Review Article Open Access
Fungi, Bacteria, Nano-particulates, Mycotoxins and Human Health in Water-Damaged Indoor Environments
Jack Dwayne Thrasher*
Advisory Committee, Chemical Impact Project, Tides Foundation, MillValley, CA, United States
- *Corresponding Author:
- Jack Dwayne Thrasher, Ph.D
Advisory Committee
Chemical Impact Project
Tides Foundation, MillValley
CA, United States
Tel: 575-937-1150
Fax: 916-827-2520
E-mail: toxicologist1@msn.com
Received date: February 29, 2016 Accepted date: March 03, 2016 Published date: March 10, 2016
Citation: Thrasher JD (2016) Fungi, Bacteria, Nano-particulates, Mycotoxins and Human Health in Water-Damaged Indoor Environments. J Comm Pub Health Nurs 2:115. doi:10.4172/2471-9846.1000115
Copyright: © 2016 Thrasher JD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community & Public Health Nursing
Abstract
Nine types of biocontaminants in damp indoor environments from microbial growth are discussed: (1) indicator molds; (2) Gram negative and positive bacteria; (3) microbial particulates; (4) mycotoxins; (5) volatile organic compounds, both microbial (MVOCs) and non-microbial (VOCs); (6) proteins; (7) galactomannans; (8) 1-3-b-Dglucans (glucans) and (9) lipopolysaccharides LPS (endotoxins). When mold species exceed those outdoors contamination is deduced. However, there are no current recommendations by the EPA, OSHA, NIOSH, WHO and the Medical and Toxicology professions as to what constitutes a safe level of indoor molds and bacteria and their toxins in a water-damaged indoor environment. The thrust of his review is to discuss the role of fungi and their toxins on the health of occupants of damp indoor spaces.
Keywords
Bacteria; Fungi; Nano-particulates; Mycotoxin
Introduction
Damp or wet building materials occur from a variety construction defects, roof leaks, HVAC condensation, water intrusion from floods, hurricanes, leaking appliances and plumbing, poorly designed foundations, e.g. basement walls that allow water seepage from wet soils, slope of the building lot leading to water accumulation under concrete slabs. We have been involved home with cracked cement slabs, bent aluminium window framing, highly contaminated wall cavities, poorly installed roofing, improperly sealed fireplaces, to mention a few. All of these situations lead to both hidden as well visual signs of fungal and bacterial growth. For simplicity, “water intrusion” will be used as all encompassing term [1-18].
Signs of Microbial Growth
Signs of water intrusion include, but are not necessarily limited to: (1) water stains on ceilings, walls and around windows; (2) increased moisture content using a moisture meter with penetrating electrodes on dry wall (e.g. wall cavities), drawl space, attics and carpeting; (3) visible fungal growth on surface of dry wall, insulation, e.g. crawl space attic, clothing, shoes and other wearing apparel, bedding, under side of carpeting; (4) musty odor from microbial volatile organic compounds; (5) The E.P.A. cautions that approximately 50% of the fungal growth can be hidden, therefore hidden from view. The identification of airborne mold spores only reveals what is present at the time of testing, not 24/7. Airborne mold testing does not necessarily reveal hidden mold, e.g. wall cavities, attic, under carpeting, ventilation ducts.
Water activity
Water concentration of substrate upon which fungi and bacteria grow dictates the species of fungi that are present. Thus, the water concentration is defined as water activity (aw) as follows: Water activity is the partial vapour pressure of water in a substance divided by the standard state partial vapour pressure of water. The standard state is most often defined as the partial vapour pressure of pure water at the same temperature. Using this particular definition, pure distilled water has a water activity of exactly one. Higher aw substances tend to support more microorganisms. Bacteria usually require at least 0.91+, and fungi at 0.7 ≥ 0.95. The fungi that are present at different water concentrations are listed in Table 1.
| Fungi Growing on Building Materials and Their aw | |||
|---|---|---|---|
| Colonizer Group | aw Range | Classification | Fungal Example |
| Primary Colonizers ( storage fungi) | <0.80 | Xerophilic/Xerotolerant | Penicilliumchrysogenum, and Aspergillusversicolor: the most common ones;A. fumigatus, niger, sydowii, ustus, Eurotium spp., P. brevicompactum, commune, corylophilum, palitans, variotta, PaecilomycesWallemiasebi |
| Secondary Colonizers | 0.80-0.9 | Mesophilic | Alternariaspp,, Cladosporium spp., Epicoccumnigrum, Phoma spp., and Ulocladium spp. |
| Tertiary Colonizers | >0.9 | Hydrophilic | Chaetomiumglobosum, Fusariam, Memnoniellaechinata, Rhizopusstolonifer, Stachybotryschartarum, Trichoderma spp. (T. atroviride, T. citrinoviride, T. harzianum, and T. longibrachiatum) |
Table 1: This table lists the species of fungi and their water activity requirements for growth.
