Research Article Open Access
Galvanic Vestibular Stimulation for Camptocormia in Parkinson's Disease: A Case Report
| Yohei Okada1*, Yorihiro Kita2, Junji Nakamura2, Megumi Tanizawa2, Shigeru Morimoto2and Koji Shomoto1 | |
| 1Faculty of Health Science, Department of Physical Therapy, Kio University, Nara, Japan | |
| 2Department of Rehabilitation, Nishiyamato Rehabilitation Hospital, Nara, Japan | |
| Corresponding Author : | Yohei Okada Faculty of Health Science, Department of Physical Therapy Kio University, 4-2-2 Umami-naka, Koryo-cho Kitakatsuragigun, Nara, 635-0832, Japan Tel: +81-745-54-1601 Fax: +81-745-54-1600 E-mail: y.okada@kio.ac.jp |
| Received July 28, 2012; Accepted August 20, 2012; Published August 23, 2012 | |
| Citation: Okada Y, Kita Y, Nakamura J, Tanizawa M, Morimoto S, et al. (2012) Galvanic Vestibular Stimulation for Camptocormia in Parkinson’s Disease: A Case Report. J Nov Physiother S1:001. doi: 10.4172/2165-7025.S1-001 | |
| Copyright: © 2012 Okada Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Background: We explored the use of galvanic vestibular stimulation (GVS) as a tool of intervention for camptocormia in a patient with Parkinson’s disease. Methods: A 73-year–old man with a 13-year history of Parkinson’s disease presented with camptocormia. Binaural monopolar GVS was applied at 1.5 mA with the patient in the supine position for 20min. His trunk flexion angle during the standing position with eyes open and with eyes closed for 60 sec each was assessed before the GVS, after the GVS, and 1.5months after the GVS. Results: The patient’s trunk flexion angle while standing after the GVS was reduced, especially with eyes closed (by 25.2°; 55.8%) compared to that before the GVS. The patient reported that standing and sitting in his daily life were improved after the GVS, and the improvement continued up to approximately 1month after the GVS. His average trunk flexion angle while standing at the follow-up test conducted 1.5 months after the GVS was increased compared to that after the GVS, but was still smaller than that before the GVS. Conclusion: The results of this case report demonstrated significant improvement of the trunk forward flexion angle in a patient with Parkinson’s disease with camptocormia. Limitations and future research suggestions were identified.
| Keywords |
| Camptocormia; Parkinson’s disease; Galvanic vestibular stimulation (GVS) |
| Introduction |
| Camptocormia is an abnormal stooped posture often seen in patients with Parkinson’s disease. Camptocormia is defined as marked (minimum 45degrees) flexion in the sagittal plane originating in the thoracolumbar spine, and almost complete resolution in the spine position [1]. Camptocormia interferes with standing, mobility and eating. Possible mechanisms involved in the development of camptocormia are dystonia in abdominal muscles and paraspinal myopathy [1-4]. There is insufficient evidence to support the effectiveness of levodopa, deep brain stimulation, botulinum toxin injection, orthosis and physiotherapy for the correction of camptocormia [1,5-10]. |
| Vestibular function is impaired in patients with Parkinson’s disease who present lateral trunk flexion, as recently reported [11]. Pollak et al. [12] reported that vestibulocollic reflexes in patients with Parkinson’s disease were often absent unilaterally or bilaterally by investigating the vestibular evoked myogenic potentials triggered by intensive sound stimulation of saccular macula. However, Pollak et al. [12] did not examine the association between the absence of vestibulocollic reflexes and abnormal postures in patients with Parkinson’s disease. We hypothesized that vestibular function may be impaired in patients with Parkinson’s disease with camptocormia. |
