Gamma Probe Assisted Axillary Lymph Node Biopsy Compared with Axillary Dissection in Breast Cancer
Received: 31-Jan-2023 / Manuscript No. JCD-23-88336 / Editor assigned: 02-Feb-2023 / PreQC No. JCD-23-88336 (PQ) / Reviewed: 16-Feb-2023 / QC No. JCD-23-88336 / Revised: 24-Apr-2023 / Manuscript No. JCD-23-88336 (R) / Published Date: 02-May-2023
Abstract
Background: Breast cancer incidence is annually increasing in various parts of the world and Sentinel Lymph Node Biopsy (SLNB) has turned into a new standard for care as a staging process in this regard. In the present study, the gamma probe technique was used for SLNB as a safe method with more accuracy and less complications. The study sought to compare the results Axillary Lymph Node Dissection (ALND) and SLNB in patients from the Western provinces of Iran.
Methods: In general, 277 cases participated in the current study. Patients were divided into those undergoing ALND and SLNB. The criteria for complete dissection or axillary biopsy using the gamma probe were based on the results of clinical examinations and the presence of palpable lymph nodes.
Results: Overall complications after surgery belonged to 58 (18.9%) cases, including 15 (25.9%) and 43 (74.1%) patients in the SLNB and ALND groups, respectively (P=0.74). Based on the findings, seroma (60.3%) was the most reported complication in each group. Most patients had tumors in the upper-outer quadrant of their left breast. The mean of the tumor dimension in the SLNB and ALND groups was 2.1 ± 1.3 cm and 3.2 ± 1.8 cm, respectively, (P=0.003).
Conclusion: The benefits of Breast Conserving Surgery (BCS) with the SLNB technique are clearly undeniable and can be considered a method with less complications and a better prognosis. Accordingly, SLNB and BCS are favorable methods that can be performed, along with gamma probe technique, which is safe and accurate.
Keywords: Breast cancer, Sentinel lymph node biopsy, Axillary lymph node dissection, Gamma probe; Tumor dimension
Introduction
The incidence of Breast Cancer (BC) is annually increasing in different parts of the world, which could be due to changes in lifestyles, more stress in today’s societies and the increasing use of screening programs [1]. Similar to other parts of the world, in Iran, BC is among the leading causes of death due to malignancy in women [2]. Overall, 52167 cases of early stage BC were reported by 2015, which equals 24.6% of all diagnosed cancers. Approximately 10,000 people are annually diagnosed with BC in Iran [3]. Factors such as the tumor dimension, the staging and grading of the tumor, hormone receptors and metastases in the axillary lymph nodes, and the rapid decision for appropriate treatment are considered essential in determining the prognosis [4,5]. Axillary surgery is a vital part of regional therapy which is important for determining the stage and therapeutic plan in BC. Axillary Lymph Node Dissection (ALND) leads to significant morbidities such as lymphedema, seroma, pain and infection from intercostal-brachial and intercostal nerve injuries and paresthesia [6]. In the mid-1990's, Sentinel Lymph Node Biopsy (SLNB) was recommended for BC as a technique to detect the first lymph node in the nodal basin that is able to contain metastases [7]. Nevertheless, recent trends have shifted from a more radical ALND to a less morbid SLNB [8].
In this method, axillary nodes are probably not involved when the sentinel lymph node is negative and ALND should not be conducted accordingly SLNB has changed into a standard for care as a staging process in patients with clinically (imaging and examination) node negative diseases. Therefore, complete ALND should be exclusively performed in patients with Sentinel Node (SN) metastases [9]. The dual technique is the standard method for SLNB through the injection of technetium labeled nano colloid (a radiolabeled tracer) and the blue dye [10]. However, blue dye injection has different drawbacks. The blue dye can blur the surgical field and regularly leaves a blue skin stain; this stain can be permanent or take months to fade. Further, there may be a slight risk of an adverse reaction to the blue dye [11]. Accordingly, some clinics stopped the routine use of blue dye. In the present research, the gamma probe technique was employed for SLNB since it is a safe method with more accuracy. As mentioned earlier, the results of the SLNB can increase the accuracy of the staging, preventing the unnecessary dissection of axillary lymph nodes and their complications while improving patients’ quality of life [12]. On the other hand, there are contradictory results regarding the influence of ALND on sentinel lymph node positive early BC [13,14]. As a result, the current study aimed to compare the results of two surgical techniques (SLNB and ALND), including epidemiological results and clinicopathological features of BC patients from the western provinces of Iran.