Xerophilic (aw < 0.8)
Species of Aspergillus and Penicillium, and Eurotium amstelodami and other fungi grow at this water activity. Several of these species produce mycotoxins that include Ochratoxin A , Aflatoxins , Sterigmatocystin , Aflatoxins and Gliotoxin . Some species of Aspergillus (fumigatus, niger, terreus and flavus are potential human pathogens, particularly A. fumigatus .
Mesophilic (aw 0.8-0.9)
Species of Alternaria , Cladosporium , Phoma and Ulocladium as well as Epicoccum nigrum . This group of fungi also produces mycotoxins, but they are not as toxic as those produced by the xerophilic and hydrophilic fungi.
Hydrophilic (aw >0.9)
The fungi at this water concentration in include Chaetomium globosum , species of Fusariam, Trichoderma, Stachybotrys chartarum , Memnoniella echinata and Rhizopus stolonifer . Trichothecenes are produced by Fusariam , Trichoderma , and Stachybotrys and Memnoniella , while Chaetomium produces chaetoglobosins A and C. All of these mycotoxins are very toxic to humans and animals.
Bacteria
A variety of bacteria have been identified in water damaged indoor environments. They require aw of ≥ 0.95. They include several species Gram positive Bacilli and Cocci (Streptococcus, Micrococcus, and Staphylococcus ) as well as Gram negative bacteria. Several of these bacteria are pathogens, while the Gram negative bacteria release endotoxins into the indoor environment. Other bacteria that have been identified in water-damaged indoor environment are the Actinobacteria: species of Streptomyces, Mycobacterium and Nocardia . The Actinobacteria produce exotoxins, e.g. Valinomycin, a mitochondrial poison. The nontuberculin Mycobacteria can cause Mycobacterium Avium Complex (MAC) in humans as reviewed by Griffin et al and published on the website of the American Thoracic Society
Particulates
Particulates shed from molds include spores, fragments of mycelia and nano-particulates. Of the mold particulates the greatest concern is the nano-particulates at or less than 0.3 microns shed from mold colonies. Field studies of water-damaged homes have shown concentrations of nano-particulates in indoor dust that are at least 1000 times or greater than the indoor air mold spore counts [12-18]. These particulates contain 1, 3-beta glucans, a variety of fungal proteins that include substrate enzymes as well as mycotoxins.
The translocation of nano-particulates with their attached toxins occurs by two mechanisms: the nervous system and the surfactants of the alveoli. The nano-particulates enter the surfactants of the lungs and are then transported across the alveolar cell membranes and enter the systemic circulation. In the alveoli they are taken up by alveolar macrophages and alveolar Type I cells. They cause the generation of reactive oxygen species and nitrogen species, release of proinflammatory cytokines and injury to nuclear DNA. Morphological changes include emphysema and granulomatous and fibrotic lesions [19-21]. The other mode of transportation is via the olfactory neurons. Nano-particulates attach to the nasal mucosa and are transported up the olfactory nerve through the cribiform plate and enter the hypothalamus/pituitary axis and spreading throughout the brain [22-25].
Mycotoxins
Mycotoxins produced by various fungi have been identified in damaged building materials, wall cavities, and dust samples from HVAC ducts (return and supply air) and refrigerator compressor (Table 2).
| Home I.D. | HVAC & Refrigerator | Aflatoxins | Ochratoxin A | Trichothecenes |
|---|---|---|---|---|
| 1 | Supply Air (Mstr bath) | 0 | 0 | 2.659 |
| Supply Air (Bdrm) | 0 | 0 | 0.109 | |
| Supply Air (Off/Den) Refrigerator | 0 | 0 | 4.082 | |
| Coil Dust | 0 | 0 | 1.36 | |
| 2 | Return Air | 0 | 0.04 | 1.15 |
| Supply air (MstrBdrm) | 0 | 0.63 | 2.634 | |
| 3 | Return Air | 0 | 0 | 0.491 |
| Supply Air (MstrBdrm) | 0 | 0 | 0.305 | |
| Refrigerator Compressor | 0 | 0 | 2.652 | |
| 4 | Return Air | 0 | 0 | 0.661 |
| 5 | Return Air | 0 | 0 | 7.417 |
| 6 | Return Air | 0 | 0 | 0.257 |
| 7 | Return Air | 0 | 0.5 | 7.753 |
| 8 | HVAC Supply Air | 0 | 0 | 26.511 |
| 9 | Refrigerator Coil Dust | 0 | 0.7 | 0.86 |
Table 2: HVAC ducts, Refrigerator compressor and HVAC Air Filter. This table summarizes the concentrations of mycotoxins in ppb detected in HVAC ducts, dust from the refrigerator compressor and one home with a dirty filter in the intake of the HVAC duct.