| Galvanic vestibular stimulation (GVS) is a method used to stimulate the vestibular system [13-15]. Electrodes are attached to the mastoids behind the subject’s ears in order to stimulate the vestibular system. Stimulation with two electrodes of different polarities placed behind the mastoids (termed “bilateral bipolar GVS”) induced the sway response of the pelvis, trunk and head toward the side of the anode electrode, and stimulation with two electrodes of the same polarity on both mastoids and remote electrodes of the other polarity is termed “binaural monopolar GVS” [13-15]. When the polarity of the mastoid electrodes is cathode and that of the electrodes on the trapezius muscles at the C7 level, the subject’s body is induced to sway backward [16]. |
| GVS has been used primarily as a research tool to investigate vestibular function. It has rarely been used clinically, but it was employed as an intervention tool for neuropsychological disorders that include a spatial component [17-19]. There are only a few reports about GVS in patients with Parkinson’s disease [20,21]. Pastor et al. [20] who used GVS as a research tool reported that there were no significant difference in GVS induced response of ground reaction force between patients with Parkinson’s disease and healthy controls. Pal et al. [21] reported that low intensity stochastic GVS did not reduce postural sway during the stimulation in normal participants, but slightly reduce postural sway in the stance condition with eyes closed in patients with Parkinson’s disease. There have been no reports about the effects of GVS on postural abnormalities in patients with Parkinson’s disease. The purpose of this case report is to describe our test of whether binaural monopolar GVS (which induces backward sway) would improve the trunk flexion angle in a patient with Parkinson’s disease with camptocormia. |
| Methods |
| Case presentation |
| The patient was a 73-year-old male outpatient treated at Nishiyamato Rehabilitation Hospital. He had been diagnosed with Parkinson’s disease thirteen years earlier; his Hoehn and Yahr stage was 3. His total Unified Parkinson’s Disease Rating Scale (UPDRS) motor subscore was 33, the subscore for rigidity (item 22) was 9, the subscore for akinesia (items 23–26) was 12, the subscore for tremor (items 20, 21) was 0, and the subscore for postural instability (item 30) was 1. He was on Carbidopa-Levodopa 100 mg six times a day, pramipexole 0.5 mg twice a day, selegiline 2.5 mg twice a day, zonisamide 25 mg once a day, trihexyphenidyl 2 mg once a day and droxidopa 100 mg three times a day. The antiparkinson drugs remained unchanged one year before and during the study. The patient had not received brain surgery such as deep brain stimulation, and he had no history of musculoskeletal or vestibular disorders. Written informed consent was obtained from the patient, and the study protocol was approved by the ethical committee of Kio University. |
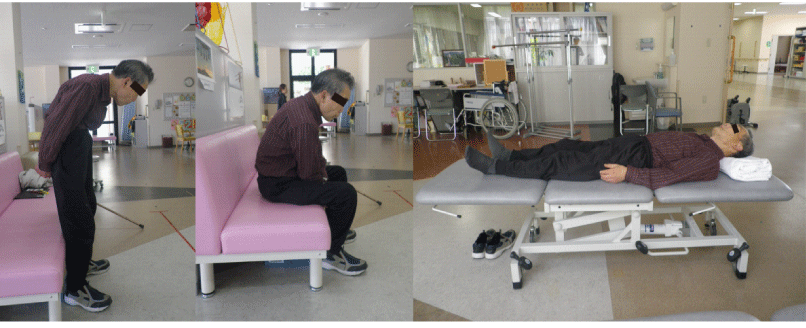
| The patient presented in July 2011 with camptocormia and for the previous 8 months. He exhibited marked (>45°) thoracolumbar flexion during standing or walking, but the flexion was almost completely resolved in the supine position (Figure 1). He reported a sensation of tightening in his abdomen during standing. The patient’s camptocormia interfered with his mobility, vision and eating; in fact, his chief complaint was the camptocormia. He had been undergoing antiparkinson drug treatment and conventional physical therapy including stretching exercises and strengthening exercises for the prior 8 months to improve his trunk control, but his camptocormia had not changed at all. |
| Galvanic vestibular stimulation |
| Binaural monopolar GVS was applied using an electrical stimulation system (Chattanooga Intelect Advanced Combo, DJO Global, Vista, CA, USA) with the patient in the supine position. Two electrode pairs were located with the cathode electrode of each pair over the mastoid process and the anode electrode over the trapezius muscle on the same side. This allocation of the electrodes was used to induce posterior sway in the standing position as in a previous study [16]. The skin behind the ears was rubbed with alcohol to reduce impedance. During the GVS, the current intensity was ramped up until 1.5mA was reached and ramped down at the end of stimulation. The duration of the GVS was 20min, and the intensity and duration of the GVS adhered to the safety criteria for transcranial direct current stimulation [22,23]. The GVS was conducted in just one session. |
