Fungal Sinusitis: Radiological Aspects
Received: 30-Aug-2019 / Accepted Date: 30-Sep-2019 / Published Date: 07-Oct-2019 DOI: 10.4172/2161-119X.1000381
Abstract
Introduction: Fungal sinusitis is a well-known entity. They are grouped into invasive forms and non-invasive forms. The diagnosis is often late and difficult given the varied and non-specific nature of the clinical signs. Invasive forms, which cause serious complications that are life-threatening, require rapid diagnosis. The objective was to illustrate the different radiological aspects of fungal sinusitis by specifying for each group its clinical and anatomo-mycological particularities.
Methods: This was a retrospective study of 30 cases of fungal sinusitis collected in service over a 20-year period (1998-2017). All patients had an imaging.
Results: Our series included 30 cases divided into 16 cases of fungal ball, 5 cases of allergic form, 5 cases of chronic invasive fungal sinusitis and 4 cases of mucormycosis. A female predominance was noted with a sex ratio of 0.3. The clinical picture was nonspecific. The functional signs were dominated by rhinorrhea, nasal obstruction and facial pain. Imaging, based on CT (Computed tomography) and/or MRI, was performed in all patients. The radiological signs varied according to the type of fungal attack. All patients were operated on. The diagnosis was mycological and/ or pathological.
Conclusion: The clinical picture of fungal sinusitis is nonspecific. However, one must know how to think of the invasive forms before any trailing sinusitis on a field of immunodepression. Imaging is of great value in the diagnostic and therapeutic approach.
Keywords: Sinus of the face; Aspergillosis; Mucormycosis; Imaging
Introduction
Fungal sinusitis is a well-known entity. Several fungi have been implicated to cause sinus infection. Aspergillus is the most commonly implicated organism causing fungal sinusitis. To date, the most widely adopted classification through several publications distinguishes two groups; invasive forms and non-invasive forms based on the presence or absence of tissue invasion by the fungal agent on histological examination [1-3]. Invasive fungal sinusitis is subdivided into acute invasive fungal sinusitis, chronic invasive fungal sinusitis, and chronic granulomatous invasive fungal sinusitis. Non-invasive fungal manifestations are allergic fungal sinusitis and fungus ball (fungal mycetoma). The diagnosis is often late and difficult given the varied and non-specific nature of clinical signs. Invasive forms, which cause serious and life-threatening complications, require a rapid diagnosis. Our series included 30 cases of fungal sinusitis collected over 20 years from January 1998 to December 2017.
The objective was to illustrate the different radiologic features of fungal sinusitis by specifying for each group its clinical and mycological particularities.
Materials and Methods
We conducted a retrospective study of 30 patients with fungal sinusitis treated over 20 years (January 1998-December 2017). We included in this study all patients operated on and followed for fungal sinusitis in the Department of Otorhinolaryngology, Rabta Hospital, Tunisia. The diagnosis was made on the pathological and/or mycological examination. Patients with no imaging were excluded (CT and/or MRI). Facial CT scan (Computed tomography) in axial and coronal sections was performed preoperatively in all patients. Magnetic resonance imaging (MRI) has been requested in addition to the scanner. This examination included T1 and T2 weighted sequences and a T1 sequence after gadolinium injection. We evaluated the images for characteristics of opacity produced by the diseased tissue, sinuses involved, expansion of sinuses, areas of bone erosion and extra-sinus extension. The Rabta Hospital’s ethical committee board approved the study.
Results
We collected 21 cases of non-invasive forms (16 cases of the fungal ball and 5 cases of allergic fungal sinusitis) and 9 cases of invasive forms (5 cases of invasive pseudotumoral form and 4 cases of mucormycosis). The average age of our patients was 45.5 years with extremes ranging from 17 to 75 years old. We noted a clear predominance of women (7 men/23 women) with a sex ratio of 0.3. Seventeen patients had dental pathology. Six patients had a history of type II diabetes. They had the local noninvasive form and acute fulminant form. Immunosuppressive treatment was found in 2 patients with the local non-invasive form and one patient with mucormycosis: this was a patient under imurel for ulcerative colitis for 1 year, a patient on long-term corticosteroid therapy for rheumatoid arthritis and a patient followed for hematological malignancy. Five patients had a history of nasosinusal polyposis resistant to medical treatment associated with asthma in one case. Allergic rhinitis has been reported in 6 cases. A history of treatment-resistant chronic sinusitis has been reported in 4 cases.
All patients with chronic invasive form were immunocompetent. The average consultation time was 2.5 years in fungal balls (range 5 months to 10 years), 1.8 years in allergic forms (range 1 to 4 years), 1 year in chronic invasive form (extremes of 9 months to 1.5 years) and 13 days (6 days to a month extreme) for fulminant forms. Functional signs were dominated by rhinological signs such as rhinorrhea (26 cases) and nasal obstruction (24 cases). Odor disorders were reported by 7 patients, 5 of whom presented with the allergic form. Hot, painful swelling of the hemisphere rapidly increasing volume associated with hemiface pain was noted in cases of mucormycosis. Ophthalmologic signs were noted in 3 patients presenting the invasive chronic form (unilateral exophthalmia in 2 cases and bilateral in 1 case, periorbital edema in 2 cases, visual blur in 1 case) and in 2 patients with fulminant form (one exophthalmos and decreased visual acuity). Anterior rhinoscopy coupled with nasal endoscopy, performed in all patients, revealed a polyp of inflammatory appearance in 6 cases and translucent polyps filling the nasal fossae related to nasosinusal polyposis in 5 cases.