The fungi present in the dust samples were identified by ERMI-36 and are listed in Table 3. Stachybotrys chartarum was identified in all dust samples along with other mycotoxin producing fungal species (work in progress).
| Home I.D | Mold Species identified by PCR-DNA IERMI-36 Tests |
|---|---|
| 1 | Aspergillusfumigatus, versicolor, penicillioides, niger, restrictus;Eurotiumamstelodami; Stachybotryschartarum; Chaetomiumglobosum; Aureobasidiumpullulans, Paecilomycesvariotta; Penicillium variable; Trichodermaviride; Wallemiasebi; Scopulariopsisbrevicaulis |
| 2 | Aspergillusfumigatus, restrictus, niger;Cladosporiumsphaerospermum; Aureobasidiumpullulans; Scopulariopsischartarum |
| 3 | Aspergillusfumigatus, restrictus, niger,Cladosporiumsphaerospermum; Eurotiumamstelodami; Penicilliumpurpurogenum; Stachybotryschartarum; Aureobasidiumpullulans; Wallemiasebi |
| 4 | Aspergillusfumigatus, penicillioides;Stachybotryschartarum; Cladosporiumsphaerospermum; Penicilliumbrevicompactum; Wallemiasebi |
| 5 | Aspergillusfumigatus, penicillioides;Cladosporiumsphaerospermum; Stachybotryschartarum;Scopulariopsischartarum; Paecilomycesvariotta; Scopulariopsischartarum; Penicilliumbrevicompactum |
| 6 | Aspergillusflavus, niger, sydowii, unguis, Chaetomiumglobosum; Cladosporiumsphaerospermum; Paecilomycesvariotta; Scopulariopsischartarum; Stachybotryschartarum |
| 7 | Aspergillusfumigatus, restrictus, penicillioides, flavus, niger;Stachybotryschartarum; Aureobasidiumpullulans; Paecilomycesvariotta; Wallemiasebi |
| 8 | Aspergillusflavus, fumigatus, niger,Ochraceuspenicillioides, ustus, versicolor; Eurotiumamstelodami; Penicilliumchrysogenum; Scopulariopsischartarum; Stachybotryschartarum; Wallemiasebi |
| 9 | Aspergillusfumigatus, ochraceus, versicolor, niger, Stachybotryschartarum; Eurotiumamstelodami; Aureobasidiumpullulans; Penicilliumcrustosum, Scopulariopsischartarum; Trichodermaviride; Wallemiasebi |
Table 3: This table summarizes the indicator species of molds identified by ERMI-36 in the dust taken from various sources in Table 1. Only the species of molds in the highest concentration are listed. Note that although Stachybotrys chartarum was not detected in home I.D. 2, it is noted that it does not readily shed its spores unless its colonies are disturbed or desiccated.
Mycotoxins and molds have been identified in urine samples from patients with ME/CFS and in autopsy materials from deceased individuals exposed to fungi in their water-damaged homes [25-34]. Three of the case studies will be briefly reviewed below. Tables 4 and 5 are from reference 30. The family of five
Tables 4 and 5 are from reference 30. The family of five (parents and 3 children) was exposed to molds and mycotoxins. The father and a daughter developed fungal sinusitis requiring surgical removal. The mother was pregnant during the first and second trimesters of the third child The family moved out of the home during her third trimester. The infant was born with a total body flare, developed Neurofibromatosis Type I, resulting in at least over 90 skin pigment blotches over her body. There was no family history of NFT1. The pet dog developed over 90 skin lesions predominantly lipomas. Family symptoms included: persistent coughing, throat irritation, headaches, severe fatigue, decreased concentration and memory, nose bleeds, loss of libido (parents), shortness of breath with wheezing, Trichothecenes ochratoxin were detected in samples of dust and other samples from various areas of the home (Table 4).
| Sample | Trichothecenes (ppb) | Aflatoxins(ppb) | Ochratoxin A(ppb) |
|---|---|---|---|
| Towel Master Bath | 11.7 | NP | 4.9 |
| Sandal Master Bdrm | 0.47 | NP | 3.4 |
| Wood Trust-Crawl Space | 1.68 | 3.5 | 5.8 |
| Gravel-Crawl Space | 7.7 | NP | 7.7 |
| Dirt-Crawl Space | 2.1 | NP | 2.1 |
| Plastic Sheet-Crawl Space | NP | NP | 2.8 |
Table 4: This table summarizes the detection of trichothecenes, aflatoxins and ochratoxin A present in bulk samples taken from the master bath, master bedroom (sandal) and crawl space. The reported date are in ppb per mycotoxin, NP: Not Present, Limit of Detection: Trichothecenes (0l2 ppb); Aflatoxins (1.0 ppb); Ochratoxin A (2.0 ppb).
