Functionalized-Graphene/Polyaniline Nanocomposites as Proficient Energy Storage Material: An Overview
Received: 03-Oct-2016 / Accepted Date: 05-Nov-2016 / Published Date: 15-Nov-2016
Abstract
Graphene-based materials owing to their unique and versatile properties, such as, larger surface area, high electrical conductivity, appreciable chemical stability and remarkable mechanical behavior, find promising applications in supercapacitors and other energy storage devices. Polyaniline (PANI), with outstanding high electrical conductivity, reversible redox, and doping/dedoping properties, and high environmental stability, has also been studied in great detail. In recent years, one- and two-dimensional (1D/2D) PANI nanostructures, including nanofibers, nanowires, nanorods, and nanotubes, and nanofilms, etc., have attracted much attention across scientific and engineering disciplines with the expectation that these materials could exhibit superior properties compared to their bulk counterparts in terms of mechanical strength, and charge transport. Thus efforts have been made to coalesce the individual properties so as to obtain high quality materials. Graphene/ polyaniline nanocomposites are easy processing, cost-effective materials. Besides, morphological variations also assist in tuning the effective area, porosity, electrical conductivity and consequently, the specific capacitance of these nanocomposites. Thus, the main focus of current research lies on designing, architecturing as well as amplifying their electrochemical behavior in such an order so to obtain promising applications in future electronics which has been highlighted in details in the present overview.
Keywords: Polyaniline; Graphene; Supercapacitor; Energy storage
19143With the ever-rising energy demands, increasing pollution and depletion of non-sustainable energy resources, the development of renewable energy production with low emission of greenhouse gases have rightly attracted a great deal attention since the last two decades. Sincere efforts have been put into developing lithium ionbased batteries and analogous secondary energy storage systems for autonomy purpose. Besides, ever-increasing demands for portable, miniatured and high power productive electrical storage vehicles too have channelized the current research onto upgrading electrochemical capacitors. In comparison to secondary batteries, electrochemical capacitors, better known as super capacitors or ultra-capacitors, are more versatile in properties as they generally exhibit faster as well as higher power density, long cycle life, enhanced thermal operating range, and low cost of maintenance [1-2]. Super capacitor operations are usually based on two energy storage mechanisms, namely the Electrical Double Layer (EDL) capacitance and the Pseudo Capacitance (PC). For EDL capacitors, carbon based materials are frequently employed, where the capacitance originates from the charge accumulation at the electrode/electrolyte interface. A variety of porous forms of carbon namely, activated carbons, carbon nanoforms, CNTs, and lately graphene, are currently preferred as the electrode materials because they have exceptionally high surface areas, superior electronic charge transport properties and acceptable production cost. The pseudocapacitors (PC) or redox supercapacitors, on the contrary, are fabricated using electrode materials based on conducting polymers and metal oxides that accumulate and store charge vide fast and reversible faradic redox reactions. Both have their merits as well as limitations and thus current effort lies on tuning both synergistically so that better properties could be achieved [1-4].
Polyaniline (PANI), a p-type conjugated polymer, has also been explored as an electrode material for energy storage due to its high theoretical capacity (assuming full doping, neglecting the mass of the anion: 294 mAh.g-1), superior charge transport, cost effectiveness and ease of synthesis. PANI stores charge through a pseudocapacitive doping/dedoping mechanism, in which anions transport in and out of the electrode as it is oxidized and reduced, respectively [5-6].
Accordingly, mass transport of the dopant ion is a potential issue, especially for dense PANI electrodes. In this regard, switchover to nanophase can show promising signatures because of their selfassembly to porous, high surface area electrodes, rapid as well as easy to synthesize in water, and remain dispersed in water-processable state for days [7]. Recently PANI nanofibers electrodes exhibited capacities in the range of 75–165 mAh.g-1 as cathodes in non-aqueous cells, demonstrating their significant ability to store charge [8]. Systematic studies of the charge storing capacities of various PANI nanostructures like nanotubes, nanowires, nanofibers, have been carried out in recent times. However, random usage of these materials in pristine form has been restricted by poor electrochemical stabilities and low cycle lives due to swelling and shrinking of the polymer backbone during continuous charging/discharging processes [9-12].
Various carbon-based materials such as activated carbons, mesoporous carbons, fullerenes and its derivatives, owing to their morphological stability have been employed in composite form with metal oxides/CPs but the obtained capacitance values are limited by micro-structural aspects of these systems [13-16]. Similar composite materials of CNTs have been tested as supercapacitor electrodes which although exhibited higher capacitance values and improved stability but very much constrained by high production cost. Moreover, recently observed electric double-layer capacitance of pristine CNTs is only up to 80 F/g have further controlled its usage in current technology [17].