Materials and Methods
Subjects
The dataset of this investigation was collected from reviewing the registered profiles of 420 BC patients referring to breast clinics in Sanandaj, Kurdistan province, Iran. This information had also been recorded using a questionnaire. The patients had undergone an operation by a surgeon at a teaching hospital in Sanandaj. At the end of the study, the outcomes were followed up by a phone call or an invitation to the clinic to sign the consent form in person. All patients referring to the breast clinic during 2017-2021 and undergoing surgery for BC participated in the present study. The exclusion criteria were patients with a history of ALND, tumor recurrence and untraceable profiles, as well as patients who had surgery in the other clinics and those with breast connective tissue disease.
Patients were divided into those undergoing complete ALND and SLNB. The criteria for complete dissection or axillary biopsy using the gamma probe were based on the results of clinical examinations and the presence of palpable lymph nodes. Among patients who referred to the breast clinic of Kurdistan university of medical sciences, Kowsar hospital, those with positive lymph nodes in the clinical examinations or radiologic findings were directly exposed to ALND and the remaining patients underwent SLNB. Moreover, patients with positive lymph nodes in SLNB underwent ALND. On the other hand, patients with negative lymph nodes and no macroscopic metastases avoided receiving the additional surgical procedure of axillary dissection.
Procedure of ALND
Twelve hours before the surgery, a radio labeled colloid (technetium-99) was injected into the peri-areolar region. Then, preoperative lymphoscintigraphy was performed to confirm sentinel lymph node absorption. The next day, after preparing the patient in a suitable position, all lymph nodes were removed; they were detected by the gamma probe as the involved nodes. It should be noted that in the area where the maximum signal with the gamma probe has been detected, the incision size must be suitable and small. Then, the sample of the biopsy should be sent for frozen sectioning and pathological investigation.
The radioisotope method was also reassessed to ensure the removal of all the involved nodes. Further, the axilla was re-evaluated by the probe to ensure the lack of any suspicious nodes. The suspicious nodes with the maximum 10% background absorption were completely removed as well. ALND should be performed in the case of macro metastasis. In the remaining patients, ALND was avoided if micro metastasis (involvement less than 2 mm) was detected or the cytologic findings were negative. The surgical plan and the possibility of a lack of performing ALND in the case of negative lymph nodes were fully explained to the patients before the surgery.
According to the research design questionnaire, epidemiological and clinicopathological characteristics, including age, tumor stage, tumor grading, progesterone receptor, tumor histology, estrogen receptor, lymphovascular invasion, and other information were recorded in the checklist for all patients.
Statistical analysis
Data were analyzed by Statistical Package for Social Sciences software, version 26.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to check the normal distribution of data. Moreover, demographic variables were presented as the number, percentage, mean, standard deviation and level of significance. Additionally, quantitative and qualitative variables such as continuous (age, number of SNs, tumor size and the like), dichotomous (estrogen and progesterone receptor status) and categorical (grade of the nucleus and histological type) variables were compared by independent t-test and Chi-square/Fisher’s exact test, respectively. Finally, their equivalent tests were conducted using an independent sample t-test and a P-value<0.05 was considered statistically significant.
Results
Overall, 420 women were identified with BC who referred to the breast clinic in Sanandaj, Kurdistan province of Iran in 2017-2021. Of this number, 318 cases underwent breast surgery and finally, 277 patients were included in the current research based on the inclusion and exclusion criteria. The profiles of these patients were complete; thus, they were included in our investigation. In general, 82 (29.6%) and 195 (70.4%) patients underwent SLNB (the SLNB group and radical mastectomy (the ALND group), respectively. Grading of the tumor, tumor node metastasis classification, receptors expression, complications and demographic characteristics of these patients were recorded for investigation. This demographic information is outlined in Table 1.