Examination of the oral cavity revealed multiple dental caries in 6 cases, scars of old extractions of sinus teeth in 8 cases, an antro-buccal fistula in one case and hard necrosis of the palate in the 3 patients with mucormycosis. Examination of the face revealed swelling of the hemiface, painful and poorly limited in all cases of mucormycosis. One patient presented a necrotic cupboard of the hemiface after 48 hours of hospitalization. A specialized ophthalmic examination was performed in the five patients with exophthalmos. It had objectified painless reducible exophthalmos in non-fulminant cases and painful in fulminant forms. A decrease in visual acuity with ophthalmoplegia was noted in two of these cases.
All our patients had a CT scan which made it possible to specify the seat, the aspect and the balance of extension to the adjacent organs. The attack was unilateral in all cases of fungal balls and interested the maxillary sinus in 13 cases. Isolated involvement of the sphenoid was noted in 2 cases (Table 1).
| Form | Affected sinus | N |
|---|---|---|
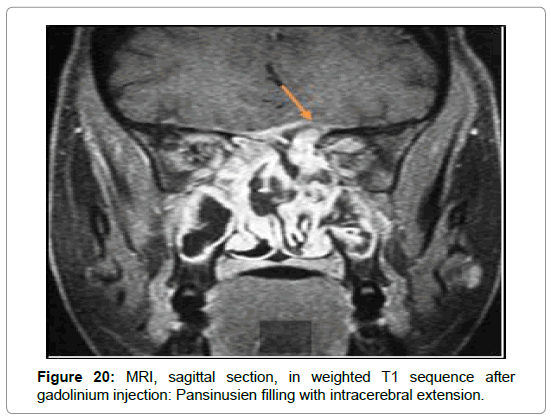
| Fungus ball | Maxillary sinus | 13 |
| Maxillary sinus and ethmoid sinus | 1 | |
| Sphenoid sinus | 2 | |
| Ethmoid sinus and maxillary sinus | 1 | |
| Frontal sinus, ethmoid sinus and maxillairy sinus | 1 | |
| Chronic invasive form | Pansinusitis | 3(bilateral: 1 case) |
| Allergic fungal sinusitis | Pansinusitis | bilateral : 4 cases |
| unilateral : 1 case | ||
| Acute invasive fungal sinusitis | Ethmoid sinus and maxillary sinus | 2 cases |
| Unilateral pansinusitis | 2 cases |
Table 1: Paranasal sinus localizations.
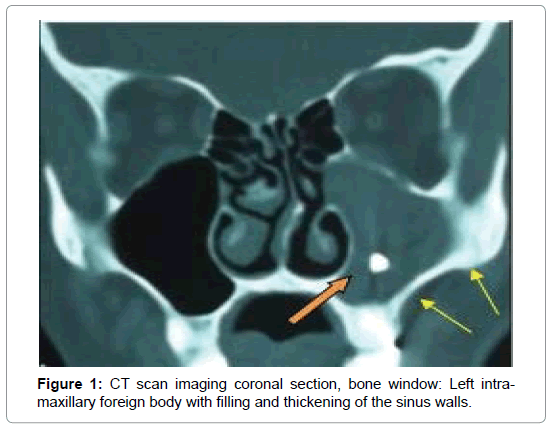
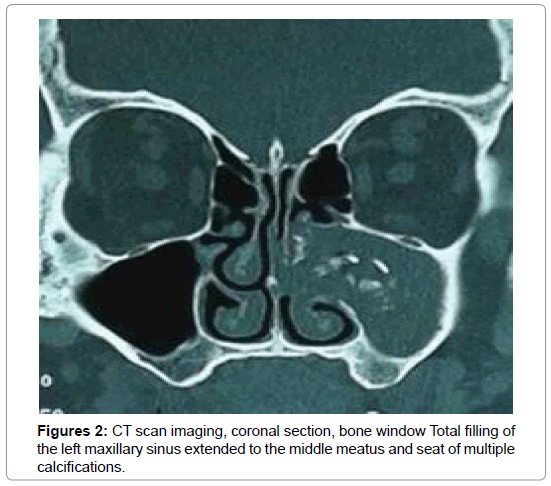
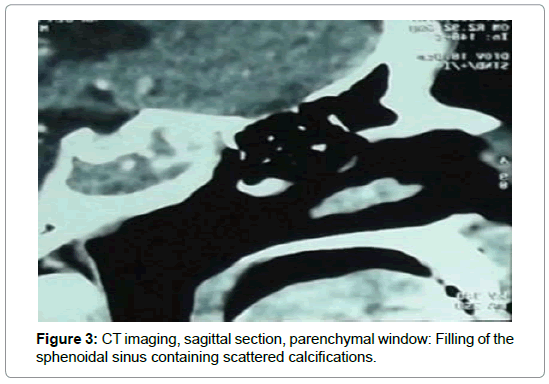