Mycotoxins were present in nasal secretions, urine, mothers breast milk, placenta and umbilical cord. The dog had trichothecenes in its urine and skin lesions. The urine of the newborn was negative for mycotoxins. However, the presence of mycotoxins in the placenta, breast milk and umbilical cord most likely are indicative of fetal exposure (Table 5).
| Specimen | Trichothecenes | Aflatoxins | Ochratoxin Z |
|---|---|---|---|
| Urine - Father | NP | NP | 18.2 |
| Nasal Secretion-Father1 | NP | 0.5 | 13 |
| Urine - Mother | NP | NP | 18.2 |
| Nasal Secretion - Mother | 1,02 | 1.2 | 1.6 |
| Urine- Daughter | 0.23 | NP | 28.0 |
| Nasal Secretion- Daughter2 | 4.68 | NP | 3.8 |
| Urine - Son | 0.2 | NP | 18.9 |
| Nasal Secretion - Son | ND | ND | ND |
| Breast Milk | 0.18 | 0.9 | 2.7 |
| Placenta | NP | NP | 4.2 |
| Umbilical Cord | NP | NP | NP |
| Urine - New Born | NP | NP | NP |
| Urine - Dog | 1.49 | NP | 25.9 |
| Ear Mass - Dog | 23.07 | 0 | 2.2 |
| Liooma - Dog | 20.9 | 0 | 1.4 |
Table 5: Mycotoxins present in body fluids and tissues of the family and pet dog. The newborn’s urine sample was negative with respect to mycotoxins. It is noted that the amniotic fluids were lost with the birth of the baby, Limits of Detection: Trichothecenes (0.2 ppb); Aflatoxins (1.0 ppb) Ochratoxin (2.0 ppb), ND – Not Done, NP – Not Present, 1: Pseudomonas aureoginosa and Penicillium were cultured from the nasal secretions. These data represent two different tests, 2: Acinetobacter sp and Aspergillus fumigatus were cultured left sphenoid sinus surgical specimens.
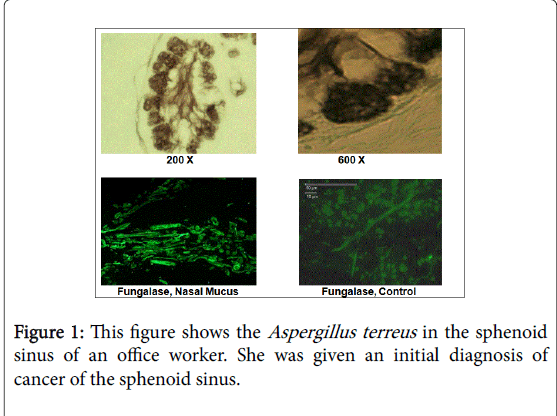
Figure 1 and Table 6 are from reference 33. This case involved a 55 year old woman who was exposed to molds in a water-damaged office building. She developed a mass in her sphenoid sinus that lead to severe headaches and fatigue. Diagnostic imaging revealed a mass that six different pathologists diagnosed as a malignancy. Sphenoid surgery, radiation, chemotherapy and radiation did not remedy the situation. Slides of the biopsy were sent to Dr. Dumanov (one of the authors). He performed differential. staining that revealed fungal growth.
| Date | Aflatoxins (ppb)1 | Trichothecenes (ppb) |
|---|---|---|
| 3/27/2007 | 21 | 1.51 |
| 3/11/2007 | 5 | 0.53 |
| 6/3/2007 | 4 | 1.38 |
| 7/10/2007 | 12 | 3.44 |
| 7/14/2007 | 9 | 0.73 |
| 8/21/2007 | 5 | 21.3 |
| 12/12/2007 | 20 | 2.9 |
Table 6: This table summarizes the mycotoxins detected in the urine on several dates after removal of the fungal aspergilloma. The data show that detoxification probably results from the liberation of stored mycotoxins, Limit of Detection Aflatoxins (1.0 ppb)’ Trichothecenes (0.2 ppb).
PCR-DNA test identified Aspergillus terreus . She was then seen by Dr. Gray, Benson, Arizona, He treated intranasally with voriconazole and cyclosporine. MRI imaging demonstrated that the mass had resolved. During the antifungal treatment her urine was tested for mycotoxins.
