Gasification and Syngas Reforming of City Waste Papers for Solid Oxide Fuel Cells
Received: 21-Sep-2017 / Accepted Date: 27-Sep-2017 / Published Date: 04-Oct-2017 DOI: 10.4172/2576-1463.1000171
Abstract
This study has investigated the thermal processes of three types of Municipal Solid Wastes (MSW) in Taipei. Three key properties of the processes are concerned, the formation of tar, the sulfur content, and the reforming of CH4 as the syngas is used for Solid Oxide Fuel Cells (SOFCs). K2CO3 is used as a catalyst to reduce the amount of tar. One of tar production from MSW is reduced from 37.2 wt% to 13.7 wt%. The raw syngas from gasification is then reformed using a Ni-CeO2 catalyst on a γ-Al2O3 support to optimize the quantity of H2 and reduce the CH4 content. H2S generated by waste paper is in a level of 1.83 ppm, and reduced to 0.04 ppm by CaCO3. Syngas with a composition of 51.3 vol% H2, 44.8 vol% CO and 3.4 vol% CH4 is used for the power generation by a selfassembled SOFC. The best power density is 269 mW.cm-2 at 800°C, which is inferior to the maximum value of 326 mW.cm-2 for a cell that uses H2 as the fuel.
Keywords: City waste; Gasification; Catalyst; Reforming; Syngas; SOFC
23156Introduction
The increase in Municipal Solid Waste (MSW) in recent years has created the problems for sustainable disposal in Taipei city, of which two Waste-To-Energy (WTE) plates are in operation at present. The recovery of papers as the renewable source is nearly 90% in Taipei city. However, there are still the paper wastes burned in the
WTE plants, occupied nearly 34.7% of the MSW in Taipei [1,2]. We are facing the dilemma, either burn those in the plant or used for better energy recovery route?
To recover the energy from the paper wastes which are wet (nearly 54% moisture) and contain dirt, the papers burn to ash in an average quantity of 6.0 wt% on a wet basis, and several percents of sulfur species. Besides, the power generation efficiency is 18% when the waste is burn in the WTE plants. The incineration of the wastes is not optimized yet.
The study of gasification of city waste to generate hydrocarbon fuel is so important [3] because of the development of cheap hydrogen energy relying on cheap fuel sources. That means the rapid increase in the production of valuable wastes in cities can be treated as a recoverable fuel, and reduce the consistent demand for fossil fuel in the mega-cities, e.g. in Taipei.
MSW in Taipei consists of mostly the papers (34.7% on a dried basis)) food waste (40.4%), plastics (15.6%) and others [1]. Those wastes contain appreciable chemical energy [4]. In this study, nonrecoverable waste papers and plastics are studied. We conduct the feasibility study of small-scale gasification/reforming of the wastes, and see the potential of the use of the syngas for solid oxide fuel cells for clean power generation.
Various fuel sources for gasification have been used to develop green technology for power generation that reduces the carbon footprint. City wastes as the renewable fuels is one of the subjects of increasing attention because the transportation cost, heat value (e.g. LHV) and the non-usable compounds in city wastes [3,4], especially in developing countries where the treatments of city waste mostly involves landfill and incineration. The emissions of the gases far exceed environmental regulations and are unfavorable economically. One feasible method is to convert city wastes into clean hydrocarbon fuels, and used to effectively generate electricity.
The gasification of wastes is a process that may involve the formation of char, tar and several residues, e.g. sulfur species and ash. The quantity of the products is mainly controlled by the gasification temperature, the properties of the wastes, the design of the reactors, and several operational factors [5]. The presence of the tar from the gasification of various sources can be as much as 40%, which could block pipeline and damage the gasification and power generation systems.
Tar can be decomposed at temperatures >800°C and can partially eliminated by the addition of different catalysts [6]. The produced gas, called syngas in this study, is a mixture of carbon monoxide (CO), hydrogen (H2), methane (CH4), and other hydrocarbon gases (CxHyOz), of which the gaseous fuels are not acceptable for the usage of the fuel for solid oxide fuel cells (SOFCs). We shall reform the hydrocarbon to the level acceptable by SOFC.
SOFCs, which effectively convert solid [7,8], liquid or gaseous fuels to electricity [9], are almost silent during operation, low pollution emission and less sensitive to impurities in fuels. The most important advantage is that SOFCs can use hydrogen and other gaseous hydrocarbon fuels [9], who have shown the combination of SOFC and the gasification of solid fuels capable of greatly reducing the cost of transportation because the wastes is directly collected from the neighboring area and nearly no transportation needed. This combination not only consumes local waste, but also supplies lowcarbon electricity.