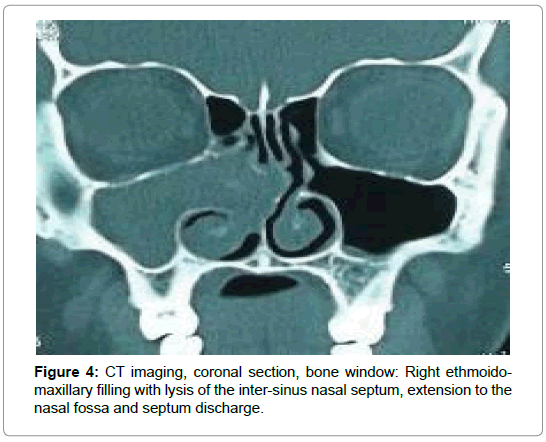
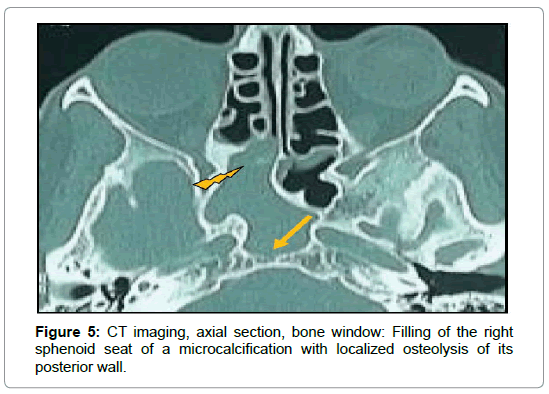
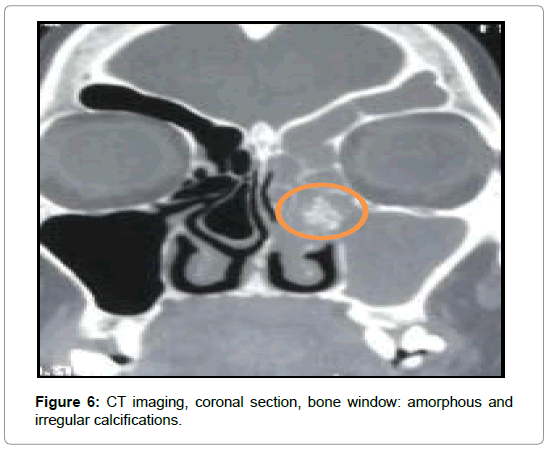
For the 16 cases of fungal balls (Table 2), CT showed typical aspects in 14 cases. It was a high density area (Figure 1) or microcalcifications within a total or partial filling of a sinus (Figures 2 and 3). In the other two cases, the CT scan showed partial filling of the maxillary sinus in one case, and heterogeneous ethmoido-maxillary filling associated with bone lysis in the other case (Figure 4). Signs related to the chronicity of sinusitis were reported as lysis of the medial wall of the maxillary sinus in 2 cases and the floor of the sphenoid in 1 case (Figure 5) and an aspect of osteosclerosis that interested the sinus walls maxillary in 8 cases (Figure 1), and sphenoid in 1 case (Figure 6).
| CT features | Fungus ball (n=16) | Chronic invasive form (n=5) | Allergic form (n=5) | Acute invasive form |
|---|---|---|---|---|
| High density area | 7 | - | - | - |
| Microcalcifications | 7 | 1 | 1 | - |
| Total or partial opacification without microcalcifications or High density area | 2 | - | - | 4 |
| Hyperattenuating filling | - | 2 | 2 | - |
| Pseudotumoral features | - | 2 | - | - |
| Extension to nasal cavity | 2 | 5 | 5 | 4 |
| Bone lysis | 2 | 5 | 2 | 4 |
| Intracranial extension | - | 3 | - | 1 |
| Orbital involvement | - | 3 | - | 4 |
Table 2: CT findings.
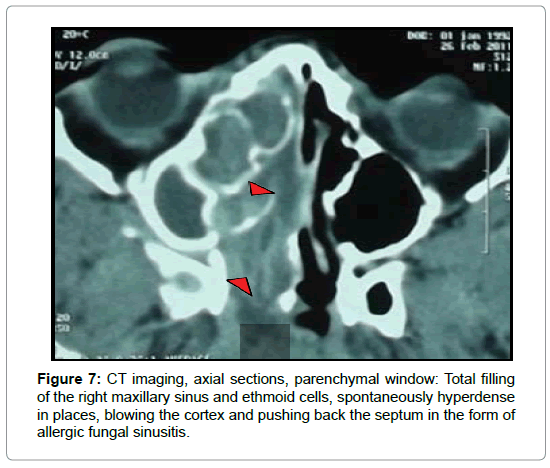
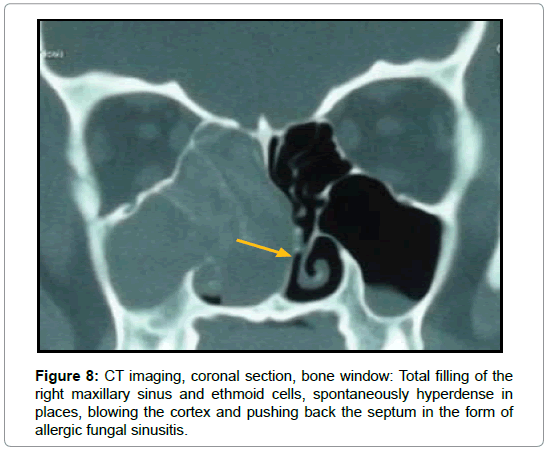
In allergic fungal sinusitis, disease tends to be bilateral with involvement of multiples sinuses. Complete opacification and expansion of the involved sinus are noted in all cases. Noncontrast CT demonstrates hyperattenuating allergic mucin within the lumen of the paranasal sinus in two cases (Figure 7 and 8).