Tables 7-9 are from reference 34. This case study is a family of five (parents and three daughters. Prior to renting a home the health history consisted of common colds and annual flu. Upon occupying a water-damaged home all became ill with a variety of symptoms. The most concerning to the family was the presence of persistent ME/CFS. ERMI-36 testing of dust samples from the refrigerator compressor/ insulation dust identified species of Aspergillus, Penicillium, Trichoderma viride , Chaetomium globosum and Stachybotrys chartarum (Table 7). Ochratoxin And trichothecenes were identified in the dust sample from the refrigerator and in urine specimens from all family members (Table 8). The diagnosis of ME / CFS was confirmed by sleep monitoring, demonstrating periods of waking, restless and unrefreshing sleep. In addition, a variety of Gram negative and positive bacteria were identified in a dust sample taken from the master bead spread at 8,400,000 CFU/g (data not shown). Thus, bacteria must also be considered as contributing factors in the ME / CFS.
| Fungal Species | Number of Spores/mg of dust |
|---|---|
| Aspergillusflavus | 8 |
| Aspergillusfumigatus | 33 |
| Aspergillusversicolor | 78 |
| Aspergillusochraceus | 151 |
| Aspergillusniger | 33 |
| Aspergillussydowii | 10 |
| Eurotiumamstelodami | 785 |
| Penicilliumpurpurogenum | 7 |
| Aureobasidiumpullulans | 114 |
| Penicilliumcorylophilum | 27 |
| Penicilliumcrustosum | 1,946 |
| Scopulariopsischartarum | 44 |
| Trichodermaviride | 12 |
| Wallemiasebi | 25 |
| Chaetomiumglobosum | 3 |
| Stachybotryschartarum | 1 |
Table 7: This table summarizes the results of the ERMI-36 test performed on the dust sample taken from the area of the refrigerator compressor and insulation. The species of each mold is given as the number of spores per milligram of dust.
| Sample I.D. | Aflatoxin | Ochratoxin | Trichothecene |
|---|---|---|---|
| Refrigerator Dust | 0 | 0.7 | 3.86 |
| Urine (Father) 32 y | 0 | 1.4 | 0.81 |
| Urine (Mother) 31 y | 0 | 2.1 | 1.26 |
| Daughter 15 y | 0 | 2.8 | 0.91 |
| Daughter12 y | 0 | 1.4 | 0.97 |
| Daughter 5 y | 0 | 1.4 | 0.22 |
Table 8: This table summarizes the mycotoxins identified in the dust sample from the refrigerator compressor area and in the urine of the five occupants of the house. The concentrations are in ppb, Urine Ochratoxin References: < 1.8 ppb (negative), 1.8-2.0 (equivocal, 2.0 ppb (positive)). Trichothecene References: ≥ 0.2 ppb (positive).
| Event | Father, 36 y | Mother,34 y | Daughter, 17 y | Daughter, 15y | Daughter, 7 y |
|---|---|---|---|---|---|
| Sleep Minutes | 477±62.5 | 508.2±68.1 | 407±67.7 | 431.6±45.9 | 551.4±62.2 |
| Times Awake | 3±1.6 | 3.4±1,3 | 2.2±1.3 | 0.8±1.3 | 1.5±1.5 |
| Restless | 19,3±3.2 | 19.6±9.5 | 13±6.5 | 9,8±5.3 | 14.8±6.2 |
| Un-refreshing Sleep | 42.8±6.7 | 38±20.8 | 25.2±18.4 | 32.6±12.2 | 26.6±12.4 |
Table 9: This table summarizes the results of sleep monitoring events in each family member using FitBit Surge monitor.
Discussion
A short review of the literature
Three case studies have been briefly reviewed with respect to chronic illness resulting from water-damage and fungal biocontaminants. However, the peer-reviewed literature is replete with published papers regarding adverse health effects on occupants in, water-damaged indoor environments with demonstrable fungal and bacterial growth. These include, but are not necessarily limited to the following:.
Upper and lower respiratory infections, bronchitis and lung disease
The health effects include infections, asthma, and hypersensitivity pneumonitis [7-10,35-40].
Fungal sinusitis
Fungi as well as bacteria in damp indoor environments do cause sinusitis as well as adversely affecting the endocrine functions of the hypothalamus/pituitary axis. In addition, intracranial invasion occurs in immunocompetent patients [41-47].
Sarcoidosis
This is systemic inflammatory illness that can affect one or all organs. It is associated with nano-particulates shed by mold that contain 1, 3-beta glucans, mycotoxins and a variety of antigenic proteins [14,21,25,48-55].
Nervous system
Neurological damage includes the following: decrease in short and long memory in adults and children, autistic spectrum disorder in young children, peripheral neuropathy, loss of balance, facial pain, glossopharengeal neuralgia, head and neck myalgias, movement disorders, and decreased visual acuity [56-64].