This study uses three types of the city waste as fuel sources. Two are collected from city garbage, and are currently not recoverable using the current garbage collection system in Taipei. The comparable case is the MSW in original state. The contents of tar and char in syngas for the gasification to 800°C are compared in after the addition of carbonate catalysts in this study. The adsorption of H2 S in syngas by various absorbents is also studied. The improvement in the H2 content and reduction in CH4 after reforming to H2 and CO are the main requirements of the produced syngas.
Experimental
Sample preparation
Waste paper, waste plastics and one MSW are the three types of city waste used for this gasification study. The MSW was first treated in an autoclave operated at 150°C to reduce the volume and to sterilize the waste. After the autoclave stage, the volume density of the MSW increased about 2 times and the water content was significantly reduced to less than 6 wt%. Wastes paper and plastics were sliced into small sizes of
The results of the approximate properties of city wastes are shown in Table 1. The amount of moisture in MSW and waste paper was around 5 wt%. The waste plastics contained 0.2 wt% moisture which was attributed to the presence of a hydrophobic surface. The MSW had high contents of fixed carbon (41.9 wt%) and ash (20.9 wt%). The ash content of waste paper and plastics was low, only 0.8% and 0.5%, respectively.
| RT – 800 °C | Municipal SolidWaste (MSW) | waste paper | waste plastics |
|---|---|---|---|
| Moisture | 5.20% | 5.20% | 0.20% |
| Fixed carbon | 41.90% | 16.20% | 1.30% |
| Ash | 20.90% | 0.80% | 0.50% |
| Volatiles | 32.00% | 77.80% | 98.00% |
| LHV (MJ kg-1) | 15.5 | 18.6 | 41.1 |
Table 1: Approximate properties of three city wastes.
MSW and waste paper had a similar heat value (-1) and the heat value of the waste plastics was 41.1 MJ. kg-1. The LHV of the waste is similar to the 14.1-15.1 MJ kg-1 of the biomass used in our work [5]. High volatiles in the waste paper and high LHV of the plastics are suitable for gasification and for producing fuel gas for SOFCs.
Preparation of K2CO3 catalyst: Regent grade K2CO3 powder was first dissolved into DI water to form a solution [5,10]. City waste was immersed in the K2CO3 solution in controlled quantities of 10 wt%. After the impregnation with K2CO3, the samples were dried at 130°C until the moisture had totally evaporated.
Preparation Ni-CeO2/γ-Al2O3: Raw gas from gasification was reformed using Ni-CeO2/γ-Al2O3 catalyst which was made modified from the reports [6,8,11,12]. Preparation of Ni-CeO2/γ-Al2O3 catalysts involved two steps: CeO2 was first coated onto porous γ-Al2O3 balls with diameters of ca. 3 mm by impregnation of 1.0 M Ce(NO3)3 aqueous solution and calcined at 500 °C for 1 h. A typical mass ratio of 30 g for the γ-Al2O3 balls impregnated in the solution gave a final figure of 7.55 g Ce(NO3)3•6H2O. After calcination, the amount of CeO2 coated on γ-Al2O3 balls was 3.0 g. The CeO2/γ-Al2O3 was then impregnated with 1.0 M Ni(NO3)2•6H2O solution and calcined at 500°C for 1 h to produce NiO-CeO2/ γ-Al2O3 balls. The mass ratio between NiO, CeO2 and γ-Al2O3 was 1.0:1.0:10.0
The other form of the catalysts, called “carboneous catalysts” was synthesized. CeO2 was coated onto porous γ-Al2O3 balls using the same impregnation method as previously described. A carbon layer was then deposited onto CeO2-Al2O3 using a sputter coater (EMITECH K450, England), to separate the Al2O3 from the Ni which could react with Al2O3 at high calcination temperature and form a NiAl2O4 spinel. The spinel degrades the effect of the catalyst [11]. The Ni-layer was then coated with Ni(NO3)2•6H2O solution as previously described.
Gas processing
The solid wastes were randomly sampled using a standard procedure and placed in a fixed-bed reactor, which consisted of quartz tube, heating element, fuel feeding inlet, muffles for sampling of gaseous species and reforming device. The reactor had a capacity of 50 gm waste in the combustion zone (GZ) and operated between room and 800°C [5], and maintained in oxygen-lean condition.
Reforming of Syngas: The raw gas from gasification was reformed using Ni-CeO2/Al2O3 balls, and then supplied to SOFCs. 20 g of Ni- CeO2/Al2O3 balls were placed in a quartz tube and this was terminated with glass fiber in a 6.0 cm-long section (the flowing distance of syngas through the reforming catalyst).
Removal of H2S: Limestone (CaCO3), CaO, MgCO3 and ZnCO3 powders were used for the adsorption of H2S. The oxide powders were obtained by calcination of the carbonates at 850°C for 2 h. 20 g of each adsorbent were fixed in a quartz tube using glass fiber, and the syngas was allowed to pass the adsorbent at 800°C.