Graphene, a two-dimensional sheet of sp2-hybridized carbon often compared to an un-rolled carbon nanotube, is particularly interesting as electrode material for thin film batteries because of its high electron mobility, excellent mechanical strength, enhanced thermal conductivity, and high surface area (theoretically up to 2630 m2g-1) [18-19]. Because of these excellent properties, graphene has been explored in various applications including sensors, solar cells, tissue engineering, drug delivery, and energy storage [20-22]. In energy storage systems, graphene has been widely employed as an electrode material itself or as a conductive additive powder showing conductivity as high as 200 Sm-1 [23]. Functionalized-graphene systems especially-O-containing functional groups accumulate charge both via EDL mechanism as well as by pseudocapacitive mechanism originating from the rapid redox reactions. Thus, systems like Graphene Oxide (GO) had high charge storing capacity but poor conductivity restricts its usage and so partially reduced GO are currently in business. Reduced Graphene Oxide (rGO) electrodes have been reported to display reversible capacities up to 120– 200 mAh.g-1 [24] Graphene/rGO have been prepared using various methods including mechanical exfoliation, chemical vapor deposition, epitaxial growth, and by wet-chemical reduction of graphene oxide [25-28]. Chemical reduction of graphene oxide to rGO using reducing agents like hydrazine, peroxides, ascorbic acid, etc. is a particularly promising method because it offers low-cost, large-scale production although its processability has been somewhat limited. Moreover toxic effects of these chemical agents are also an important environmental issue. Lately, the electrochemical reduction of processed GO sheets has been demonstrated, thus eliminating the need for harsh and toxic reducing agents [29-30]. Very recently observed appreciable values of EDL capacitance (~21 μFcm-2) and origin of quantum capacitance in single layer and double-layer graphene have opened up immense hopes regarding its application in energy storage devices [31]. It has also been established that as far as electrochemical properties are considered, single layered graphene is no good than multi-layered graphenes [32]. So it is wise to use low-costing multi-layered graphenes rather than expensive single-layered graphene systems.
In this review, the author highlights on the charge storing capacity of the functionalized-Graphene/polyaniline nanocomposites so obtained from different fabrication methods as that reported till date. It is important to understand that the morphological variations that arise due to different fabrication techniques, appreciably assist in tuning the surface area, porosity, electrical conductivity, charge storing capacity and hence, modifying the target parameter-specific capacitance? of the nanocomposites. The different observations of specific capacitance of the above composite have been collected from literature and on-going through these available reports, the author has tried to sort out the optimum and requisite conditions for achieving ideal charge storing capacity for the above composite. Besides, the future prospect of this nanocomposite in this field has also been narrated.