| Variables | Axillary surgery | |||||
|---|---|---|---|---|---|---|
| SLNB | ALND | Total | P-value | |||
| Age of diagnosis | (Mean ± SD) | 48.70 ± 10.135 year | 51.15 ± 13.279 year | 0.14 | ||
| BMI | (Mean ± SD) | 29.19 ± 3.905 | 28.44 ± 4.848 | 0.31 | ||
| Menarche age | (Mean ± SD) | 13.54 ±1.535 year | 13.80 ±1.217 year | 0.14 | ||
| Age of the first pregnancy | (Mean ± SD) | 22.31 ± 5.805 year | 21.49 ± 5.209 year | 0.28 | ||
| Total lactation duration | (Mean ± SD) | 62.36 ± 40.456 | 68.31 ± 43.803 | 0.33 | ||
| Menopause age | (Mean ± SD) | 46.70 ± 5.165 year | 47.80 ± 6.631 year | 0.34 | ||
| Educational level | Illiterate | (Count ± percentage) | 13 (20.0%) | 52 (80.0%) | 65 | <0.0001* |
| Under high school | 34 (35.1%) | 63 (64.9%) | 97 | |||
| High school diploma | 2 (20.0%) | 8 (80.0%) | 10 | |||
| University diploma | 20 (62.5%) | 12 (37.5%) | 32 | |||
| Marriage status | Widowed | (Count ± percentage) | 2 (7.7%) | 24 (92.3%) | 26 | 0.07 |
| Married | 69 (29.9%) | 162 (70.1%) | 231 | |||
| Single | 6 (40.0%) | 9 (60.0%) | 15 | |||
| Divorced | 2 (40.0%) | 3 (60.0%) | 5 | |||
| Tumor size and type (Non-inflammatory and inflammatory) | Right breast (N.I) | (Count ± percentage) | 43 (39.1%) | 67 (60.9%) | 110 | <0.0001* |
| Left breast (N.I) | 34 (31.2%) | 75 (68.8%) | 109 | |||
| Bilateral (N.I) | 0 (0.0%) | 6 (100.0%) | 6 | |||
| Right breast (I) | 0 (0.0%) | 15 (100.0%) | 15 | |||
| Left breast (I) | 0 (0.0%) | 25 (100.0%) | 25 | |||
| Bilateral (I) | 0 (0.0%) | 3 (100%) | 3 | |||
| Cancer type | Inflammatory | (Count ± percentage) | 0 (0.0%) | 43 (100.0%) | 43 | <0.0001* |
| Non inflammatory | 77 (34.2%) | 148 (65.8%) | 225 | |||
| Complication | Yes | (Count ± percentage) | 15 (25.9%) | 43 (74.1%) | 58 | 0.74 |
| No | 64 (29.2%) | 155 (70.8%) | 219 | |||
| Type of complication | Inflammation or abscess | (Count ± percentage) | 3 (50.0%) | 3 (50.0%) | 6 | 0.44 |
| Lymphedema | 2 (50.0%) | 2 (50.0%) | 4 | |||
| Scar | 1 (33.3%) | 2 (66.7%) | 3 | |||
| Hematoma | 1 (33.3%) | 2 (66.7%) | 3 | |||
| Seroma | 8 (22.9%) | 27 (77.1%) | 35 | |||
| Margin positive | 0 (0.0%) | 2 (100.0%) | 2 | |||
| Other | 0 (0.0%) | 5 (100.0%) | 5 | |||
| Age of diagnosis | Lower than 50 | (Count ± percentage) | 49 (35.0%) | 91 (65.0%) | 140 | 0.03* |
| 50 and more | 30 (22.7%) | 102 (77.3%) | 132 | |||
| Pregnancy at diagnosis | Yes | (Count ± percentage) | 0 (0.0%) | 2 (100.0%) | 2 | |
| No | 19 (18.8%) | 82 (81.2%) | 101 | |||
| Lactation at diagnosis | Yes | (Count ± percentage) | 1 (16.7%) | 5 (83.3%) | 6 | |
| No | 78 (29.7%) | 185 (70.3%) | 263 | |||
| Abortion | Yes | (Count ± percentage) | 17 (23.6%) | 55 (76.4%) | 72 | |
| No | 9 (17.6%) | 42 (82.4%) | 51 | |||
| Family history | Yes | (Count ± percentage) | 3 (15.8%) | 16 (84.2%) | 19 | 0.2 |