Fabrication of SOFC
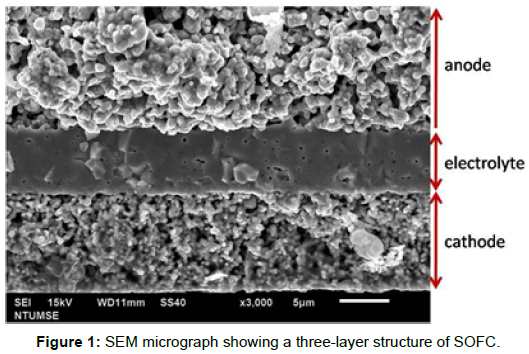
The preparation of SOFC followed the detail procedure shown as below. A cell with a three-layer structure, Ni-8YSZ (8 mol% yttria doped zirconia)/8YSZ/LSCF-6428 (La0.4Sr0.6Co0.2Fe0.8O 3-δ), was fabricated, in the thicknesses of 300 μm, 6 μm and 10 μm, respectively. The electrolyte with a density of 98.7% T.D. (theoretical density) showed dense characteristics (Figure 1).
Characterization
Approximate analysis of wastes: The amounts of moisture, tar, char and ash in city waste for gasification were measured for different conditions. City waste was dried at 130°C for 1 h to measure the mass loss due to moisture. Char was determined by pyrolyzing city waste at 800°C for 1 h in N2. Ash was obtained by the oxidation of city waste at 800 oC for 1 h in air. Because the decomposition temperature of tar was greater than 400 oC, tar was collected using a cold trap with glass fiber at room temperature.
Thermal analysis: Samples were heated in Ar from room temperature to 800°C at a heating rate of 5°C min-1 to 20°Cmin-1. Mass loss was recorded as a fraction of the initial mass.Differential thermogravimetry (DTA, SETSYS Evolution TGA-DTA/DSC SETARAM, France) was used to record the mass change over time, which allowed the reaction rate to be determined.
Temperature programmed reduction (TPR): Samples of 50 mg powder in a U-shaped quartz tube were heated from room temperature to 800°C at 10°C min-1 in a flowing gas with a fixed H2/Ar ratio of 1:9 for the reduction tests of NiO and NiO+CeO2. H2 consumption at the test temperature was recorded using a TCD (thermal conductivity detector). When the H2 composition varied, a different thermal conductivity to that for the standard carrier gas of H2/Ar gas was detected.
Analysis of gas composition: The gases were injected into the column of a gas chromatography (GC2000, China Chromatography, China) and diluted with He or Ar carrier gas. The carrier gas acted as a standard that the TCD detector can distinguish from other samples. Different species of H2, CO, CH4 and CO2 flowing in the column were separated by different flow rates and detected by the TCD individually. The concentration of every gas was calculated using calibration curve for the individual gas species.
Temperature distribution during gasification: Measurements were taken at two positons: the top (waste surface) and the bottom of the furnace. The temperature distribution was recorded using two thermal couples, which instantaneously showed the variation in temperature over time.
Syngas for SOFC testing: After reforming and cleaning, the syngas was supplied to SOFCs. Fuel cells of Ni+8YSZ/8YSZ/LSCF-6428 were tested, fueled with pure hydrogen (99.9%) or syngas. The NiO at the anode side of the cell was reduced by exposure to hydrogen at 600 °C for 1 h, and the flow rate for the power-output tests was 50 ml min-1. On the other side, the air-flow rate in the cathode was maintained at 250 ml.min-1. The output of the cell power was recorded from 600°C to 800° C until an open circuit voltage (1.1 V) was observed for the cell.