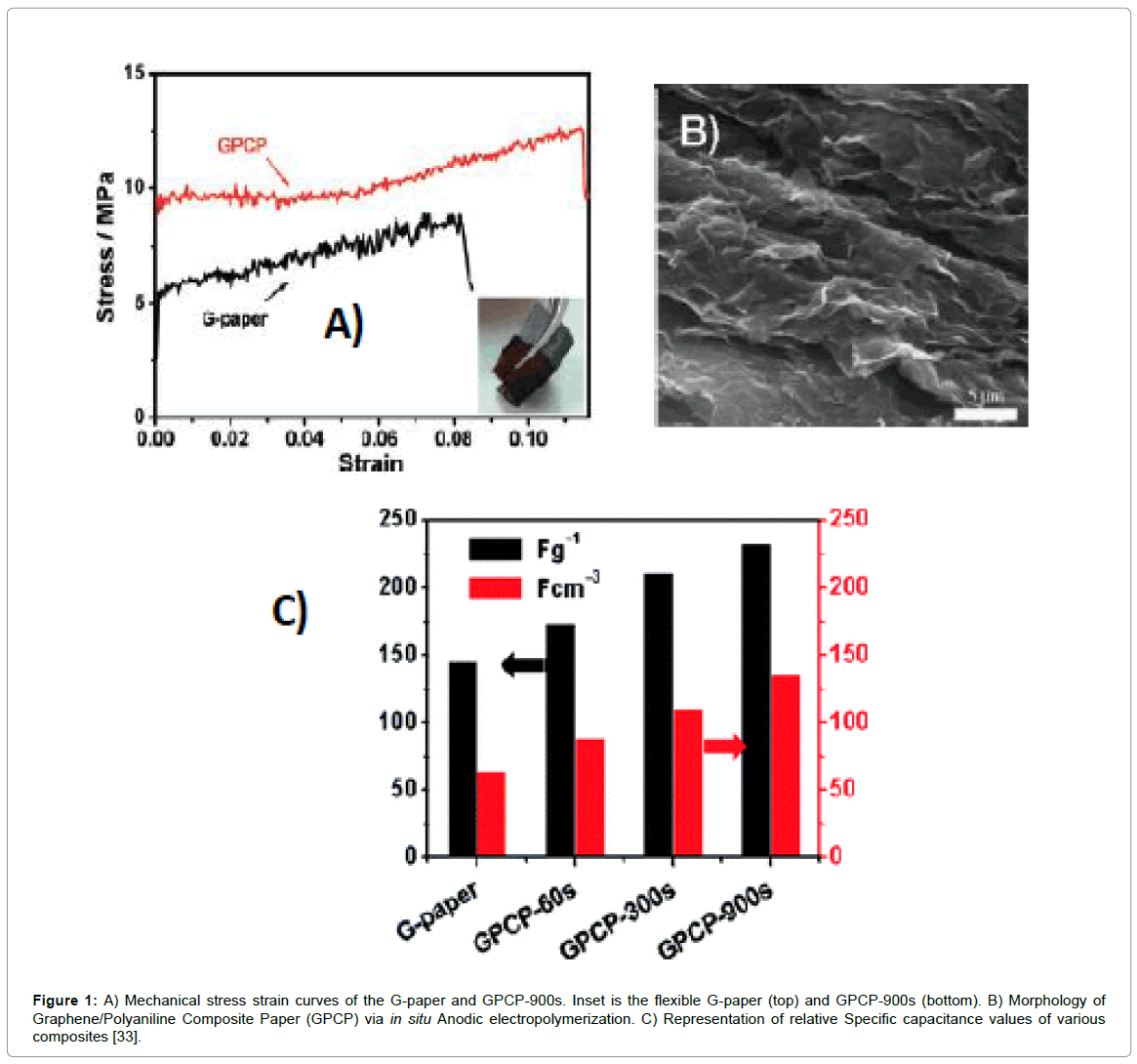
To accomplish graphene-conducting-polymer-based materials in supercapacitor applications, earlier works were primarily based on forming/coating polyaniline layers onto both graphene as well as functionalized graphene sheets adopting various synthetic procedures. In an early work, electrochemical capacitance ~233 Fg-1 was obtained by in-situ anodic electro-polymerization of aniline on graphene paper, with improved mechanical strength of the latter compared to its components (Figure 1). However, the specific capacitance was mainly dominated by the pseudo-capacitance from the polyaniline films coated on the graphene paper surface rather than electric double layer contribution from graphene sheet due to agglomerated layer like structure of the latter [33]. Progressively, many works based on doping of graphene oxide/graphene nanosheets into polyaniline matrix and vice-versa were reported as summarized in Table 1. Optimum doping amount of insulating GO (e.g., 1%) is required in these cases because excess GO tend to reduce the conductivity of the composite [34-36]. Composites with PANI doped with small amount of GO exhibited specific capacitance to as high as 531 Fg-1 in the 1M H2SO4 electrolyte, was mainly attributed to π—π stacking between PANI backbone and GO nanosheets [37]. The rGO/PANI composite electrodes exhibit higher specific capacitance and conductivity than those of GO/PANI composite electrode. This is obvious as GO has insulating characteristics due to topological defects and numerous oxygenated groups compared to rGO. Feng and co-workers reported sulfonated-graphene/polyaniline composites prepared via in-situ chemical polymerization in absence of inorganic or organic acids [38]. The surface sulfonate (—SO3-) groups on graphene played important roles-increased the aqueous solubility; enhanced the total capacitance through pseudocapacitance effects, as well as improved the wettability of porous carbon with electrolytes. Moreover, these sulfonate groups also helped in doping of PANI because of its proton doping mechanism. The composite displayed maximum specific capacitance of ~676 Fg-1 at 0.1 Ag-1 of current density [38].
Figure 1: A) Mechanical stress strain curves of the G-paper and GPCP-900s. Inset is the flexible G-paper (top) and GPCP-900s (bottom). B) Morphology of Graphene/Polyaniline Composite Paper (GPCP) via in situ Anodic electropolymerization. C) Representation of relative Specific capacitance values of various composites [33].
| Material | PANI morphology |
Electrolyte | Specific capacitance |
Cyclic performances |
References |
|---|---|---|---|---|---|
| Inkjet-printed graphene/PANI |
Bulk | 1M H2SO4 | 82 Fg-1 @ 10 mVs1 |
82 F g-1 after 1000 cycles |
[39] |
| PANI–GO | Bulk films | 1 M H2SO4 | 475 Fg-1 @ 0.4 Ag-1 |
96% after 500 cycles. |
[34] |
| PANI GRAPHENE (PG100:1) |
PANI film | 0.5 M H2SO4 aqueous solution |
25 mF cm-2 @ 5m Vs-1 |
retains 53.1% after 1000 cycles. |
[8] |
| PANI–GO | Bulk | 0.5 M H2SO4 | 25 mF cm-2 @ 5mVs-1 |
91% after 200cycles |
[45] |
| PANI–GONS | PANI film | 1 M H2SO4 | 547 Fg-1 @ 0.01mA |
- | J. Power Sources 224 (2013) 195 |
| PANI-GO | Bulk | 1 M H2SO4 | 355 Fg-1 @ 0.5 Ag-1 |
- | [15] |
Table 1: Summary of works based on functionalized graphene/ bulk-PANI composites.