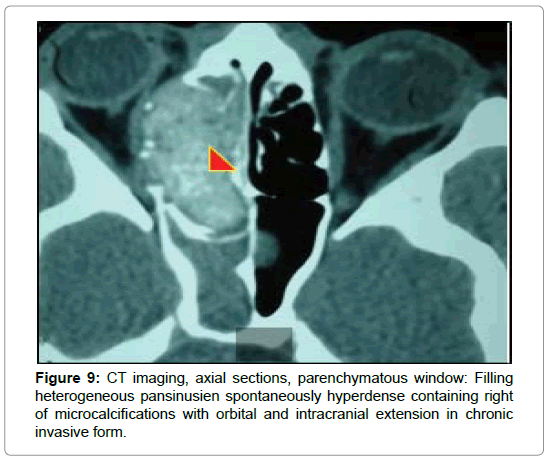
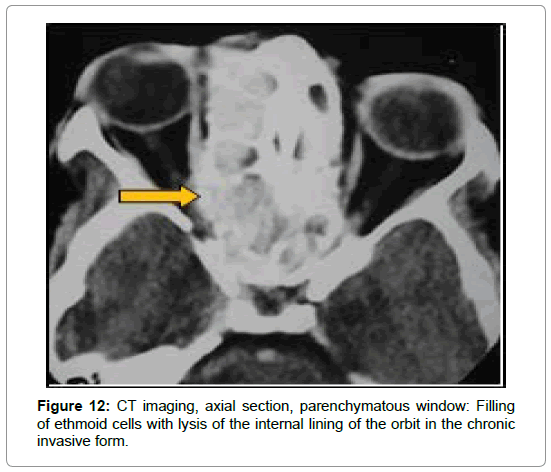
In cases of chronic invasive sinusitis, CT showed pansinusal filling with calcium-rich hyperdense plaques in 2 cases strongly suggestive of fungal origin (Figures 9-11), an expansive, heterogeneous tissue process, taking the product contrast in 2 cases (Figure 12) and homogeneous filling in one case. It allowed to establish the assessment of extension to the adjacent organs by showing a lysis bone interesting ethmoidal partitions, the papery leaf (3 cases), the nasal septum and the intersinunasal partition (1 case), the floor of the frontal sinus (1 case), the lateral wall of the sphenoid (1 case) (Figure 10). Intracranial and orbital extension were noted in 3 cases (Figures 9,11,13) and cavernous sinus invasion in 1 case (Table 2).
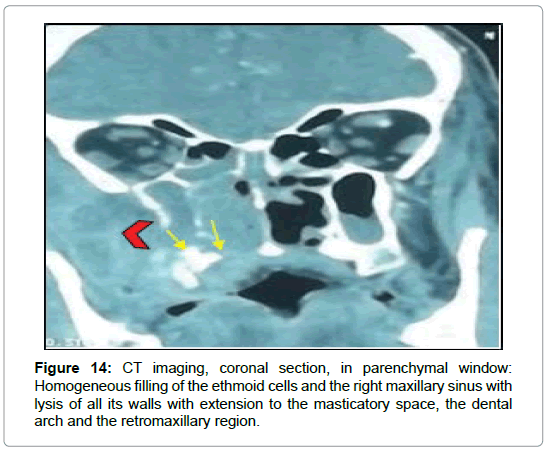
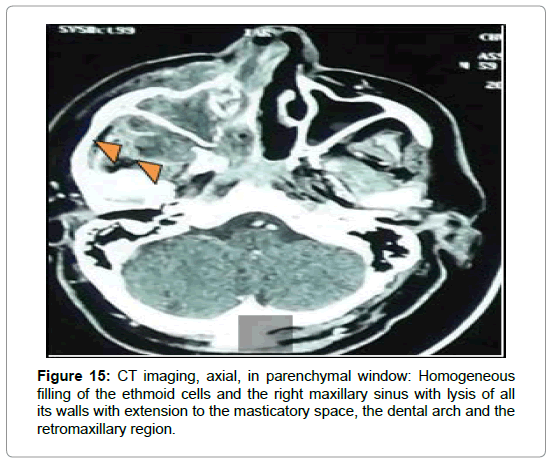
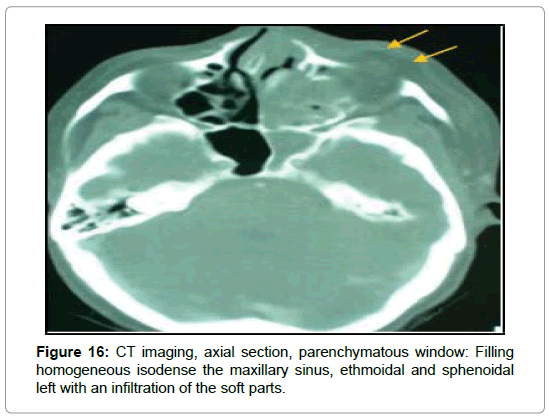
For the cases of mucormycosis, all the patients had a CT of the facial solid mass in urgency. She had shown homogeneous ethmoidomaxillary filling in 2 cases (Figures 14 and 15) and pansinusal in 2 cases (Figure 16). Cellulitis of the orbitofacial region was noted in all cases as well as an extension to the deep spaces of the face and multiple bone lysis. Thrombosis of the right cavernous sinus and right internal carotid artery was noted in one case. Complement with craniofacial MRI was requested in 5 cases, in 3 cases of chronic invasive form, in 1 case of the fungal ball and 1 case of mucormycosis.