Results And Discussion
Properties of city waste
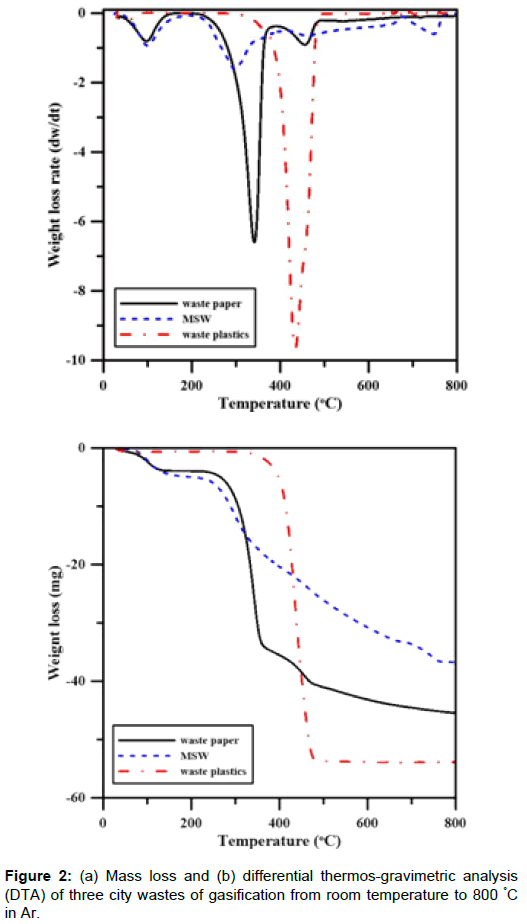
Thermal analysis: Mass loss of city wastes during gasification from room temperature to 800°C in Ar was determined using TGA and the results are shown in Figure 2(a). The differential thermogravimetric analysis (DTA) is shown in Figure 2(b). The peaks shown in the DTA represent three stages of mass loss, depending on the temperature at which the gasification reaction occurs. The initial mass loss occurs due to the evaporation of moisture, about 5 wt% in the MSW and waste paper. In the second stage, i.e. 200°C to 480°C, there are either one or two significant mass loss peaks, respectively, for MSW or waste plastics and paper. At temperature greater than 480°C, the variation in the residual mass is relatively minor.
Gas contents from gasification: The TGA and GC results show that the gasification can be divided into three gasified ingredients. The contents of tar, char and gas are shown in Table 2. Tar was mainly formed from room temperature to 400°C and it decomposed into the gaseous form at higher temperatures. The decomposition of tar resulted in a low tar content in the range of 480°C to 800°C, because main decomposition reaction occurred between 300°C and 480°C, as previous TGA results showed. In general, the high content of tar in the wastes must be reduced.
| Tar | Char | Gas | |
|---|---|---|---|
| MSW | 37.20% | 36.60% | 26.20% |
| Waste paper | 40.30% | 16.10% | 42.80% |
| Waste plastics | 57.20% | 0.80% | 42.00% |
Table 2: Mass contents in dry-based city wastes tested to 800°C.
The gas composition for MSW, waste paper and plastics is shown in Table 3. The GC analysis was also conducted in two temperature regions: from room temperature to 400°C and from 400°C to 800°C. From room temperature to 400°C, the main gases produced by the three types of waste were CO, and only a little H2. The 3rd gas species, CH4, did not form below 400°C, but was the main product of waste plastics from 400°C to 800°C. At higher temperatures, the tar with longer hydrocarbon chains decomposed and formed CH4, CO and H2. Most of the fuel gases were produced between 200°C to ~500 °C, which was the principal temperature region where gasification reactions occurred.
| Samples | CH4 | CO | H2 |
|---|---|---|---|
| MSW | 21.50% | 45.20% | 33.30% |
| Waste paper | 25.70% | 51.70% | 22.60% |
| Waste plastics | 59.20% | 19.60% | 21.20% |
Table 3: Gas compositions of dry-based city wastes by gasification to 800°C.
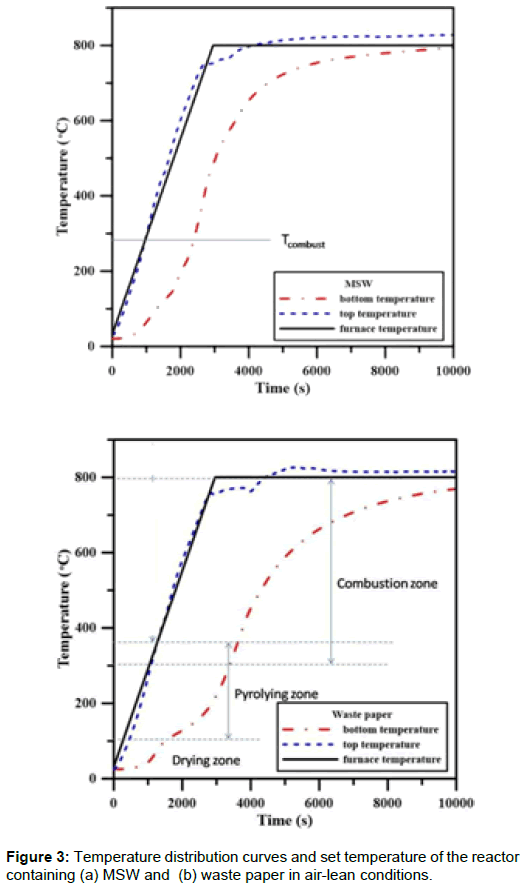
Temperature profile for batch gasification: The temperature distributions at the top and bottom of the waste in the reactor during the gasification tested in air are shown in Figure 3. They can be divided into three zones: a Drying Zone (DZ), a Pyrolyzing Zone (PZ) and a Combustion Zone (CZ). At a temperature of 100°C physically absorbed water was evaporated. In the DZ, an endothermic reaction occurred at the beginning of gasification. The length of the drying period depends on the water content in the waste. In the PZ, an endothermic reaction in the MSW and paper would produce volatile matter and char.