Another approach to improve the specific capacitance of the material is to increase the effective surface area which was successfully accomplished by fabricating mesoporous PANI film on ultra-thin graphene nanosheets (G-mPANI) hybrid by in situ polymerization using graphene-mesoporous silica composite as template [39]. Due to its mesoporous structure, over-all conductive network, G-mPANI electrode displays a substantial specific capacitance of 749 Fg-1 at 0.5 Ag-1 with exceptionally high rate capability. Gupta et al electro-deposited multilayer films of polyaniline and graphene oxide alternatively on indium tin oxide (ITO) electrode to increase the effective active sites for electrochemical performances [40]. In this regard, homogeneity of films is an important issue. Wei et al. synthesized graphene/PANI composite where graphene (~15 wt %) was homogeneously coated on to PANI sheets, that exhibited high specific capacitance of 1046 Fg-1 at current density of 10Ag-1, with capacitance retention ~96% on increasing the current density from 10 to 100 Ag-1, indicating a superior electrochemical feature [41]. The uniform, homogeneous G– PANI-Nafion film based supercapacitor on graphite electrodes using in N-Methyl-2-pyrrolidone (NMP) solvent showed tuning of specific capacitance of 300–500 Fg-1 at a current density of 0.1Ag-1, obtained for various compositions of the nanocomposite material [42]. However, these systems also faced poor electrochemical stabilities and low cycle rates similar to the pristine components.
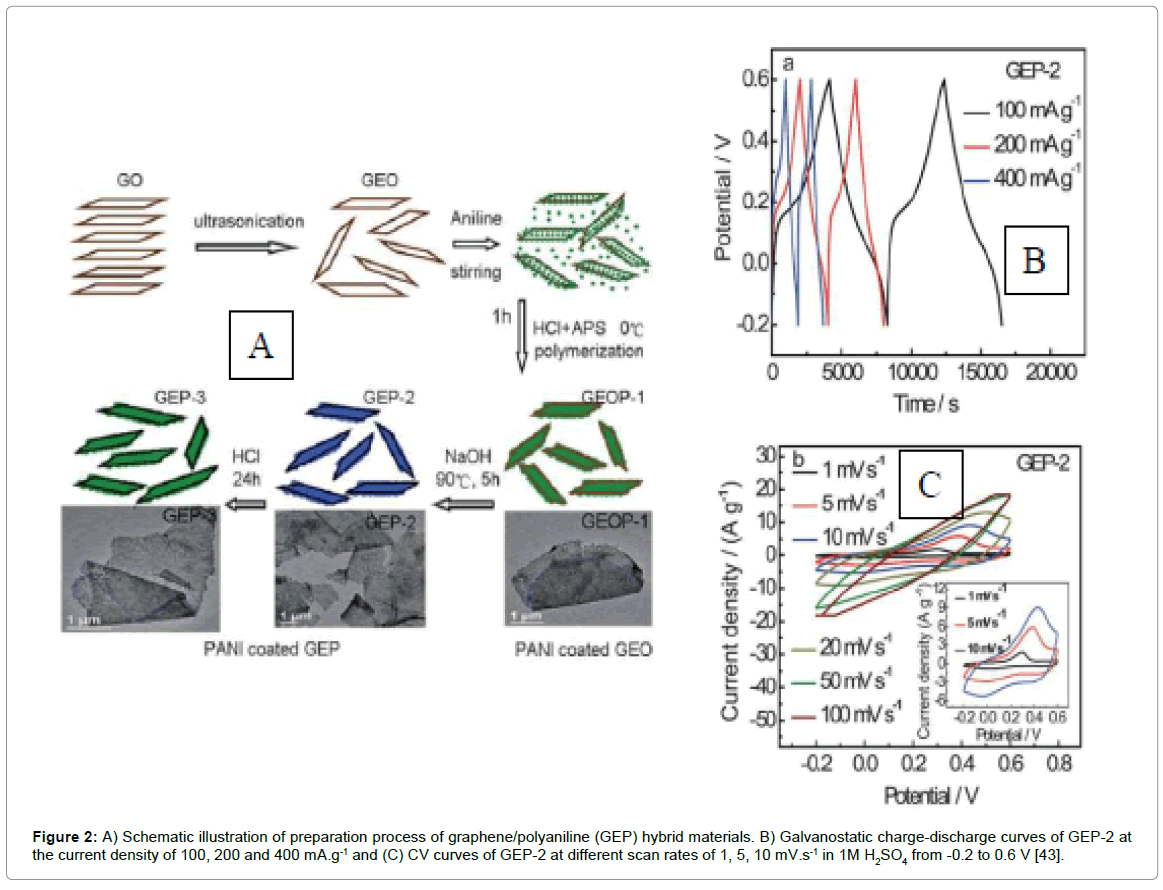
To cope up with and address the above-faced difficulties; and also further enhance the effective surface area as well as improve charge transport properties, researchers emphasized on fabricating nanostructured PANI besides functionalized graphene in a cost-effective route. Based on the above strategy, graphene/PANI hybrid materials, designed using three step-in-situ-polymerization-reduction/dedopingredoping processes, shown in Figure 2, renders specific capacitance as high as 1126 Fg-1 at a scan rate 1 mVs-1 with appreciable cyclic performances [43]. Various morphologies of PANI nanostructures anchored onto graphene/functionalized systems have been fabricated and better tuning of capacitive results have been reported that is summarized in Table 2.