Kidney disease
Kidney disease has been associated with exposure to Ochratoxin A produced by several; species of Aspergillus. In European Asian counties it is referred to as endemic Balkan nephropathy associated with oral consumption of affected foods. In addition, a recent review of Ochratoxin A has associated inhalational exposure to kidney disease, including focal segmental glomerulosclerosis [65,66].
Conclusion
Exposure to water-damaged indoor environments and subsequent fungal and bacterial growth leads to a variety of symptoms that are often overlooked by the medical profession. Most likely this results from the fact that a medical doctor with a busy practice has not kept up with the peer reviewed literature on this subject. Often, the office and/or field nurses are the first individuals to interview and interact with the patient. With respect to the nurse in the field, I highly recommend that awareness of water intrusion and microbial growth are often present in homes and retirement facilities. Therefore, you are encouraged to look for signs of water-damaged and fungal growth. For example, I just had a conversation with a mother, age 32, who has a ten month old infant with chronic rasping cough, Also, her two older children have repeated upper and lower respiratory infections. The mother has ME / CFS as well as chronic sinus-nasal congestion. The rented home has had water intrusion via a faulty roof and plumbing leaks.
References
- Campbell AW, Thrasher JD, Gray MR, Vojdani A (2004) Mold and mycotoxins: effects on the neurological and immune systems in humans. AdvApplMicrobiol 55: 375-406.
- Indoor air Pollution (2009) Dampness and Mould. World Health Organization
- NIOSH Mold Alert (2013) Preventing Occupational Respiratory Disease from Exposures Caused by Dampness in Office Buildings, Schools, and other Nonindustrial Buildings.
- Thrasher JD, Crawley S (2009) The biocontaminants and complexity of damp indoor spaces: More than meets the eyes. Toxicology Industrial Health 25: 583-615.
- Damp Indoor Spaces and Health (2004) Institute of Medicine of the National Academies. The National Academic Press.
- Pestka JJ, Yike I, Dearborn DG, Ward MD (2008)HarkemaStachybotryschartarum, trichothecenemycotoxins, and damp building-related illness: new insights into a public health enigma. ToxicolSci 104:4-26.
- Fisk WJ, Eliseeva EA, Mendell MJ (2010) Association of residential dampness and mold with respiratory tract infections and bronchitis: a meta-analysis. Environ Health 9: 72.
- Fisk WJ, Lei-Gomez Q, Mendell MJ. (2007) Meta-Analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 17: 284-296.
- Park JH, Cox-Ganser JM (2011) Mold exposure and respiratory health in damp indoor environments. Front Biosci (Elite Ed) 3: 757-771.
- Park JH, Cox-Ganser JM (2011)Mold exposure and respiratory health in damp indoor environments. Frontiers Biosci E3:757-7571.
- Gosh BH, Lal JH, Srivastava A (2015) Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environmental International 85:254-272.
- Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, et al. (2002) Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol68: 3522-3531.
- Cho S-H, Seo S-Cm Schmechel D, Grinshpun SA, Reponen T (2005) Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ 39:5454-5465.
- Reponen T, Seo S-C, Grimsley F, Lee T, Crawford C, et al. (2007) Fungal fragments in moldy houses: A field study in homes in New Orleans and Southern Ohio. Atmos Environ41:8140-8149.
- Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, et al. (2002) Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol68: 3522-3531.
- Adhikari A, Jung J, Reponen T, Lewis JS, DeGrasse EC, et al. (2009) Aerosolization of fungi, (1-->3)-beta-D glucan, and endotoxin from flood-affected materials collected in New Orleans homes. Environ Res 109: 215-224.
- Thrasher JD, Crawley S (2009) The biocontaminants and complexity of damp indoor spaces: more than what meets the eyes. ToxicolInd Health 25: 583-615.
- Straus DC (2009) Molds, mycotoxins, and sick building syndrome. ToxicolInd Health 25: 617-635.
- Mühlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, et al. (2008) Interactions of nanoparticles with pulmonary structures and cellular responses. Am J Physiol Lung Cell MolPhysiol 294: L817-829.
- Peters A, Veronesi B, Calderon-Garciduenas L, Gerhr P, Chen LC, Geiser M, et al. (2006) Translocation and potential neurological effects of fine and ultrafine particles a critical update. Particle Fibre Toxicol 3:13.
- Rylander R (1997) Investigations of the relationship between disease and airborne (1-->3)-beta-D-glucan in buildings. Mediators Inflamm 6: 275-277.
- Genc S, Zadeoglulari Z, Fuss SH, Genc K (2012) The adverse effects of air pollution on the nervous system. J Toxicol 2012: 782462.