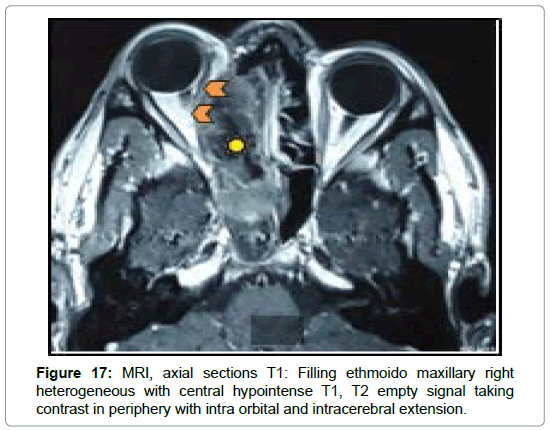
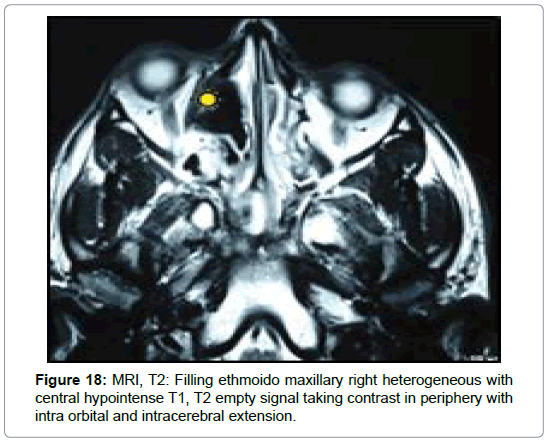
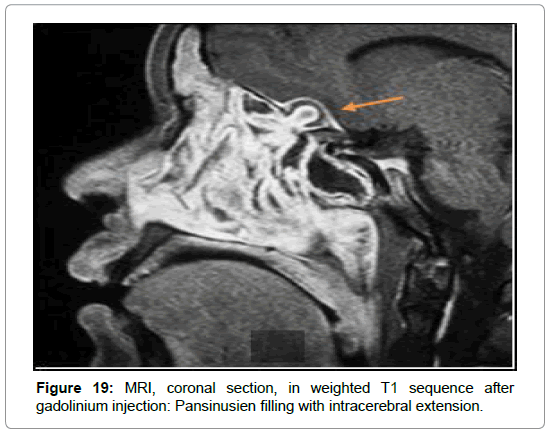
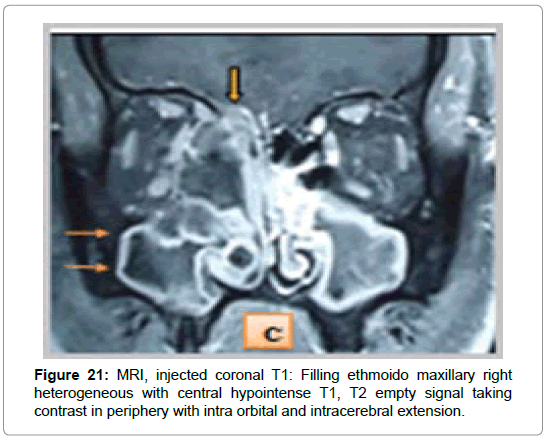
In the 3 cases of chronic invasive form, MRI was requested in front of a pansinusal filling with multiple bones lyses and endocranial extension in 2 cases (Figures 9-11), and an ethmoido-maxillary tissue filling extended to the nasal cavity with lysis of the intersinusonasal septum and nasal septum in one case. It showed a heterogeneous pansinusial filling in hypo or isosignal T1, T2 signal void very suggestive of fungal involvement (Figures 17 and 18) and confirmed the intracranial and orbital extension (Figures 19-21) in 2 cases.
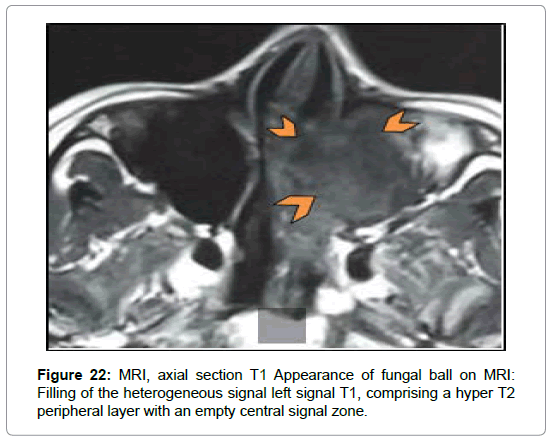
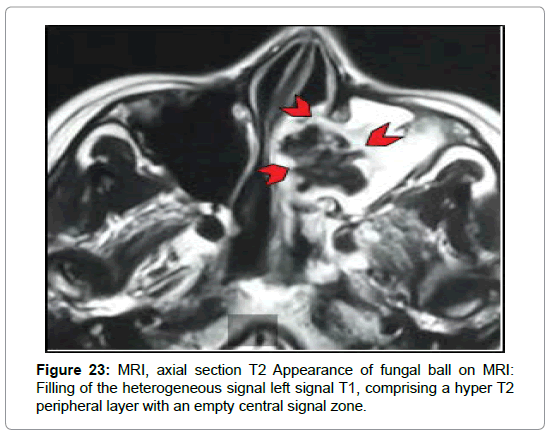
In the case of the fungal ball, MRI was requested in the presence of nonspecific left ethmoido-maxillary filling with lysis of the intersinusonasal septum at the CT associated with polyploid filling of the nasal cavity at the endoscopy in one case (Figure 4). It found an aspect of a complicated fungal graft and left maxillary mucocele (Figures 22 and 23).