Figure 2: A) Schematic illustration of preparation process of graphene/polyaniline (GEP) hybrid materials. B) Galvanostatic charge-discharge curves of GEP-2 at the current density of 100, 200 and 400 mA.g-1 and (C) CV curves of GEP-2 at different scan rates of 1, 5, 10 mV.s-1 in 1M H2SO4 from -0.2 to 0.6 V [43].
| Material/PANI morphology | Techniques | Electrolyte | Specific capacitance |
Cyclic performances |
References |
|---|---|---|---|---|---|
| Nano-PANI/GO PANI Nanoparticles |
Electrochemical reduction | 1.0 M HCL | 15 mF cm-2 | 71% after 10000 cycles | [15] |
| PANI-rGO PANI nanothorn arrays |
solvothermalin-situ polymerization in a reverse microemulsion system |
1 M H2SO4 | 595 Fg−1 @ 0.5 Ag−1 | 76% over 1000charge/ discharg e cycles 76% over 1000charge/ discharg e cycles |
Carbon (2014) |
| Self-assembled hierarchical graphene/polyaniline hybrid aerogels |
hydrothermal route | 1 M H2SO4 | 520.3 Fg−1 @ 0.25 Ag−1 | - | [34] |
| PANI nanowires nanorod- PANI–Graphene nanorod- PANI |
electrochemical polymerization in reverse micelle electrolyte |
1 M HClO4 | 878.57 Fg−1 @ 1 Ag−1 | Above 80% after 1000 cycles |
[44] |
| PANI-NF/GO PANI nanofibres | interfacial polymerization | 1 M H2SO4 | 346 Fg−1 @ 0.5 Ag−1 | - | [19] |
| GO–PANI | 1 M H2SO4 | 773 Fg−1 @ 1 mVs-1 | capacitance of 507 Fg−1 at the first cycle and 612 Fg−1 at the 200th cycle capacitance of 507 Fg−1 at the first cycle and 612 Fg−1 at the 200th cycle |
[45] | |
| PANI nanowires–GO PANI nanowires |
Chemical oxidative polymerization method |
1 M H2SO4 | 555 Fg−1 @ 0.2 Ag−1 | 92% of its initial capacitance, 2000 cycles |
[52] |
| PANi nanorods /graphene nanoribbons PANi nanorods |
Chemical oxidative polymerization method |
1 M H2SO4 | 340 Fg−1 @ 0.25 Ag−1 | 90% retention after 4200 cycles |
[45] |
| Stretchable wavy shaped graphene/PANi supercapacitor |
H3PO4/PVA | 261 Fg−1 @ 0.38 Ag−1 | 89% retention after 1000 cycles |
[45] | |
| Freestanding Graphene Paper Supported 3D- Porous Graphene − PANI Nanocomposite nanostructures |
inkjet printing synthesis | 1 M H2SO4 | 864 Fg−1 @ 1 Ag−1 | 96% retention after 5000 cycles |
[52] |
| Graphene/PANi PANI nanoparticles |
In-situ-polymerization | 6 M KOH | 1046 Fg−1 @ 1 mV s−1 | Carbon (2010) | |
| PANi nanofibers /3D graphene framework PANI nanofibers |
Chemical oxidative polymerization | 1 M H2SO4 | ~1024 Fg−1 @ 10 mVs−1 | ~86.5% retention after 5000 cycles |
[45] |
| PANI-NFs-GO PANI nanofibers |
In situ polymerization | 1 M Na2SO4 | 530 Fg−1 @ 200 mAg−1 | - | [36] |
| GO–PANI PANI microfibers | In situ polymerization | 1 M H2SO4 | 531 Fg−1@ 200 mAg−1 | - | [37] |