| No | 76 (30.5%) | 173 (69.5%) | 249 | |||
| Relative degree | First degree | (Count ± percentage) | 0 (0.0%) | 5 (100.0%) | 5 | |
| Second degree | 3 (21.4%) | 11 (78.6%) | 14 | |||
| Age of relative at diagnosis | (Mean ± SD) | 53.67± 23.245 | 43.69 ± 10.719 | 0.26 | ||
| Biopsy | Core needle | (Count ± percentage) | 29 (26.4%) | 81 (26.4%) | 110 | |
| Open biopsy | 2 (20.0%) | 8 (80.0%) | 10 | |||
| Side of tumor | Right | (Count ± percentage) | 46 (37.1%) | 78 (62.9%) | 124 | 0.01* |
| Left | 31 (23.7%) | 100 (76.3%) | 131 | |||
| Bilateral | 0 (0.0%) | 8 (100.0%) | 8 | |||
| Number of dissected nodes | (Mean ± SD) | 2.85 ± 1.83 | 10.45 ± 5.81 | <0.0001* | ||
| Number of positive nodes | (Mean ± SD) | 0.37 ± 0.82 | 2.41 ± 3.59 | 0.003* | ||
| Diameter of tumor | (Mean ± SD) | 2.15 ± cm 1.34 cm | 3.28 cm ± 1.87 cm | 0.003* | ||
| Note: BMI: Body Mass Index; SD: Standard Deviation; SLNB: Sentinel Lymph Node Biopsy; ALND: Axillary Lymph Node Dissection. | ||||||
Table 1: Patients and clinicopathological characteristics: SLNB vs. ALND.
Most patients had tumors in the upper-outer quadrant of their left breast. The tumor size of the dominant lesion was in the range of 2 mm to 10 cm with a mean of 1.8 cm ± 3 cm. The mean of the tumor dimension in the SLNB and ALND groups was 2.1 cm ± 1.3 cm and 3.2 cm ± 1.8 cm, respectively. In addition, the tumor dimension demonstrated a significant difference between the two groups (P=0.003). Most patients had grade 2 in both groups (P=0.08).
The total count of dissected lymph nodes ranged from 26 to 1 with a mean of 9.21 ± 6. The mean of nodes was 2.7 ± 1.8 and 10.67 ± 5.7 in the SLNB and ALND groups, respectively (P<0.001). Overall complications after surgery were related to 58 (18.9%) cases, including 15 patients (25.9%) in the SLNB group and 43 patients (74.1%) in the ALND group (P=0.74). Based on the results, seroma (60.3%) was the most reported complication in each group. The patients of the SLNB group had a higher educational level compared to those of the ALND group and single women were more interested in Breast Conserving Surgery (BCS) than married ones.
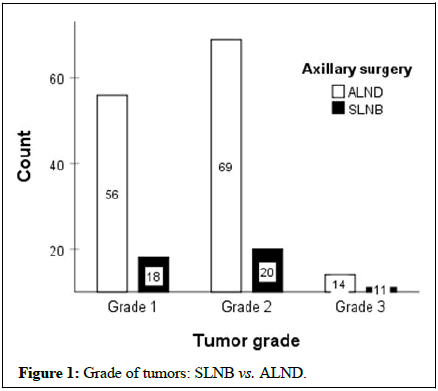
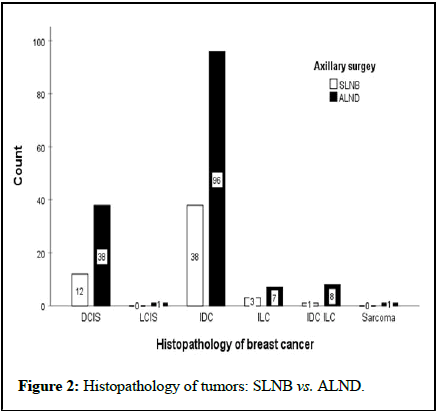
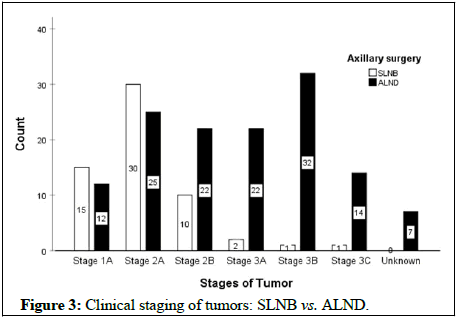
The mean age of the first pregnancy and number of pregnancies were 21.8 ± 5.4 years and 3.67 ± 2.2, respectively. In this regard, the results demonstrated no significant differences between the two groups (P>0.05). Figure 1 displays the frequencies of the tumor grade between the two groups that it shows the most tumors were in G2 (p=0.087). Also, most tumors showed histopathologic invasive ductal carcinoma in both groups (Figure 2). The clinical stages of tumors were 2A and the prognostic stages were 1A and 1B in both groups (Figure 3).
