a rare complication of gastric duplication. The three cases presented in this paper have three different scenarios: the first case presented, a well to moderate differentiated adenocarcinoma arose from the cystic lesion, limited to the submocosa of the lesion without the invasion of the gastric wall. The gastric wall remained normal. In the second case, high neoplasia involved with the epithelium of the adjacent gastric wall was noted while neither carcinoma in situ nor precancerous lesion was found in the cyst. The third case revealed the presence of early cancer arising from both the duplication cyst and the adjacent gastric wall. No evidence of invading adenocarcinoma was detected in the muscularis mucosae or the smooth muscle around the cystic lesions indicating that the two adenocarcinomas developed independent of each other. To the best of our knowledge, only 13 instances of malignant tumors arising from gastric duplications in adults have been reported since 1955, case 1 and case 3 in this paper included in Table 1 [5-14]. However, no predictor of the malignant change has been found, including the symptoms, size, location, tumor markers or macroscopical founding. The mechanism of malignant transformation is not clear.
| Author | Age/Sex | Symptoms | Wrong Diagnosis | Gastric Duplication | Malignant Transformations | Follow Up | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size (cm) | Location | Mucosa | Macroscopical | Microscopic | Invasion | |||||
| Mayo [6] | 64/ F | Weakness, weight loss | Gastric carcinoma | 6.0 | Antrum | Gastric mucosa | Polyploid | Well differentiated adenocarcinoma | the gastric muscular wall | DFS at 12 months |
| Trieger [7] | 50/ M | Vomitting, weight loss | unknown | 17.0 | unknown | unknown | ulcerative | Infiltration epithelial carcinoma | the gastric muscular wall | unknown |
| Coit [8] | 72/F | Abdominal pain, weight loss | Gastric carcinoma | 4.0 | antrum | Intestinal mucosa, gastric mucosa and pancreatic tissue | granular | Mucinous papillary adenocarcinoma | Submucosa of the stomach, peritonial nodules | DFS at 72 months |
| Ishikawa [3] | 56/M | Vomitting, weight loss | Pancreatic Cyst | 10.0 | Between the fundus and the pancreatic tail | Cilliated columnar epitheliumand pyloric glands | Supperficial depressed | Well differentiated adenocarcinoma | Mucosa of the cyst | DFS at 28 months |
| Mamiya [12] | 71/F | Abdominal pain, poor appetite | unknown | 8.0 | unknown | unknown | Supperficial elevated | papillary adenocarcinoma | wall of the cyst | DFS at 1 month |
| Kuroaka [3] | 40/M | Fewer, back pain | Splenic cyst | 7.0 | Anterior wall of fundus | Pseudostratified ciliated columnar epithelium | granular | Well differentiated adenocarcinoma | The whole gastric wall | Liver metastasis at 7 months |
| Horne [4] | 40/M | Acute abdominal pain, poor appetite | GIST | 12.0 | Posterior wall of fundus | Pseudostratified ciliated columnar epithelium | protruded | Well differentiated neuroendocrine carcinoma | wall of the cyst | Peritonial metastasis at 14 months |
| Barussaud [5] | 67/F | Abdominal pain, weight loss | Preatic carcinoma | 18.0 | antrum | Mixed adenocarcinoma and squamous cell carcinoma | unknown | Mixed adenocarcinoma and squamous cell carcinoma | Gastric wall and peritoneal nodules | Liver metastasis at 6 months |
| Jf. Zheng [13] | 25/M | Asymptomatic (CEA elevation) | adenocarcinoma | 8.0 | Greater curvature of the body | Fundic type gastric mucosa and focally columnal epithelium | protruded | Infiltrative, moderatly differentiated tubular carcinoma | The serosa of the cyst wall and the gastric muscular wall | DFS at 13 months |
| Kang [20] | 56/M | Asymptomatic (check up) | Submucosal tumor | 5.5 | Greater curvature of the body | Gastric foveolar epithelium | Thickened granular | adenocarcinoma | Muscle layer of the cyst | unknown |
| Lewitowicz [21] | 73/M | Epigastric pain and gastrointestinal bleeding | tumor | 4.0 | subcrardial | Gastric foveolar epithelium | Growing arround | Gastro intestinal stromal tumor (lacking mitotic activity) | Around or from gastric duplicatin cyst | unknown |
| Case 1 | 62/M | Chronic abdominal pain | GIST | 4.0 | Greater curvature of the body | Gastric mucosa | granular | Well differentiated neuroendocrine carcinoma | Sub-mucosa of the cyst | DFS till now |
| Case 3 | 72/M | Regurgitation distention | Adenocarcinoma | 2,0 | Posterior wall of antrum | Gastric mucosa | Superficial flat | Well differentiated neuroendocrine carcinoma | Sub-mucosa of the cyst | Not followed |
Table 1: Characteristic of malignant transformation of the gastric duplication cyst.