Table 2: Summary of works based on functionalized graphene/ nanostructured-PANI composites.
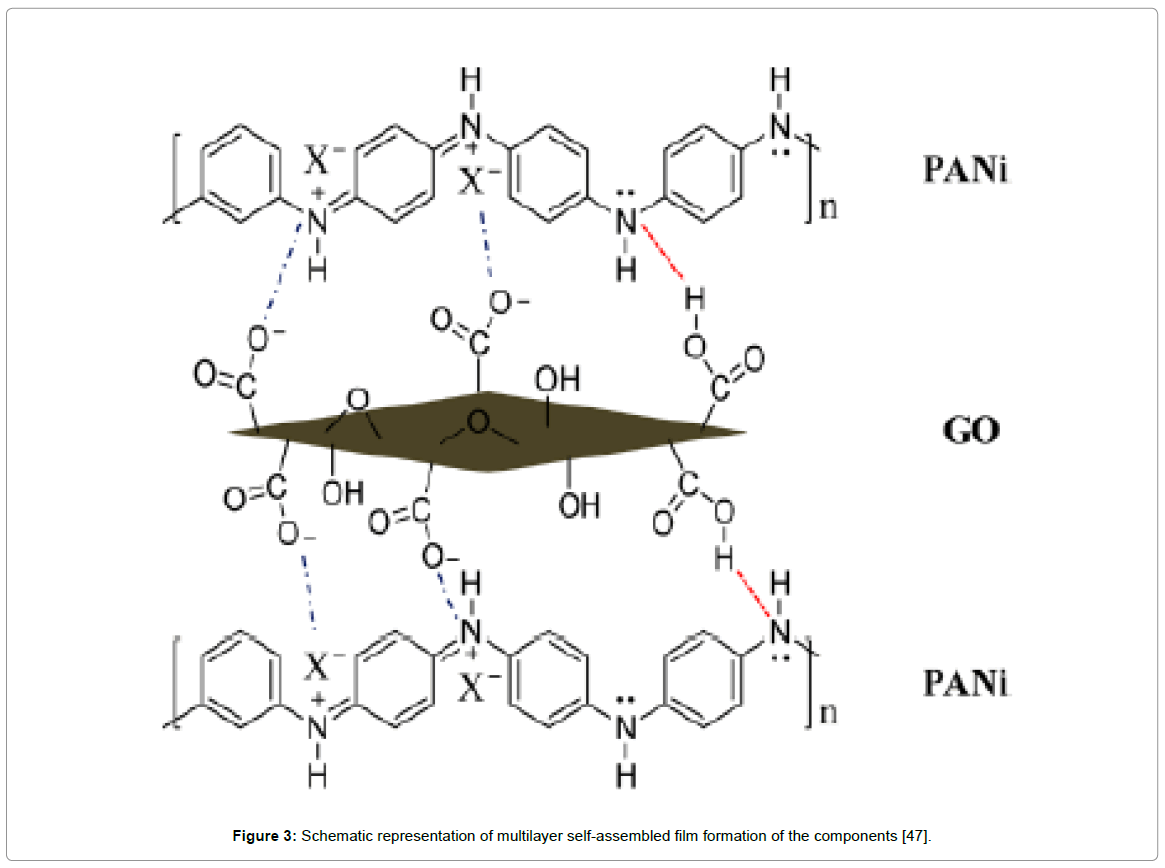
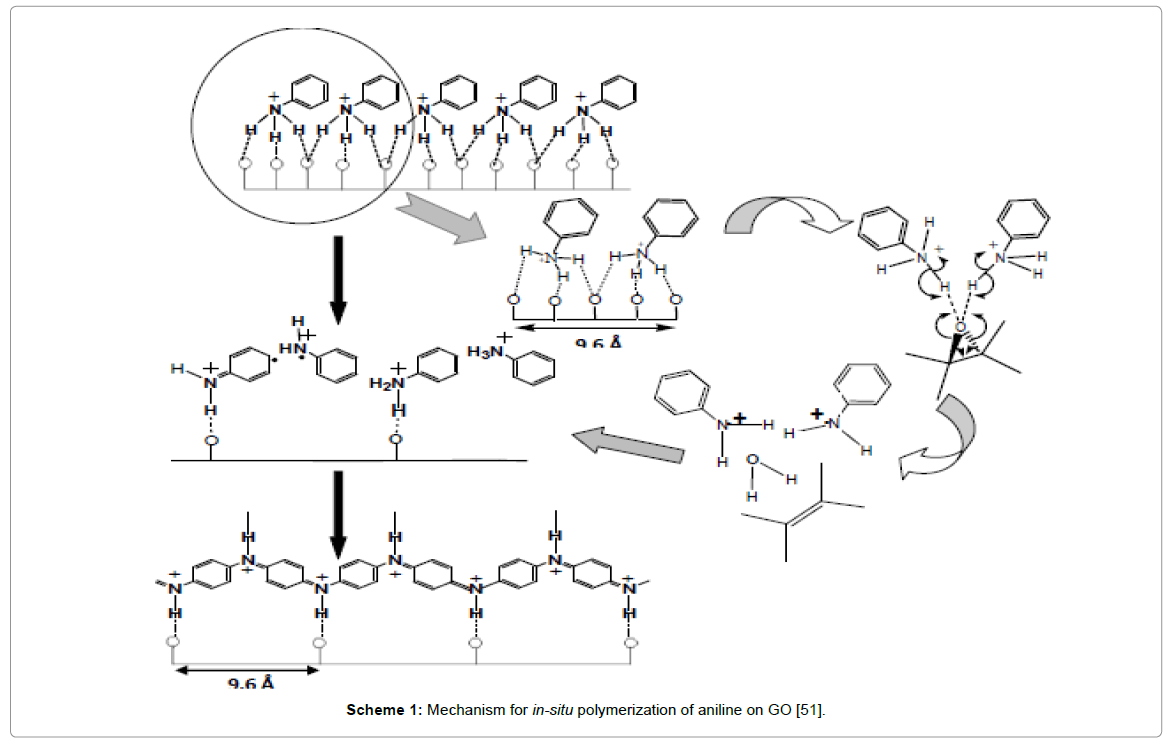
In many instances, it was often difficult to control the agglomeration of the PANI nanostructures during the polymerization of aniline, which could lead to a decrease in capacitance and the cycle durability of the graphene/PANI composites. Less agglomerated PANI nanostructures such as nanorod/nanowires exhibiting fibrous morphology with appropriate pore size distribution provided good contact with the electrolyte, resulting in faster doping–dedoping rate [44]. Lately, Tang et al reported composites with uniform distribution of PANI nanoparticles on GO sheets having strong interactions that exhibited a capacitance with a good stability during long-term cyclic voltammetric measurements [45]. In another method, porous graphene oxide/polyaniline composite was obtained by one-step electrochemical co-deposition method with specific capacitance as high as 1136.4 Fg-1 at a scan rate of 1 mVs-1 with a good cycling stability, with ~89% capacitance retention after 1000 cycles shown in Sarker and Hong [46]. Sarkar and Zhao groups independently fabricated layer-by-layer rGO sheets/polyaniline nano-structures by electrodepositing nanofibers of PANI onto electrically conductive graphene nanosheets to form a multilayer configuration with improved electrochemical performances shown in Figure 3 [46,47]. Morphological studies revealed that PANI nanofibers not only were coated on the surface but also intercalated into GO sheets that were responsible for increased surface area of the composite which significantly contributed to such enhanced specific capacitance value. Chen and co-workers designed homogeneous, three-dimensional porous sandwich-like, electrochemically reduced graphite oxide-polyaniline composite based supercapacitors which achieved the maximum specific capacitance value of 716 Fg-1 at 0.47 Ag-1 [48]. Parveen et al. reported high crystalline polyaniline-graphene nanosheets composite with excellent interfaces that exhibited specific capacitance of 613 Fg-1 at a scan rate of 10 mV/s [49]. Such unique features may be attributed to epitaxial growth of PANI onto rGO sheets due to simultaneous reduction of graphene oxide (GO) by aniline, followed by in-situ polymerization as shown in Scheme 1 [50]. Similar works on PANI nanofibers/graphene composite fabricated by Jia et al. exhibited enhanced specific capacitance of 965 Fg-1 at 0.5 Ag-1 and the capacity retention was 90% after 2000 cycles [51].