The volatile matter and the char that are produced by pyrolyzing started to burn in the combustion zone, between 300°C and 800°C if the atmosphere is rich in O2. It is noteworthy that 300°C is the minimal temperature to start up the gasification (drying and pyrolyzing reactions) in furnace. Because the combustion reaction is exothermic, the heating rate rapidly increased, which resulted in a greater topposition temperature. Most tar was decomposed in this zone because of the high temperatures. The reaction in the PZ proceeds at higher temperatures if insufficient oxygen is supplied.
Effects of purification and reforming
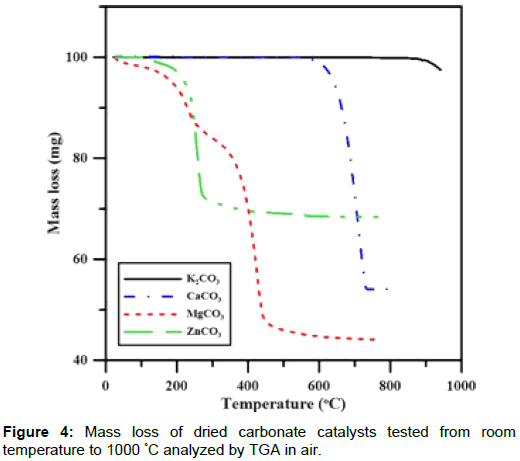
Properties of catalysts: Carbonate has a catalytic effect in removing tar and H2S during gasification [13]. The decomposition temperatures of four catalysts were studied and compared to identify their catalytic effects during gasification. The decomposition rates for K2CO3, CaCO3, MgCO3 and ZnCO3 in air are shown in Figure 4. ZnCO3 powder would completely decompose at 300°C, and the respective decomposition temperature of MgCO3 and CaCO3 was 500°C and 730°C. The decomposition of K2CO3 resulted in slightly mass lost at 900°C, which did not finish until the temperature reached 1000°C. These dry-based carbonates underwent thermal decomposition and turned to metallic oxides, with a remaining oxide mass of 68.4%, 44.1%, 54.2% and 97.5%, respectively, at 950°C. K2CO3 is soluble in water, but CaCO3, MgCO3 and ZnCO3 are not. Insolubility in water is a major drawback for these three carbonates, which could not undergo a simple solutionprecipitation process by an aqueous route. Therefore, K2CO3 was the only catalyst that is used to catalyze the tar in this study. CaCO3 and ZnCO3 were later used as absorbents for H2S adsorption.
Composition of syngas by catalyst: A comparison of the gaseous products in city waste, before and after the addition of catalysts during gasification is shown in Table 4. The impregnation method for waste paper could precipitate fine K2CO3 particles that are uniformly distributed on the surface of the biomass. After the addition of 10 wt% K2CO3 catalyst, MSW was significantly reduced from 37.2% to only 13.7% and the gas content was increased from 26.2% to 48.7%, which is due to K2CO3 active in the decomposition of tar. Similar improvements were seen for waste paper, but not for waste plastics. Previous TGA evidence (Figure 4) indicated that only slighly CO2 was released from K2CO3 at the temperatures of less than 800°. Therefore, tar decomposition might be enhanced by the catalytic effect of fine K2CO3 precipitates. This catalytic effect was not obvious for waste plastics because of the hydrophobic characteristics of the plastic surface, on which no cover of fine grain K2CO3 observed by SEM.
| RT – 800 °C 10 °C/min | Tar | Char | Gas |
|---|---|---|---|
| MSW without catalyst | 37.20% | 36.60% | 26.20% |
| MSW with 10 wt% K2CO3 | 13.70% | 37.60% | 48.70% |
| Waste paper without catalyst | 40.30% | 16.10% | 42.80% |
| Waste paper with 10 wt% K2CO3 | 16.80% | 16.90% | 65.50% |
| Waste plastics without catalyst | 57.20% | 0.80% | 42.00% |
| Waste plastics with 10 wt% K2CO3 | 55.80% | 1.30% | 42.90% |
Table 4: Products of city wastes with or without K2CO3 by gasification.