| Assessment |
| The patient’s sagittal standing posture was captured by a digital video camera (Optico P80, Pentax, Tokyo, Japan) from the side at 30Hz. The digital video camera was set 5m away from the standing point. The patient stood for 60 sec in two conditions (eyes open, eyes closed). He was instructed to stand with his feet 10cm apart and his arm lying along the trunk. A frame-by-frame video-based analysis was performed to assess the patient’s forward flexion angle during static standing posture. The trunk forward flexion angle was calculated as the angle formed between the line joining the marker positioned on the C7 spinous process and the midpoint of the right and left posterior superior iliac spines and a vertical reference line every 30 frames and averaged. The patient’s average trunk forward flexion angle during the static standing position was assessed before the GVS, immediately after the GVS and 1.5months after the GVS. |
| Results |
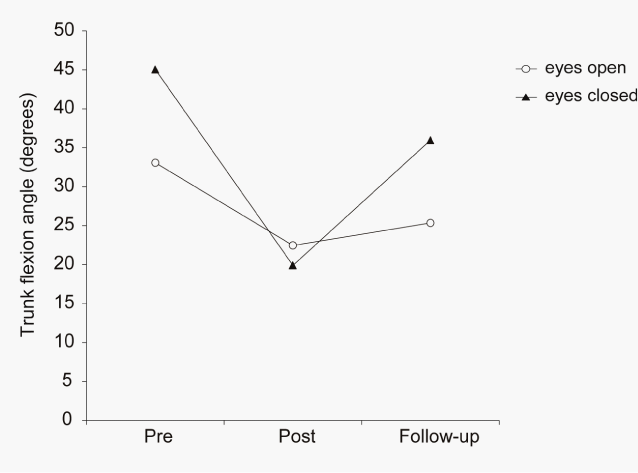
| Before the GVS, the patient’s trunk flexion angle during the standing position with eyes closed was larger than that with eyes open, by 12.0° (26.6%). The patient completed the GVS. He complained of slight nausea immediately after the GVS, but it was resolved within 1hour after the GVS. No other side effect was observed. Immediately after the GVS, the patient’s standing posture was markedly improved (Figure 2). His average trunk forward flexion angle during the standing position with eyes open was reduced by 10.6° (32.1%) immediately after the GVS compared to the angle before the GVS. The angle during the standing position with eyes closed was markedly reduced by 25.2° (55.8%) immediately after the GVS compared to the angle before the GVS. In weekly telephone surveys, the patient reported that his standing and sitting positions in daily life were improved after the GVS and that the improvement continued up to 1month after the GVS. At the follow-up test conducted 1.5 months after the GVS, the patient’s average trunk forward flexion angles while standing with eyes closed and open were increased compared to those immediately after the GVS, but they were still smaller than those before the GVS (Figure 3). |
| Discussion |
| Our patient’s trunk forward flexion angle while standing with his eyes closed was larger than that with his eyes open. This phenomenon indicated that his visual dependence in keeping his posture upright was increased, consistent with previous studies concerning about the visual dependence of posture and locomotion in patients with Parkinson’s disease [24]. Therefore, increased visual dependence in postural control might be characteristic of patients with Parkinson’s disease in general. |
| Postural control depends on vestibular, visual, and somatosensory information. Increased visual dependence in patients with Parkinson’s disease has recently been interpreted as an adaptive strategy to compensate for impaired proprioception [25,26]. Increased visual dependence in the case suggests that the processing of vestibular and/or somatosensory information might be impaired. This does not conflict with our hypothesis that vestibular function is impaired in patients with Parkinson’s disease with camptocormia. Vestibular function was not assessed quantitatively in the present study, and thus the existence of vestibular dysfunction was not clarified. Quantitative assessments of vestibular function and further investigations examining the association between vestibular dysfunction and camptocormia are needed. |