Figure 3: Schematic representation of multilayer self-assembled film formation of the components [47].
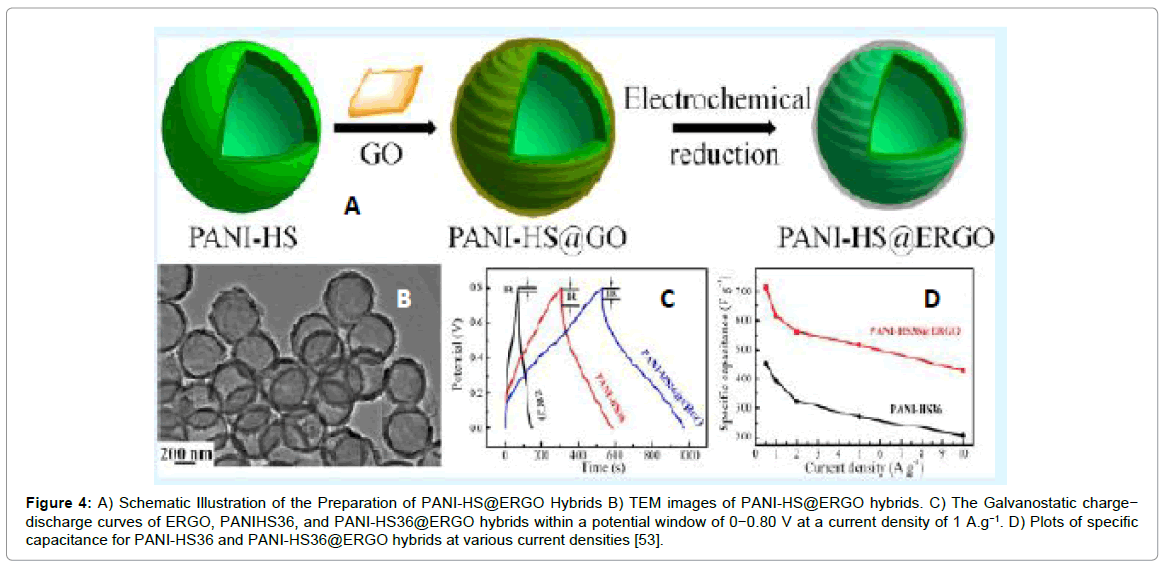
Polyaniline hollow spheres with core shell structures embedded onto electrochemical rGO hybrids have been fabricated by solution-based co-assembly process as shown in Figure 4. The specific capacitance of the resultant composite reached ~614 Fg-1 at a current density of 1 Ag-1 [52]. The hollow nanostructured morphology increased the effective surface area of electro-active regions, shortened diffusion lengths for transport of charge and ions simultaneously. Moreover, wrapping of graphene sheets on the hollow spheres provided highly conductive pathways facilitating the high rate and better cycling performance of supercapacitors.
Figure 4: A) Schematic Illustration of the Preparation of PANI-HS@ERGO Hybrids B) TEM images of PANI-HS@ERGO hybrids. C) The Galvanostatic charge− discharge curves of ERGO, PANIHS36, and PANI-HS36@ERGO hybrids within a potential window of 0−0.80 V at a current density of 1 A.g−1. D) Plots of specific capacitance for PANI-HS36 and PANI-HS36@ERGO hybrids at various current densities [53].
Scheme 1: Mechanism for in-situ polymerization of aniline on GO [51].