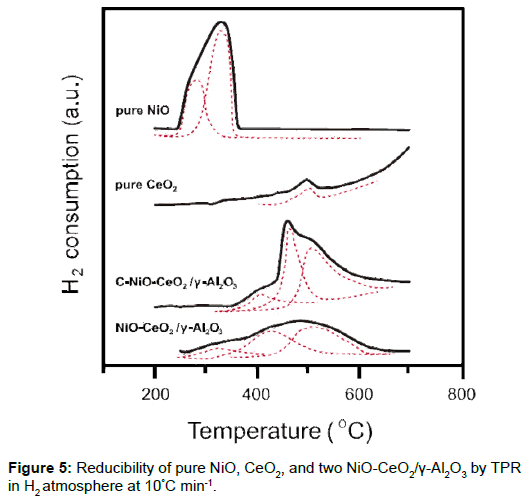
TPR Test for catalytic materials: The reduction ability of three oxides and mixtures was characterized by TPR and the results are shown in Figure 5. Each curve was deconvoluted to correspondent reaction cuves shown in dot lines in the figure. There were two possibly reduction peaks for pure NiO at 275°C and 325°C. The lower and higher reduction peaks imply that there are two bonding states in NiO [14]. The other catalyst, pure CeO2 had a reduction peak at 500 oC, which might be due to the reduction of Ce4+ to Ce3+.
Two catalytic materials, NiO-CeO2/γ-Al2O3 and C-NiO-CeO2/γ- Al2O3, showed three reduction peaks. The reduction temperatures of first two peaks for NiO increased for both materials, representing a strong bonding or strong interaction between NiO and the Al2O3 support. The carbon-coated case, C-NiO-CeO2/γ-Al2O3, retarded the reduction of NiO to temperatures >370°C. The third reduction peak (the peaks at ~500°C) coincided with the reduction of pure CeO2, implying the conversion of Ce4+ to Ce3+, but over a broader temperature range.
The surface of the C-Ni-CeO2/γ-Al2O3 catalysts was examined by SEM. The average particle sizes for CeO2 and NiO were 1.8 μm and 0.25 μm, respectively. NiO particles were deposited on the surface of CeO2, where the Ni↔NiO reaction could occur in the presence of H2 and could be retarded by residual carbon. If the concentration of CH4 is high, the gas content is not suitable for used in SOFCs [8]. To upgrade the content of H2 and reduce the content of CH4, two methods were used in this study: Impregnation of fine K2CO3 catalyst into the waste, and the use of Ni-CeO2/γ-Al2O3 to reform the syngas.
The H2 content of the syngas from waster paper and plastics increased about 6%-10% after impregnation with 10 wt% K2CO3 catalyst. The results are shown in Table 5. The gas from the gasification of waste paper and plastics was then dry-reforming with the Ni-CeO2 catalyst on γ-Al2O3. The main gas compositions after reforming were H2 and CO. The final content of CH4 was greatly reduced to only 3.4% and 8.3%, respectively, for paper and plastics. Most CH4 was decomposed to CO and H2 after the reforming.
| Waste paper RT – 800 °C | CH4 | CO | H2 | CO2 |
|---|---|---|---|---|
| Without K2CO3 | 25.70% | 51.70% | 22.60% | ND |
| With K2CO3 | 20.60% | 46.90% | 32.40% | ND |
| With K2CO3 & reforming | 3.40% | 44.80% | 51.30% | 0.53% |
| Waste plastics RT – 800 °C | CH4 | CO | H2 | CO2 |
| Without K2CO3 | 59.20% | 19.60% | 21.20% | ND |
| With K2CO3 | 57.80% | 14.80% | 27.40% | ND |
| With K2CO3 & reforming | 8.30% | 45.40% | 46.30% | ND |
Table 5: Gas compositions of waste paper and plastics after gasification in different catalytic conditions using 20 gm catalytic balls.
To further reduce the concentration of CH4, masses of 30 g and 40 g of NiO-CeO2/γ-Al2O3 were used for another reforming test. The results are shown in Table 6. The concentration of CH4 was significantly reduced to less than 0.03 vol% when the mass of catalytic materials was increased to 40 g. However, a possibly formation of water-gas reaction would occur ( CO2+H2 CO+H2O ) and consume H2 concentration in this case. This explained why the H2 content reduced from 51.3% to 41.9% as more catalytic material was used
| Mass of catalytic materials | CH4 | CO | H2 | CO2 |
|---|---|---|---|---|
| 20 g | 3.40% | 44.80% | 51.30% | 0.53% |
| 30 g | 0.10% | 58.80% | 41.00% | 0.10% |
| 40 g | <0.03% | 58.00% | 41.90% | 0.10% |
Table 6: Gas compositions (vol%) of waste paper tested from RT to 800°C in different amounts of catalytic materials.