- Block ML, Calderón-Garcidueñas L (2009) Air pollution: mechanisms of neuroinflammtion and CNS disease. Trends Neurosci 32: 506-516.
- Calderon-Garciduenas L, Franco-Lira M, Torres-Jardon R, Henriquez-Roland c, Barragán-Mejía G, et al (2007) Pediatric respiratory and systemic effects of chronic air pollution exposure: Nose, lung heart, and brain pathology. ToxicolPathol 35:154-162.
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jordan R, Nuse V, et al. (2008) Long-term air pollutions is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, an accumulation of β-42 and α-synuclein in children and young adults. ToxicolPathol 36:289-310.
- Gray MR, Thrasher JD, Crago R, Madison RA, Campbell AW, et al. (2003) Mixed moldmycotoxicosis: Immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health 59:410-420.
- Hooper DG, Bolton VE, Guilford FT, Straus DC (2009) Mycotoxin detection in human samples from patients exposed to environmental molds. Int J MolSci 10: 1465-1475.
- Brewer JH, Thrasher JD, Straus DC, Madison RA, Hooper D (2013) Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins (Basel) 5: 605-617.
- Gray MR, Hooper D, Thrasher JD (2014) Molds and mycotoxins in autopsy specimens in a death related to fungal pneumonia and pancytopenia, marijuana usage and a water-damaged home: A case report. Internat J ClinToxicol 2:11-20
- Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A (2012) A water-damaged home and health of occupants: a case study. J Environ Public Health 2012: 312836.
- Thrasher JD, Hooper DH, Taber J (2014) Family of six, their health and the death of a 16 month old male from pulmonary hemorrhage: Identification o mycotoxins and mold in the home, lungs, liver and brain of the deceased infant. Inter J ClinToxicol 2:1-10.
- Gray MR, Thrasher JD, Hooper D, Crago R (2014) A case of Reye’s-like syndrome in a 68-day old infant: Water damage home, mold, bacteria and Aflatoxins. Inter J ClinToxicol 2:42-54
- Gray MR, Thrasher JD, Dennis D, Dumanov M, Cravens H, et al. (2015) Sphenoid Aspergilloma: Diagnosed as a malignancy: A case report. Otolaryngology 2015:3
- Thrasher JD, Prokop C, Roberts C, Hooper D (2015) Family with ME/CFS following exposure to molds, mycotoxins and bacteria in a water-damaged home: A case report. Inter J ClinToxicol3:18-28.
- Kurup VP, Zacharisen MC, Fink JN (2006) Hypersensitivity pneumonitis. Indian J Chest Dis Allied Sci 48: 115-128.
- Vesper SJ, Wymer L, Kennedy S, Grimsley LF (2013) Decreased pulmonary function measured in children exposed to high environmental relative moldiness index homes. The Open Respir Med J 7: 83-86.
- Cox-Ganser JM, White SK, Jones R, Hilsbos K, Storey E, et al. (2005) Respiratory morbidity in office workers in a water-damaged building. Environ Health Perspect 113: 485-490.
- Blanc PD, Quinlan PJ, Katz PP, Balmes JR, Trupin L (2013) Higher environmental relative moldiness index values measured in homes of adults with asthma, rhinitis, or both conditions. Environ Health122: 98-101.
- Tercelj M, Salobir B, Narancsik Z, Kriznar K, Brzetic-Romcevic T, et al. ((2012) Nocturnal asthma and domestic exposure to fungi. Indoor Built Environ 22: 876-880.
- White SK, Cox-Ganser JM, Benaise LG, Kreiss K (2013) Work-related peak flow and asthma symptoms in a damp building. Occup Med (Lond) 63: 287-290.
- Dennis DP (2003) Chronic sinusitis: defective T-cells responding to superantigens, treated by reduction of fungi in the nose and air. Arch Environ Health 58: 433-441.
- Dennis D, Robertson D, Curtis L, Black J (2009) Fungal exposure endocrinopathy in sinusitis with growth hormone deficiency: Dennis-Robertson syndrome. ToxicolIndustToxicol 55: 669-680.
- Gorovoy IR, Kazanjian M, Kersten RC, Kim HJ, Vagefi MR (2012) Fungal rhinosinusitis and imaging modalities. Saudi J Ophthalmol 26: 419-426.
- Mossa-Basha M, Ilica AT, Maluf F, Karakoç Ö, Izbudak I, et al. (2013) The many faces of fungal disease of the paranasal sinuses: CT and MRI findings. DiagnIntervRadiol 19: 195-200.
- Siddiqui AA, Shah AA, Bashir SH (2004) Craniocerebralaspergillosis of sinonasal origin in immunocompetent patients: clinical spectrum and outcome in 25 cases. Neurosurgery 55: 602-611.