| GVS markedly improved our patient’s camptocormia while standing. During the binaural monopolar GVS applied in this study, the cathode electrode of each pair was over the mastoid process and the anode electrode was over the trapezius muscle at the C7 level on the same side. The GVS applied to our patient with camptocormia may have activated both sides of the vestibular afferents to induce posterior body sway, even after the end of the GVS. The effects of GVS on the patient’s camptocormia were seen during the standing position with eyes closed, in particular. The results suggest that GVS might improve postural control by contributing to the processing of vestibular and somatosensory information. Although a quantitative assessment of the patient’s standing posture was not done, he reported in weekly telephone surveys that his standing and sitting in daily life were improved and the improvement continued up to 1 month after the GVS. In light of other such reports by patients as “Standing and sitting positions in daily life were improved after the GVS and that the improvement continued up to 1 month after the GVS”, GVS may induce a plastic change in postural control. Our patient’s camptocormia worsened again 1.5 months after the GVS, although it was still better than before the GVS. The optimal intervention frequency for GVS should be examined in a future study. |
| The results of this case report demonstrated significant improvement of the trunk forward flexion angle in a patient with Parkinson’s disease with camptocormia. GVS has the potential to be a novel intervention in physiotherapy to improve camptocormia in patients with Parkinson’s disease. Therefore the feasibility and safety of GVS need to be fully identified. Our patient complained of slight nausea after undergoing GVS, but it eased within 1hour, and no other side effect was observed. The intensity and duration of the GVS were set at 1.5mA for 20min, in accord with the safety criteria for transcranial direct current stimulation [22,23]. However, it is possible that the intensity and duration of GVS was not suitable for our patient and the optimal intensity and duration of GVS should be explored in future studies. |
| The limitations of this study are as follows: first, there was no sham condition, and therefore the possibility of a placebo effect cannot be excluded. Placebo effects in patients with Parkinson’s disease were reported previously [27]. Comparisons of the effects of GVS and sham conditions are needed. A second limitation is the small sample size; a large randomized controlled trial is necessary to clarify the effects of GVS. Third, the mechanism of the GVS-induced improvement of camptocormia was not clarified. Quantitative evaluations of subjects’ trunk muscle activity while standing and their vestibular function are needed to clarify the possible mechanisms of the GVS-induced improvement of camptocormia. |
| Conclusion |
| The results of this case report demonstrated that GVS could improve trunk flexion angles in a patient with Parkinson’s disease with camptocormia. Our present findings suggest that GVS has the potential to be a novel intervention in physiotherapy to improve camptocormia in patients with Parkinson’s disease. Limitations and future research suggestions were identified. |
| Acknowledgements |
| We thank the patient for his cooperation and his consent to the use of both information about his treatment and the photographs in this article. |
References
- Doherty KM, van de Warrenburg BP, Peralta MC, Silveira-Moriyama L, Azulay JP, et al. (2011) Postural deformities in Parkinson's disease. Lancet Neurol 10: 538-549.
- Djaldetti R, Mosberg-Galili R, Sroka H, Merims D, Melamed E (1999) Camptocormia (bent spine) in patients with Parkinson's disease--characterization and possible pathogenesis of an unusual phenomenon. Mov Disord 14: 443-447.
- Sawek J, Derejko M, Lass P (2003) Camptocormia as a form of dystonia in Parkinson's disease. Eur J Neurol 10: 107-108.
- Laroche M, Cintas P (2010) Bent spine syndrome (camptocormia): a retrospective study of 63 patients. Joint Bone Spine 77: 593-596.