Removal of Sulfur: The sulfur (S) contents in three wastes, MSW, waste paper and plastics- were 1.24%, 4.05% and 1.03%, respectively. Only the H2S content in the syngas produced from waste paper was tested and reported in this study, as shown in Table 7. In the reducing atmosphere during gasification, the sulfur content in city waste, which possibly converts to H2S gas, can be removed by the following reactions:
| Absorbent | Non H2S (ppm) | ZnO H2S (ppm) | ZnCO3 H2S (ppm) | Limestone H2S (ppm) | CaO H2S (ppm) |
|---|---|---|---|---|---|
| *1 without catalyst | 183.00% | 0.5 | 1.23 | 0.1 | 1.12 |
| *2 with 10wt% K2CO3 | 0.87 | 0.275 | 0.63 | 0.04 | 0.56 |
| with 10 wt% K2CO3 and 20 ml/min CO2 | 1.23 | 0.43 | 1.15 | 1.76 | 1.09 |
| BET of adsorbent (m2 g-1) | — | 1.6 | 188.7 | 1.9 | 9.2 |
*2Gas composition: 20.6% CH4, 46.9% CO, 32.4% H2
Table 7: Concentration (ppm) of H2S produced from the syngas prepared from waste paper by various adsorbents.
MCO3(s)+ H2S(g) = MS(s)+H2O(l)+CO2(g) ------------------------- (1),
and
MxOy(s)+yH2S(g) → MxSy(s)+yH2O(g)-------------------------(2).
After gasification, the concentration of H2S in the gas from waste paper was 1.83 ppm. When the raw gas passed through four types of absorbents, the H2S concentration was reduced to 0.1 ppm by the CaCO3 case and the H2S content in syngas was reduced to 0.04 ppm when waste paper was impregnated with a K2CO3
catalyst. CaCO3+K2CO3 removed H2S most effectively. In an additional test, a CO2 flow rate of 20 ml min-1 CO2 gave the concentration of H2S of 1.76 ppm because CO2 would suppress the formation of H2S gas.
Performance of SOFC
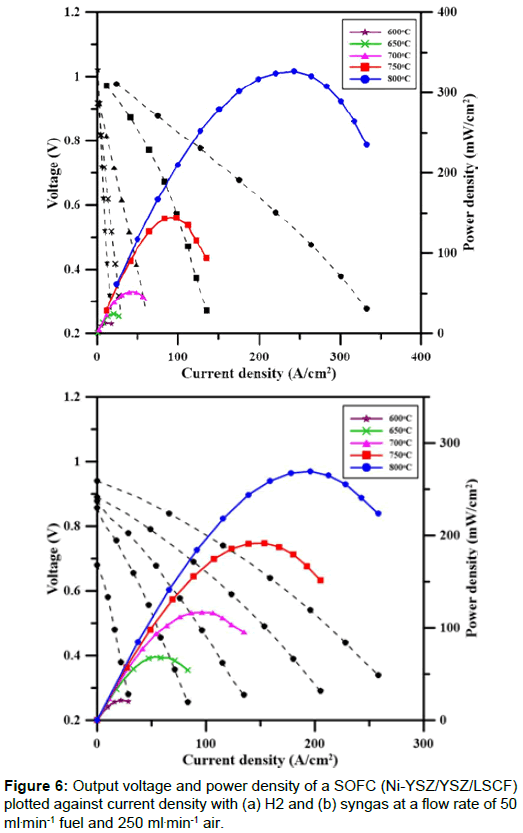
The current density-voltage (I-V) and powder output for an anodesupported single cell was tested. The testing conditions were fixed, with the exception of the fuels. The power density and IV for a single cell are shown in Figure 6. The open circuit voltage (OCV) was 0.98 V, and the maximum power density was 326 mWcm-2 for H2 fuel at 800°C, which was close to the value of 400 mWcm-2 for a previous work which used LaSrFeCuO3-δ as the cathode and testing up to 800°C [15].
The performance of the fuel cell (Ni-YSZ/YSZ/LSCF) that was fueled by syngas is shown in Figure 6(b). The syngas with a composition of 51.3% H2, 44.8% CO, 3.4% CH4 and 0.53% CO2 was supplied to the SOFC at same rate of 50 ml min-1. The OCV was 0.94V and the maximum power was 269 mWcm-2 at 800°C. The maximum power density for the cell fueled by syngas showed ca. 20% lower than that for the H2-fuelled cell at 800°C. A decrease in the temperature, produced a gradual reduction in the maximum power density which might be caused by a reduction in the ionic conductivity of the electrolyte at lower temperature.
Summary and Conclusion
In this study, three types of city waste are used for the gasification and purification of syngas, and the power generation of a SOFC which is fueled with syngas and H2 are compared. K2CO3 and NiO-CeO2 catalysts are used to reduce the amount of tar and to increase the concentration of H2. H2S is absorbed using different adsorbents. Finally, the syngas is used to fuel a SOFC to compare the power output.