The frequency of lymphovascular involvement was 15 (17%) and 73 (83%) in the SLNB and ALND groups, respectively, indicating a lower involvement in sentinel biopsy than axillary dissection (P=0.008). Table 2 provides the frequencies of estrogen and progesterone receptor expression.
| Tumor receptors and pathologic characteristics | Axillary surgery | ||||
|---|---|---|---|---|---|
| SLNB | ALND | P-value | |||
| Estrogen receptor | Yes | (Count ± percentage | 45 (72.6%) | 122 (76.3%) | 0.6 |
| No | 17 (27.4%) | 38 (23.8%) | |||
| Progesterone receptor | Yes | (Count ± percentage) | 43 (69.4%) | 115 (71.9%) | 0.74 |
| No | 19 (30.6%) | 45 (28.1%) | |||
| HER2 receptor | Yes | (Count ± percentage) | 10 (17.5%) | 47 (30.7%) | 0.08 |
| No | 47 (82.5%) | 106 (69.3%) | |||
| Lymphovascular invasion | Yes | (Count ± percentage) | 15 (34.1%) | 73 (58.4%) | 0.008* |
| No | 29 (65.9%) | 52 (41.6%) | |||
| Note: SLNB: Sentinel Lymph Node Biopsy; ALND: Axillary Lymph Node Dissection. | |||||
Table 2: Tumor receptors and pathologic characteristics: SLNB vs. ALND.
Discussion
Modified radical mastectomy was the gold standard technique in BC surgery until the 1970's, which has been questioned by two thorough investigations, namely, the Kings/Cambridge and NSABP-04 [15]. They randomly included patients with a clinically node negative axilla in either the early or delayed axillary treatment group. Then, a new concept was suggested in axillary surgery in the mid-1980s [16]. Regarding the latest treatment technique, SLNB is the method of choice in various clinical cases instead of classical axillary lymphadenectomy [17]. It was demonstrated that patients could undergo this procedure in case they were negative for lymph node involvement in the clinical examination and imaging techniques and were in the first and second stages of BC, while patients with negative sentinel lymph nodes received no additional benefits from ALND [18]. The results of SLNB can increase the accuracy of staging and prevent unnecessary dissection of the axillary lymph nodes and their complications [19]. These complications include shoulder pain, lymphedema, seroma, and limited range of motion of the shoulder joint following axillary lymph node dissection [20]. Furthermore, making a decision based on the results of the SLNB is a method that has been used in recent years to prevent unnecessary ALND [21].
In our study, after examining patients undergoing BC surgery, the incidence of complications such as seroma was lower in the SLNB group compared to the ALND group; nonetheless, no significant difference was observed in this regard. Furthermore, ALND was related to higher morbidity such as seroma, lymphedema, pain, inflammation and infection than the SLNB group. Our results are consistent with those of previous papers, representing that the SLNB method had significantly lower morbidity than the ALND technique [22,23]. In addition, Wang et al evaluated the effect and safety of ALND in early BC and reported no significant differences in the overall survival, regional lymph node recurrence and disease free survival for sentinel lymph node positive patients [24]. Likewise, Ram, et al., found that the former criteria did not significantly vary between SLNB and ALND techniques. In terms of overall survival, loco regional recurrence and disease free survival between these two groups, our findings conform to the results of a meta-analysis by Li, et al. [25].
Due to several therapeutic reasons, in our clinic, the gamma probe method was used instead of the blue dye for SLNB. The first reason for using this method was to reduce the side effects of blue dye injection, which was reported in previous research. Further, the use of radioisotope and gamma probe methods for identifying axillary nodes is accurate, causing no serious complications. Thus, this technique was also preferable for patients.