- Srinivasan US (2008) Intracranial aspergilloma in immunocompetent patients successfully treated with, radical surgical intervention and antifungal therapy – Case series. Ann Acad Med Singapore 37: 783-787.
- Gupta AK, Gupta AK (2009) Postgraduate institute management protocol for invasive Aspergillusflavus: Internat J Infectious Dis 13:134-139.
- TerÄÂ�elj M, Salobir B, Harlander M, Rylander R (2011) Fungal exposure in homes of patients with sarcoidosis - an environmental exposure study. Environ Health 10: 8.
- TerÄÂ�elj M, Salobir B, Zupancic M, Wraber B, Rylander R (2014) Inflammatory markers and pulmonary granuloma infiltration in sarcoidosis. Respirology 19: 225-230.
- TerÄÂ�elj M, StopinÅ¡ek S, Ihan A, Salobir B, SimÄÂ�iÄÂ� S, et al. (2011) In vitro and in vivo reactivity to fungal cell wall agents in sarcoidosis. ClinExpImmunol 166: 87-93.
- Tercelj M, Salobir B, Zupancic M, Rylander R (2011) Antifungal medication is efficient in the treatment of sarcoidosis. TherAdvRespir Dis 5: 157-162.
- Gerke AL (2014) Morbidity and mortality in sarcoidosis. CurrOpinPulm Med 20: 472-478.
- Rao DA, Dellaripa PF (2013) Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am 39: 277-297.
- Engelhard SB, Patel V1, Reddy AK1 (2015) Intermediate uveitis, posterior uveitis, and panuveitis in the Mid-Atlantic USA. ClinOphthalmol 9: 1549-1555.
- Wilson NJ, King CM (1998) Cutaneous sarcoidosis. Postgrad Med J 74: 649-652.
- Anyanwu EC, Campbell AW, Vojdani A (2003) Neurophysiological effects of chronic indoor environmental toxic mold exposure on children. ScientificWorldJournal 3: 281-290.
- Kilburn KH, Thrasher JD, Immers NB (2009) Do terbutaline- and mold-associated impairments of the brain and lung relate to autism? ToxicolInd Health 25: 703-710.
- Kilburn KH (2009) Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals. ToxicolInd Health 25: 681-692.
- Empting LD (2009) Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. ToxicolInd Health 25: 577-581.
- Campbell AW, Thrasher JD, Gray MR, Vojdani A (2004) Mold and mycotoxins: effects on the neurological and immune systems in humans. AdvApplMicrobiol 55: 375-406.
- Carey SA, Plopper DG, Hyde SM, Islam Z, Pestka JJ, et al. (2012) Satratoxin-G from the black moldStachybotryschartarum induces rhinitis and apoptosis of olfactory neurons in the nasal airways of Rhesus monkeys. ToxicolPathol 40:887-898.
- Jedrychowski W, Maugeri U, Stigter L, Jankowski J, Butscher M, et al. (2011) Cognitive function of 6-yeawr old children exposed to mold-contaminated homes in early postnatal period, prospective birth control study in Poland. PhysiolBehav 104:989-995.
- Campbell AW, Thrasher JD, Madison RA, Vojdani A, Gray MR, et al. (2003) Neural autoantibodies and neurophysiologic abnormalities in patients exposed to molds in water-damaged buildings. Arch Environ Health 58: 464-474.
- Doi K, Uetsuka K (2011) Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J MolSci 12: 5213-5237.
- Grollman AF, Jelkovic B (2007) Role of environmental toxins in endemic (Balkan) nephropathy. J Am SocNephrol 18:2817-2883.
- Hope J, Hope BE (2012) A review of the diagnosis and treatment of Ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J Environ Pub Health.
Relevant Topics
- Chronic Disease Management
- Community Based Nursing
- Community Health Assessment
- Community Health Nursing Care
- Community Nursing
- Community Nursing Care
- Community Nursing Diagnosis
- Community Nursing Intervention
- Core Functions Of Public Health Nursing
- Epidemiology
- Epidemiology in community nursing
- Health education
- Health Equity
- Health Promotion
- History Of Public Health Nursing
- Nursing Public Health
- Public Health Nursing
- Risk Factors And Burnout And Public Health Nursing
- Risk Factors and Burnout and Public Health Nursing
Recommended Journals
- Epidemiology journal
- Global Journal of Nursing & Forensic Studies
- Global Nursing & Forensic Studies Journal
- global journal of nursing & forensic studies
- journal of community medicine& health education
- journal of community medicine& health education
- Palliative Care & Medicine journal
- journal of pregnancy and child health
Article Tools
Article Usage
- Total views: 12396
- [From(publication date):
May-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11478
- PDF downloads : 918