- Lepoutre AC, Devos D, Blanchard-Dauphin A, Pardessus V, Maurage CA, et al. (2006) A specific clinical pattern of camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 77: 1229-1234.
- Ho B, Prakash R, Morgan JC, Sethi KD (2007) A case of levodopa-responsive camptocormia associated with advanced Parkinson's disease. Nat Clin Pract Neurol 3: 526-530.
- Sako W, Nishio M, Maruo T, Shimazu H, Matsuzaki K, et al. (2009) Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson's disease. Mov Disord 24: 1076-1079.
- Yamada K, Goto S, Matsuzaki K, Tamura T, Murase N, et al. (2006) Alleviation of camptocormia by bilateral subthalamic nucleus stimulation in a patient with Parkinson's disease. Parkinsonism Relat Disord 12: 372-375.
- Azher SN, Jankovic J (2005) Camptocormia: pathogenesis, classification, and response to therapy. Neurology 65: 355-359.
- de S√?¬®ze MP, Creuz√?¬© A, de S√?¬®ze M, Mazaux JM (2008) An orthosis and physiotherapy programme for camptocormia: a prospective case study. J Rehabil Med 40: 761-765.
- Vitale C, Marcelli V, Furia T, Santangelo G, Cozzolino A, et al. (2011) Vestibular impairment and adaptive postural imbalance in parkinsonian patients with lateral trunk flexion. Mov Disord 26: 1458-1463.
- Pollak L, Prohorov T, Kushnir M, Rabey M (2009) Vestibulocervical reflexes in idiopathic Parkinson disease. Neurophysiol Clin 39: 235-240.
- Fitzpatrick RC, Day BL (2004) Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96: 2301-2316.
- Utz KS, Dimova V, Oppenl√?¬§nder K, Kerkhoff G (2010) Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology--a review of current data and future implications. Neuropsychologia 48: 2789-2810.
- Day BL, S√?¬©verac Cauquil A, Bartolomei L, Pastor MA, Lyon IN (1997) Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol 500 : 661-672.
- Bortolami SB, Inglis JT, Castellani S, DiZio P, Lackner JR (2010) Influence of galvanic vestibular stimulation on postural recovery during sudden falls. Exp Brain Res 205: 123-129.
- Wilkinson D, Nicholls S, Pattenden C, Kilduff P, Milberg W (2008) Galvanic vestibular stimulation speeds visual memory recall. Exp Brain Res 189: 243-248.
- Saj A, Honor√?¬© J, Rousseaux M (2006) Perception of the vertical in patients with right hemispheric lesion: effect of galvanic vestibular stimulation. Neuropsychologia 44: 1509-1512.
- Utz KS, Keller I, Kardinal M, Kerkhoff G (2011) Galvanic vestibular stimulation reduces the pathological rightward line bisection error in neglect-a sham stimulation-controlled study. Neuropsychologia 49: 1219-1225.
- Pastor MA, Day BL, Marsden CD (1993) Vestibular induced postural responses in Parkinson's disease. Brain 116: 1177-1190.
- Pal S, Rosengren SM, Colebatch JG (2009) Stochastic galvanic vestibular stimulation produces a small reduction in sway in Parkinson's disease. J Vestib Res 19: 137-142.
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, et al. (2005) Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 64: 872-875.
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, et al. (2003) Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol 56: 255-276.
- Azulay JP, Mesure S, Amblard B, Pouget J (2002) Increased visual dependence in Parkinson's disease. Percept Mot Skills 95: 1106-1114.
- Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP (2007) Impaired vertical postural control and proprioceptive integration deficits in Parkinson's disease. Neuroscience 146: 852-863.
- Vaugoyeau M, Hakam H, Azulay JP (2011) Proprioceptive impairment and postural orientation control in Parkinson's disease. Hum Mov Sci 30: 405-414.
- Goetz CG, Leurgans S, Raman R, Stebbins GT (2000) Objective changes in motor function during placebo treatment in PD. Neurology 54: 710-714.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 11286
- [From(publication date):
specialissue-2012 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 6601
- PDF downloads : 4685