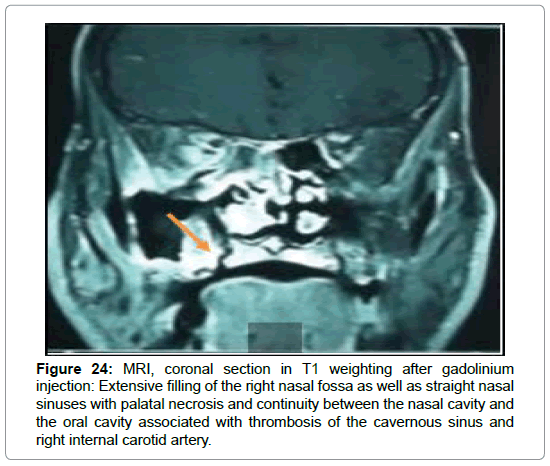
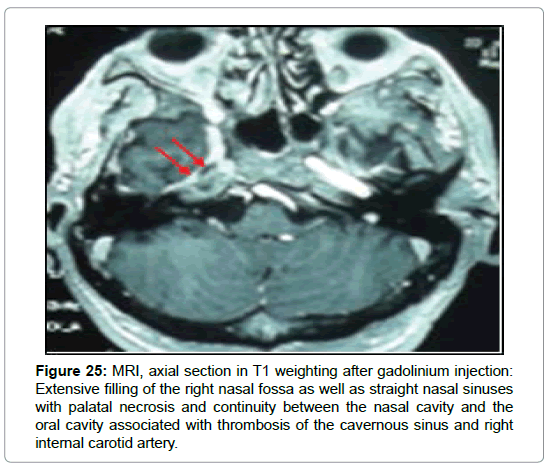
MRI was performed in a case of mucormycosis with suspicion of thrombosis of the cavernous sinus and internal carotid artery. She confirmed this diagnosis by showing the thrombosis of the cavernous sinus associated with extensive thrombosis of the right internal carotid artery with an irregular appearance of the anterior and Sylvian ipsilateral communicating arteries (Figures 24 and 25).
Figure 24: MRI, coronal section in T1 weighting after gadolinium injection: Extensive filling of the right nasal fossa as well as straight nasal sinuses with palatal necrosis and continuity between the nasal cavity and the oral cavity associated with thrombosis of the cavernous sinus and right internal carotid artery.
Figure 25: MRI, axial section in T1 weighting after gadolinium injection: Extensive filling of the right nasal fossa as well as straight nasal sinuses with palatal necrosis and continuity between the nasal cavity and the oral cavity associated with thrombosis of the cavernous sinus and right internal carotid artery.
All patients had surgical treatment. Functional endoscopic sinus surgery was performed in 24 patients. External approach was performed in one case in collaboration with neurosurgeons in a patient with orbital and endocranial extension. The Caldwell Luc pathway was performed in a single case of maxillary fungus bull operated in 1998. The paralateronasal route was performed in a case of mucormycosis with a massive extension to the soft tissues and intraorbital with hemiface necrosis. A mycological examination of intraoperative specimens was performed in 22 cases (12 cases of the fungal ball and all cases of the chronic and invasive form of the allergic form).
The direct examination was positive in 14 cases showing mycelial filaments in all cases. The culture was positive in 10 cases and isolated one Aspergillus fumigatus in 5 cases and one Aspergillus flavus in 5 cases. The appearance of friable and fetid blackish truffles was found in 21 patients. The presence of dental paste or calcium concretions was noted in 6 cases. An endoscopic appearance of allergic mucin was found in all five cases of the allergic form. Anatomo-pathological examination, performed in all cases, confirmed the diagnosis of allergic fungal sinusitis by showing mycelial filaments in 22 cases. In the remaining 4 cases, the diagnosis was retained on the result of the mycological study.
Pathological examination of allergic mucin revealed eosinophilic polynuclear cells in all cases and mycelial filaments in 3 cases. The crystals of charcot and leyden have not been objectified in any case. Invasion of the mucosa by the fungal agent with areas of ulceration was noted in 5 cases of chronic invasive fungal sinusitis. Because of the clinical suspicion of mucormycosis, the biopsy of the nasosinusal mucosa was made urgently with a mycological and histological study. The direct examination was positive in all cases thus allowing the identification of the fungal agent by showing mycelial filaments. Histological examination showed non-septal mycelial filaments.
Discussion
The diagnosis of fungal sinusitis is based on the association of suggestive clinical and paraclinical signs. The clinical picture is nonspecific. In addition to the clinical exploration, CT of the facial area, in axial and coronal sections, was performed in all our patients. The radiological signs varied according to the type of fungal attack. We have been able, through this study of 30 cases of fungal sinusitis, to analyze the different radiological characteristics of these subgroups and to illustrate each form by a rich iconography. Computed tomography (CT), in axial and coronal sections, in bony and parenchymal windows, is the morphological examination of choice that must be performed as first-line treatment in the event of chronic sinusitis that is resistant to medical treatment [4-7]. This examination allows a strong diagnostic suspicion, a good analysis of the bone structures and a study of the invasion of the neighborhood organs.
In the literature, the fungal nature of sinusitis is evoked in front of [7-9] a heterogeneous, unilateral sinus filling, a metallic tonality image suggesting a foreign body, microcalcifications or spontaneously hyperdense plaques, a thickening of the bony walls of the sinus involved. According to Dhong [10], the presence of metallic opacity or microcalcifications within a filling of sinus cavities has a positive predictive value of 60% in fungal balls. However, it should be noted that these images of calcifications, although evocative, are not pathognomonic of fungal sinusitis because they can be seen in other pathologies [6]. The impact of the maxillary sinus is by far the most frequent, followed by isolated sphenoidal localization [6,7,9,11]. Bilateral forms are very rare [12]. We noted bone lysis in 3 cases. These images of bone lysis can be seen in the case of the fungal ball but are rare and most often localized. They are considered as signs related to the chronicity of sinusitis [6,7].