The mean age of patients undergoing SLNB surgery was lower than that of those who underwent axillary dissection surgery, indicating that age was an essential parameter in determining the type of surgery; nonetheless, this difference was not significant [26]. Tumor grading demonstrated no significant difference in patients between the two groups. The tumor grading and receptor expression play a major role in prognostic staging, but in this study, no significant difference was found between the two groups with regard to estrogen, progesterone and human epidermal growth factor receptor 2 expressions. Hence, these factors may not be employed for determining the prognosis of the disease. Moreover, lymphovascular involvement was lower in the SLNB group, highlighting a significant difference in this respect. No recurrence was observed in patients who underwent breast maintenance surgery; however, three cases of metastasis occurred in this group. Based on the findings, patients with a higher level of education showed a greater tendency for BCS. Furthermore, patients with a lower educational level did not intend to accept the ALND if the lymph node cytologic finding was positive after the SLNB surgery. In other words, patients with lower literacy rates had more tendency to have a radical mastectomy than to maintain the breast. On the other hand, single patients were more inclined to conserve their breast using the SLNB than married patients, which could be for beauty reasons, social situations and cultural reasons. In our study, the percentage of patients with inflammatory cancer was significantly higher than in the other regions of the world, which would be investigated in the future. It should be indicated that performing SLNB surgery to conserve the breast or radical mastectomy depends on various factors such as those related to the patients, clinical features and tumor imaging results and the prognosis of SLNB in each patient [27]. Therefore, the benefits of BCS with the SLNB technique are undeniable and BCS can be considered a method with less complications and a better prognosis for several reasons. In spite of our findings regarding the lack of significant differences between the two methods, in the early stage of BC and at a lower clinical stage and lower tumor grading, it seems that SLNB is safe and has a better prognosis and consequences.
Conclusion
In general, SLNB and BCS are favorable methods that can be performed with the gamma probe and have less complications such as seroma and inflammations. Additionally, using the gamma probe is safe and accurate; thus, it is proposed that surgeons use this technique to gain some new experiences in this regard. However, the careful selection of patients for SLNB after neoadjuvant chemotherapy instead of completion ALND has an important place in BC surgery. In the current study, the sample size was small, but current evidence indicated that complications and prognostic factors (e.g. hormone receptors and grading) could not play a role in making decisions about breast and axillary surgery. It is also necessary to investigate this issue in future studies with a larger sample size, containing homogenous patients and well matched controls.
Acknowledgment
We would like to thank Mr. Rasooli M the statistic consultant in Kurdistan university of medical sciences for his contributions to this study.
Author’s Contribution
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Barthelmes L, Goyal A, Newcombe RG, McNeill F, Mansel RE (2010) Adverse reactions to patent blue V dye-The new start and ALMANAC experience. Eur J Surg Oncol 36: 399-403.
[Crossref] [Google Scholar] [PubMed]
- Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, et al. (2009) Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node positive breast cancer. J Clin Oncol 27: 2946-2953.
[Crossref] [Google Scholar] [PubMed]
- Bray F, McCarron P, Parkin DM (2004) The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 6: 229-239.
[Crossref] [Google Scholar] [PubMed]
- Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW (2017) Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev 1.
[Crossref] [Google Scholar] [PubMed]
- Charalampoudis P, Markopoulos C, Kovacs T (2018) Controversies and recommendations regarding sentinel lymph node biopsy in primary breast cancer: A comprehensive review of current data. Eur J Surg Oncol 44: 5-14.
[Crossref] [Google Scholar] [PubMed]
- hehade HEH, Headon H, El Tokhy O, Heeney J, Kasem A, et al. (2016) Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am J Surg 212: 969-981.
[Crossref] [Google Scholar] [PubMed]
- Chu KU, Turner RR, Hansen NM, Brennan MB, Bilchik A, et al. (1999) Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection?. Ann Surg 229: 536-541.
[Crossref] [Google Scholar] [PubMed]
- Dabakuyo TS, Fraisse J, Causeret S, Gouy S, Padeano MM, et al. (2009) A multicenter cohort study to compare quality of life in breast cancer patients according to sentinel lymph node biopsy or axillary lymph node dissection. Ann Oncol 20: 1352-1361.