Based on the observations of the three cases reported here, it can be concluded that gastric adenocarcinoma can arise from the duplication cysts located in the submucosa or the adjacent gastric wall. The mechanisms of malignant transformation in those two layers might be different. Studies demonstrated a clear association between Hp infection and gastric adenocarcinoma [15,16-18]. Thus, Hp infection may play a role in malignant transformation of epithelium of gastric wall with gastric duplication [15,19]. The exits of cysts may draw some factors to the canceration of the adjacent gastric wall, such as cystic pressure and epithelium metabolism of the cysts. There are many possible explanations for the gastric duplication canceration. Gastric duplication has been reported with ectopic gastric or pancreatic mucosa that containing gastric acid and peptic enzymes, which may cause ulceration and perforation [18]. These persistent irritants together with events, such as increase of intracystic pressure and oxygen deficiency in the local microenvironment, may cause chronic inflammation, repeated apoptosis and regeneration of the epithelium that could ultimately lead to the malignant transformation process in the gastric duplication [20,21]. However, such a proposal needs further studies to confirm this assumption.
In conclusion, the gastric duplication is usually a benign lesion, but has the potential to turn malignant from either the duplication itself or the adjacent gastric wall. The mechanism of such malignant transformation process is poorly understood. In the event of presence of any suspicion of malignant transformation process in the gastric duplication, it is recommend surgical resection of the lesion once detected.
Acknowledgments
This research was supported by grants from the National Health Key Special Fund (No. 200802112), the Health Department Fund (No. 2007A093), the Traditional Chinese Medicine Bureau Fund (No. 2007ZA019), and the Natural Science Fund of Zhejiang Province (No. Y2080001, Y12H160121 and Z2080514), and the Key Project of Zhejiang Province (No. 2009C03012-5).
References
- Yang MC, Duh YC, Lai HS, Chen WJ, Chen CC, et al. (1996) Alimentary tract duplications.J Formos Med Assoc 95: 406-409.
- Rowling JT (1959) Some observations on gastric cysts.Br J Surg 46: 441-445.
- Kuraoka K, Nakayama H, Kagawa T, Ichikawa T, Yasui W (2004) Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: a case report with literature review.J Clin Pathol 57: 428-431.
- Horne G, Ming-Lum C, Kirkpatrick AW, Parker RL (2007) High-grade neuroendocrine carcinoma arising in a gastric duplication cyst: a case report with literature review.Int J Surg Pathol 15: 187-191.
- Barussaud ML, Meurette G, Cassagnau E, Dupasc B, Le Borgne J (2008) Mixed adenocarcinoma and squamous cell carcinoma arising in a gastric duplication cyst.Gastroenterol Clin Biol 32: 188-191.
- Mayo HW JR, Mckee EE, Anderson RM (1955) Carcinoma arising in reduplication of the stomach (gastrogenous cyst): a case report.Ann Surg 141: 550-555.
- Treiger M, Rubens J, Chindler J, Lobão M, Keiserman I, et al. (1969) [Stomach duplication. Report of a 2d case in literature complicated by a peptic ulcer and malignant neoplasms].Hospital (Rio J) 75: 1-10.
- Coit DG, Mies C (1992) Adenocarcinoma arising within a gastric duplication cyst.J Surg Oncol 50: 274-277.
- Rice CA, Anderson TM, Sepahdari S (1986) Computed tomography and ultrasonography of carcinoma in duplication cysts.J Comput Assist Tomogr 10: 233-235.
- Horie H, Iwasaki I, Takahashi H (1986) Carcinoid in a gastrointestinal duplication.J Pediatr Surg 21: 902-904.
- Hata H, Hiraoka N, Ojima H, Shimada K, Kosuge T, et al. (2006) Carcinoid tumor arising in a duplication cyst of the duodenum.PatholInt 56: 272-278.
- Mamiya N, Karasawa Y, Kojima N, Takemoto T, Kondoh N, et al. (1996) [A case of gastric duplication cyst containing papillary adenocarcinoma].Nihon ShokakibyoGakkaiZasshi 93: 34-38.
- Kang HJ, Jang SJ, Park YS (2014) Adenocarcinoma arising in gastric duplication cyst.Korean J Pathol 48: 159-161.
- Lewitowicz P, Matykiewicz J, Koziel D, Gluszek SZ, Sosnowski Z, et al. (2015) Gastric gastrointestinal stromal tumor with incomplete duplication cyst - a case with possibility of neoplasia in fetal-period malformed tissues. Polish journal of pathology : official journal of the Polish Society of Pathologists 66: 86-91.
- Zheng J, Jing H (2012) Adenocarcinoma arising from a gastric duplication cyst.Surg Oncol 21: e97-101.
- Huang JQ, Sridhar S, Chen Y, Hunt RH (1998) Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer.Gastroenterology 114: 1169-1179.
- Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ (1999) Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis.Am J Gastroenterol 94: 2373-2379.
- Crowe SE (2005) Helicobacter infection, chronic inflammation, and the development of malignancy.CurrOpinGastroenterol 21: 32-38.
- Petersen AM, Krogfelt KA (2003) Helicobacter pylori: an invading microorganism? A review.FEMS Immunol Med Microbiol 36: 117-126.
- Camoglio FS, Forestieri C, Zanatta C, Capelli P, Pecori S, et al. (2004) Complete pancreatic ectopia in a gastric duplication cyst: a case report and review of the literature.Eur J Pediatr Surg 14: 60-62.
- Iwanaga T, Koyama H, Takahashi Y, Taniguchi H, Wada A (1975) Diffuse submucosal cysts and carcinoma of the stomach.Cancer 36: 606-614.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 11028
- [From(publication date):
December-2015 - Aug 17, 2024] - Breakdown by view type
- HTML page views : 10350
- PDF downloads : 678