A heterogeneous filling with hypodense zones and hyperdense zones (double signal) containing calcifications were noted in 2 cases in our series. This aspect was suggestive of allergic fungal sinusitis in the presence of a particular clinical context. Indeed, this aspect is considered by some authors as pathognomonic of allergic fungal sinusitis [13]. For others, CT imaging is suggestive but not specific [14]. These hyperdensities, visible on non-injected sequences, are explained by the presence of allergic mucin whose composition is rich in protein and poor in water [15-17]. These same findings have been reported in different series [16,18]. Unilateral forms [19,20], as well as bilateral forms [5,21,22], have been reported. Bony deformities and erosions are found in 30 to 50% of cases [23,24], most commonly at the level of the ethmoidal sinus. These aspects have been found in all our patients. The exact mechanism responsible for this bone erosion is unclear [19]. According to Nussenbaum et al. [25], this fact can be explained by a hyper pressure on the bone walls stimulating their resorption or by the osteolytic action of certain enzymes secreted by the fungal agent. Extension to Perisinusian regions, orbit and / or endocranium is frequent at 20-50% [26,27]. For invasive fungal sinusitis, the radiological aspect is not specific. However, in a particular clinical context, they should be evoked in the presence of a multiple sinus lesion and especially if they are associated with orbital and/or endocranial involvement [28,29].
The most commonly found appearance is total filling or mucosal nodular thickening of the sinus cavities, usually unilateral with a predilection for the maxillary and ethmoidal sinuses followed by the sphenoid [5,21,28]. In our series, the involvement was pansinusal in 2 cases and ethmoido-maxillary in the other 2 cases. Bone lysis was noted in all cases. In addition to these signs, the infiltration of peri-maxillary fat is a very early sign that precedes bone lysis. Its presence makes it possible to suspect the diagnosis in front of any unilateral sinusitis on a weakened ground [15].
Complications are common. We noted in one case the thrombosis of the cavernous sinus associated with extensive thrombosis of the internal carotid artery. Posterior ethmoid or sphenoid involvement increases the risk of intracranial complications such as cavernous sinus thrombosis, internal carotid occlusion (13%) and brain abscess (12%). This extension can be achieved in the absence of bone lysis by diffusion of the fungus via perivascular ducts [30].
A heterogeneous hyperdense filling with calcifications associated with bone lysis and orbital and endocranial invasion was noted in 2 cases among our patients with chronic invasive fungal sinusitis. This is the most frequently found aspect. Nevertheless, this CT appearance is nonspecific and can be confusing with a tumor process [5,15,31]. MRI is not indicated in the usual forms of chronic sinusitis. It finds its indications in invasive forms for studying of extension to neighboring organs (orbit, the base of skull, deep spaces of a face, cavernous lodge etc.) and for differential diagnosis with neoplasias [32,33].
The main and common feature of the various forms of Aspergillus sinusitis is the very hypointense aspect of the T2-weighted sequences which is due to the presence of ferromagnetic elements and essentially zinc in high concentration. Homogeneous enhancement is noted within the mass and its possible cerebral and orbital extensions after gadolinium injection. This enhancement is only annular in case of mass infected or necrotic center [34].
In allergic fungal sinusitis, the affected sinuses appear as central hyposignal in T1 and T2 weighted mode and as hyperintense mainly in peripheral T2 [13,15]. The contrast enhancement is mainly linear peripheral, sparing the center of the sinus. It corresponds to the inflammatory sinus mucosa. This aspect was noted in one case of our series. According to some authors, it allows this entity to be distinguished from other neoplasias [16]. The intracerebral extension is often limited and remains extradural.
In the literature, several studies have investigated the role of MRI in the early diagnosis of acute invasive sinusitis. Early in the disease process, imaging results can not be distinguished from those of common rhinosinusitis. Early signs that should suggest this diagnosis are the infiltration of fat from the retromaxillary region and soft tissues of the nasopharynx. Thanks to its high resolution in the study of soft tissues, MRI can demonstrate the first findings of fulminant fungal invasion with a negative predictive value of 60 to 100% [15]. It allows in the advanced stages to study the intraorbital and cerebral extension and to highlight the thromboses of the cavernous sinus, the internal carotid artery and its branches on the angiographic sequences [28,30,35].
In our study, MRI was performed in one case with suspicion of thrombosis of the cavernous sinus and internal carotid artery.
Conclusion
The presentations of fungal sinusitis on imaging are quite variable depending on its contents. Practicians should know the typical as well as atypical findings. Special care should be taken with regard to differential diagnosis.
Conflict of Interest
We declare that there is no conflict of personal interest incompatible with the objectives of this work.
References
- Chakrabarti A, Denning D, Ferguson B, Ponikau J, Buzina W, et al. (2009) Fungal rhinosinusitis: a categorazation and definitionol schema addressing current controversies. Laryngoscope 119: 1809-1818.
- Klossek J, Kauffmann-Lacroix C, Dufour X (2001) Sinusites fongiques: Classification, méthodes diagnostiques et prise en charge. J Mycol Med 11: 216-221.
- Montone K, Livolsi V, Feldman M, Palmer J, Chiu A (2012) Fungual rhinosinusitis, a retrospective microbiologic and pathologic rewiew of 400 patients at a single university medical center. Int J Otolaryngol 6: 1-9.
- Farié J, Klossel J (2003) L’imagerie des sinus de la face et du massif facial: stratégie d’exploration. J Radiol 84: 963-967.
- Roopkamal S, Nirali M, Bhavesh D, Harshad S, Asutosh D, et al. (2014) Clinico-radiological manifestations of invasive and non-Invasive fungal infections in sinuses and respiratory tract. GCSMC J Med Sc 3: 42-47.
- Braun J, Bourjat P (2000) CT imaging of fungal and nonfungal caseous sinusitis. A report of 50 casesl. J Radio 81: 227-231.