[Crossref] [Google Scholar] [PubMed]
- Ersoy YE, Kadioglu H (2018) Review of novel sentinel lymph node biopsy techniques in breast cancer patients treated with neoadjuvant chemotherapy. Clin Breast Cancer 18: e555-e559.
[Crossref] [Google Scholar] [PubMed]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917.
[Crossref] [Google Scholar] [PubMed]
- Gherghe M, Bordea C, Blidaru A (2015) Sentinel Lymph Node Biopsy (SLNB) vs. Axillary Lymph Node Dissection (ALND) in the current surgical treatment of early stage breast cancer. J Med Life 8: 176-180.
[Google Scholar] [PubMed]
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, et al. (2009) Thresholds for therapies: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20: 1319-1329.
[Crossref] [Google Scholar] [PubMed]
- Hack TF, Cohen L, Katz J, Robson LS, Goss P (1999) Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol 17: 143-149.
[Crossref] [Google Scholar] [PubMed]
- Hwang RF, Gonzalez-Angulo AM, Yi M, Buchholz TA, Meric-Bernstam F, et al. (2007) Low loco regional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer 110: 723-730.
[Crossref] [Google Scholar] [PubMed]
- Jazayeri SB, Saadat S, Ramezani R, Kaviani A (2015) Incidence of primary breast cancer in Iran: Ten years national cancer registry data report. Cancer Epidemiol 39: 519-527.
[Crossref] [Google Scholar] [PubMed]
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, et al. (2007) Technical outcomes of sentinel lymph node resection and conventional axillary lymph node dissection in patients with clinically node negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8: 881-888.
[Crossref] [Google Scholar] [PubMed]
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, et al. (2010) Sentinel lymph node resection compared with conventional axillary lymph node dissection in clinically node negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11: 927-933.
[Crossref] [Google Scholar] [PubMed]
- Langer I, Guller U, Berclaz G, Koechli OR, Schaer G, et al. (2007) Morbidity of Sentinel Lymph Node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: A prospective Swiss multicenter study on 659 patients. Ann Surg 245: 452-461.
[Crossref] [Google Scholar] [PubMed]
- Li CZ, Zhang P, Li RW, Wu CT, Zhang XP, et al. (2015) Axillary lymph node dissection versus sentinel lymph node biopsy alone for early breast cancer with sentinel node metastasis: A meta-analysis. Eur J Surg Oncol 41: 958-966.
[Crossref] [Google Scholar] [PubMed]
- Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, et al. (2007) Surgical complications associated with Sentinel Lymph Node Dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American college of surgeons oncology group trial Z0011. J Clin Oncol 25: 3657-3663.
[Crossref] [Google Scholar] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, et al. (2006) Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC trial. J Natl Cancer Inst 98: 599-609.
[Crossref] [Google Scholar] [PubMed]
- Petrek JA, Pressman PI, Smith RA (2000) Lymphedema: Current issues in research and management. CA Cancer J Clin 50: 292-307.
[Crossref] [Google Scholar] [PubMed]
- Ram R, Singh J, McCaig E (2014) Sentinel node biopsy alone versus completion axillary node dissection in node positive breast cancer: Systematic review and meta-analysis. Int J Breast Cancer 2014: 513780.
[Crossref] [Google Scholar] [PubMed]
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, et al. (2003) A randomized comparison of sentinel node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349: 546-553.
[Crossref] [Google Scholar] [PubMed]
- Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, et al. (2010) Sentinel lymph node biopsy in breast cancer: Ten years results of a randomized controlled study. Ann Surg 251: 595-600.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Wu LC, Chen JQ (2011) Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: A meta-analysis. Breast Cancer Res Treat 129: 675-689.
[Crossref] [Google Scholar] [PubMed]
- Zahoor S, Haji A, Battoo A, Qurieshi M, Mir W, Shah M (2017) Sentinel lymph node biopsy in breast cancer: A clinical review and update. J Breast Cancer 20: 217-227.
[Crossref] [Google Scholar] [PubMed]
Citation: Foroutan MA, Moayeri H, Sabooni K, Ardeshiri MR (2023) Gamma Probe Assisted Axillary Lymph Node Biopsy Compared with Axillary Dissection in Breast Cancer. J Cancer Diagn 7: 184.
Copyright: © 2023 Foroutan MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1271
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1028
- PDF downloads: 243