The results are summarized as below.
1. Sulfide impurity in the syngas from waste paper is 1.83 ppm. The desulfurization of H2S by CaCO3+K2CO3 is the most effective of the four H2S absorbents. In a CO2-rich atmosphere, the effect of the removal of sulfur is suppressed. The concentration of H2S is reduced to 0.04 ppm when waste paper is treated using CaCO3+K2CO3.
2. Impregnating with the K2CO3 catalyst, reduces the amount of tar in MSW to 13.7 wt%. The K2CO3 promotes the formation of H2 and the decomposition of CH4. Syngas prepared by the reforming using NiO-CeO2/γ-Al2O3 has a higher H2 content of 51.3 vol% and the concentration of CH4 is reduced to 3.4 vol%.
3. The reducibility if NiO-CeO2/γ-Al2O3 by TPR indicates that there may be oxygen ion transfer between Ni and CeO2, which refreshes the catalyst.
4. A Solid Oxide Fuel Cell (SOFC) with a layer structure of Ni- 8YSZ/8YSZ/ LSCF-6428 that is used for power generation has a power density of 326 mW.cm-2 at 800°C when fueled by pure H2, and 269 mW.cm-2 when fueled by syngas.
Acknowledgement
The authors like to thank the funding given by MOST, Taiwan by a contract NSC102-2221-E-002-061-MY2, and also thank the analysis equipment offered by Professor C. Y. Chang at Institute of Environmental Science & Engineering, National Taiwan University.
References
- Chen YC, Wang CT (2017) Municipal solid waste incineration’s potential contribution to electricity production and economic revenue in Taiwan. J Taiwan Energy 4: 93-106.
- Bellomare F, Rokni M (2013) Integration of a municipal solid waste gasification plant with solid oxide fuel cell and gas turbine. Renew Energy 55: 490-500.
- Doherty W, Reynolds A, Kennedy D (2015) Process simulation of biomass gasification integrated with a solid oxide fuel cell stack. J Power Sources 277: 292-303.
- Wei WCJ, Cheong UH, Cheng KY, Chang CY (2017) Gasification and syngas reforming of lignin biomasses in Taiwan for SOFC applications. J Taiwan Energy 4: 77-92.
- Roh HS, Eum IH, Jeong DW (2012) Low temperature steam reforming of methane over Ni-Ce(1-x)Zr(x)O2 catalysts under severe conditions. Renew Energy 42: 212-216.
- He M, Xiao B, Hu Z, Liu S, Guo X, et al. (2009) Syngas production from catalytic gasification of waste polyethylene: influence of temperature on gas yield and composition. Intern J Hydrogen Energy 34: 1342-1348.
- Rokni M (2014) Thermodynamic and thermoeconomic analysis of a system with biomass gasification, Solid Oxide Fuel Cell (SOFC) and stirling engine. Energy 76: 19-31.
- Mogensen M, Kammer K (2003) Conversion of hydrocarbons in solid oxide fuel ells. Annu Rev Mater Res 33: 321-331.
- Michael JV, Alexis TB (1978) Effect of alkali metal catalysis on gasification of coal char. Fuel 57: 194-200.
- Li G, Hu L, Hill JM (2006) Comparison of reducibility and stability of alumina-supported Ni catalysis prepared by impregnation and co-precipitation. Applied Catalysis A: General 301: 16-24.
- Samashima S, Hirata Y, Sato J, Matsunaga N (2008) Synthesis of hydrogen and carbon monoxide from methane and carbon dioxide over Ni-Al2O3 catalyst. J Ceramic Soci Jpn 116: 374-379.
- Sutton D, Kelleher B, Ross JRH (2001) Review of literature on catalysts for biomass gasification. Fuel Process Technol 73: 155-173.
- Koo KY, Roh HS, Jung UH, Yoon WL (2009) CeO2 promoted Ni/Al2O3 catalyst in combined steam and carbon dioxide reforming of methane for Gas to Liquid (GTL) process. Catal Lett 130: 217-221.
- Zhou Q, Xu L, Guo Y, Jia D, Wei WCJ, et al. (2012) La0.6Sr0.4Fe0.8Cu0.2O3-δ perovskite oxide as cathode for IT-SOFC. Intern J Hydrogen energy 37: 11963-11968.
Citation: Ke SY, Chen YY, Wei WCJ (2017) Gasification and Syngas Reforming of City Waste Papers for Solid Oxide Fuel Cells. Innov Ener Res 6: 171. DOI: 10.4172/2576-1463.1000171
Copyright: ©2017 Ke SY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3124
- [From(publication date): 0-2017 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 2361
- PDF downloads: 763