- Ariband M, McCoy V, Bazan C (2007) Imaging features of invasive and non-invasive fungal sinusitis: a review. Radiographics 27: 1283-1296.
- Braun J, Burjat P, Gentine A, Koehl C, Veillon F, et al. (1997) Caseous sinusitis Clinical, x-ray computed, surgical, histopathological, biological, biochemical and myco-bacteriological aspects. A propos of 33 cases. Ann Otolaryngol Chir Cervicofac 114: 105-115.
- Kazuhiro N, Daiya A, Tsuguhisa N, Yoshinori M, Tsuyoshi Y (2013) Sinus fungus ball in the japanese population. Clinical and imaging characteristics of 104 cases. Int J Otolaryngol 14: 1-4.
- Dhong H, Jung J, Park J (2000) Diagnostic accuracy in sinus fungus balls: CT scan and operative findings. Am J Rhinol 14: 227-230.
- Rispail P, Jarry D, Tomas C (1995) Mycose sinusiennes: champignons impliqués, leur position dans la classification, épidémiologie et pathogénie. Cahiers d'ORL 31: 459-467.
- Klossek J, Fourcroy P, Bodson I, Fontanel J (1995) Mycoses sinusiennes non invasives du sinus maxillaire. Cahiers d’ORL 31: 473-476.
- Manning S, Merkel M, Kriesel K, Vuitch F, Marple B (1997) Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope 107: 170-176.
- Braun J, Riehm S, Veillon F (2008) Intérêt de la tomodensitométrie dans la sinusite fongique allargique. J Radiol 89: 480-486.
- Gorovoy I, Kazanjian M, Kersten R, Kim H, Vagefi M (2012) Fungal rhinosinusitis and imaging modalities. Saudi J Ophthalmol 26: 419-426.
- Panjabi C, Shah A (2011) Allergic aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac Allergy 1: 130-137.
- Schubert M (2009) Allergic fungal               sinusitis: pathophysiology, diagnosisand management. Med Mycol 47: 324-330.
- Desmots F, Gabaudan C, Geffroy Y, Cassagneau P, Varoquaux A (2012) Allergic fungal sinusitis. Answer to march e- quid. Diagn Interv Imaging 93: 413-419.
- Bent J, Kuhn F (1994) Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg 111: 580-588.
- Sohail M, Khabouri M, Hyder J, Verma A (2004) Allergic fungal sinusitis: can we predict the recurrence? Otolaryngol Head Neck Surg 131: 704-710.
- Mossa-Basha M, Ilıca A, Maluf F, Karakoç O, İzbudak I, et al. (2013) The many faces of fungal disease of the paranasal sinuses: CT and MRI findings. Diagn Interv Radiol 19: 195-200.
- Surayie H, Dousary A (2008) Allergic fungal sinusitis: radiological and microbiological features of 59 cases. Ann Saudi Med 28: 17-21.
- Bozeman S, Deshazo R, Stringer S, Wright L (2011) Complications of allergic fungal sinusitis. Am J Med 124: 359-368.
- Corey J, Delsupehe K, Ferguson BJ (1995) Allergic fungal sinusitis: allergic, infectious, or both? Otolaryngol Head Neck Surg 113: 110-119.
- Nussenbaum B, Marple B, Schwade N (2001) Characteristics of bony erosion in allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg 124: 150-154.
- Berlucchi M, Pedruzzi B (2012) Allergic Fungal Sinusitis in Children. J Aller Ther 5: 1-5.
- Mohamad R, Chaabana D, Jayant M, Pintoa C, Colin S, Cheng H, Jessie A.
- Mnif N, Hmaied E, Oueslati S, Rajhi H, Hamza R, et al. (2005) L'imagerie dans la mucormycose rhinocérébrale. J Radiol 86: 1017-1020.
- Trabelsi S, Marrakchi J, Aloui D, Lahiani R, Sellami A, et al. (2013) Rhino-orbito-cerebral mucormycosis. La Tunisie medicale 91: 164.
- Herrera DA, Dublin AB, Eleanor LO, Aminpour S, Howell LP (2009) Imaging findings of rhinocerebral mucormycosis. Skull Base 19: 117-125.
- Oukabli M, Ennouali H, Chahdi H, Qamouss O, Damiria A, et al. (2010) Rhinosinusite chronique invasive aspergillaire chez une patiente immunocompétente. J Myc Med 20: 120-123.
- Breen D, Clifton A, Wilkins P, Uttley D, Westmor G (1993) Invasive aspergilloma of the skull base. Neuroradiol 35: 216-217.
- Razek A, Sieza B, Maha B (2009) Assessment of nasal and paranasal sinus masses by diffusion-weignted MR imaging. J Neuroradiol 36: 206- 211.
- Siddiqui A, Bashir S, Shah A, Sajjad Z, Ahmed N, et al. (2006) Diagnostic MR imaging features of craniocerebral aspergillosis of sino-nasal origin in immunocompetent patients. Acta Neurochir 148: 155-166.
- Martin-Moro J, Calleja J, Garcia M, Carretero J, Rodriguez J (2008) Rhinoorbitocerebral mucormycosis: a case report and literature review. Med Oral Patol Oral Cir Bucal 13: 792-795.
Citation: Jihène M, Maha M, Samia M, Zainine R, Mohamed BA, et al. (2019) Fungal Sinusitis: Radiological Aspects. Otolaryngol (Sunnyvale) 9:381. DOI: 10.4172/2161-119X.1000381
Copyright: © 2019 Jihène M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4629
- [From(publication date): 0-2019 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3804
- PDF downloads: 825