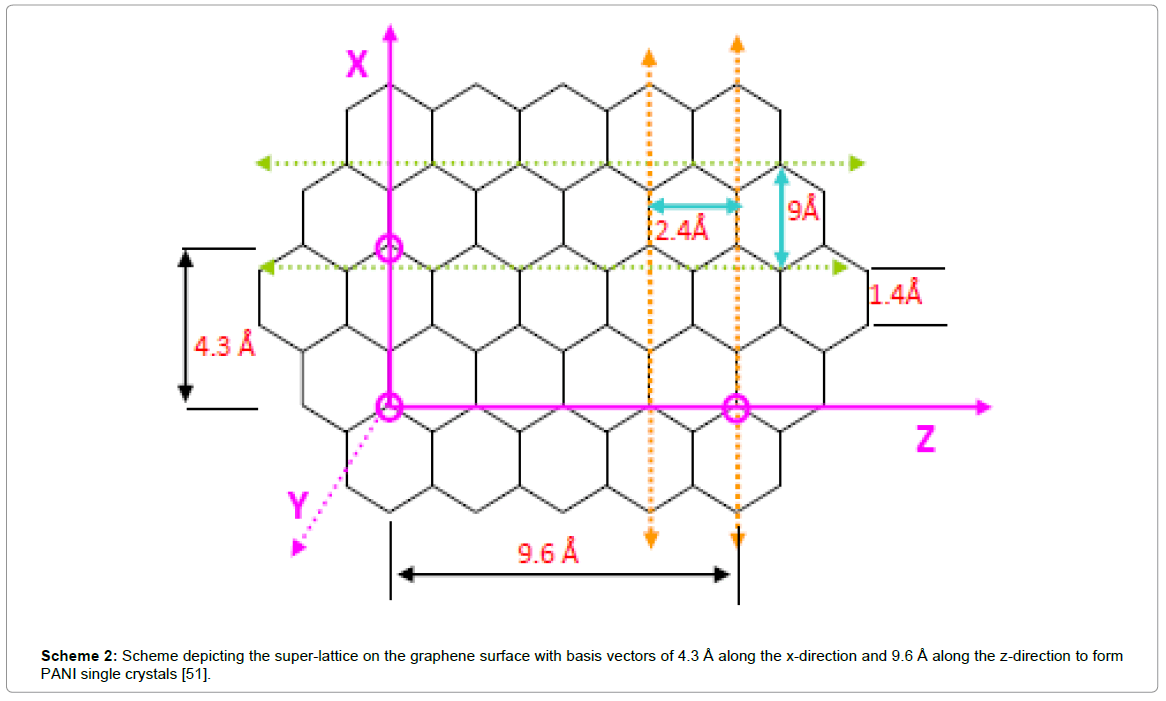
Scheme 2: Scheme depicting the super-lattice on the graphene surface with basis vectors of 4.3 Å along the x-direction and 9.6 Å along the z-direction to form PANI single crystals [51].
Graphene oxide/polyaniline nanocomposite, synthesized in supercritical carbon dioxide (SC-CO2), exhibited appreciable specific capacitance about 425 Fg-1 at a current density of 0.2 Ag-1 has been recently reported [53]. The SC-CO2 increases the dispersion of aniline monomer onto ethanol, also enhances the wettability of the GO basal planes, which trigger uniform distribution of PANI onto GO sheets in this case.
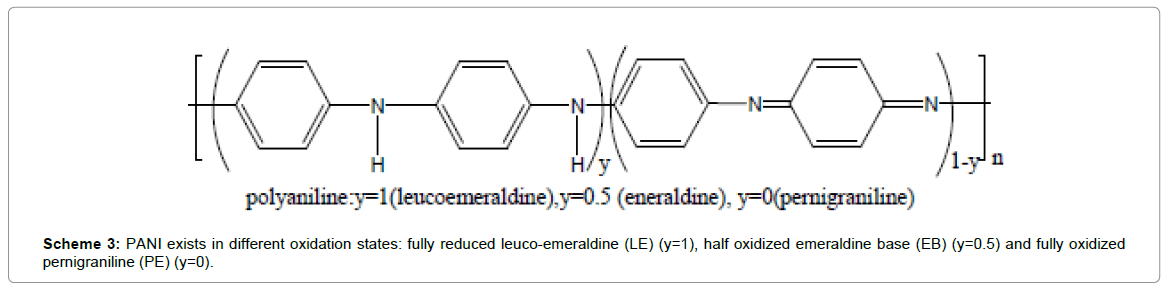
From the above studies we infer that the specific capacitance of functionalized-graphene/PANI composites vary over a large range depending upon morphological diversity. This is obvious, resulting from adopting different synthetic methodologies and modes of composite fabrication. Various important factors like homogeneity, porosity play key roles in determining the performances of the electrode materials. It is also obvious that as 1D nanostructures like nanorods/nanowires exhibit better charge transport properties than corresponding nanoparticles/granulated structures which is observed in the above stated systems. However, in some cases, the values are appreciably higher! It is very true that the synergistic contribution of capacitance of the two components is prime reason. However, one also has to consider that doped PANI behaves as p-type semiconductor while graphene systems owing to their π-electron density, act as n-type semiconductors. Thus the interface forms p-n junction and has a junction capacitance contributes to the overall capacitance of the system. However, the contribution of junction capacitance becomes significant and highly dependent on the quality of the interface between Graphene and PANI. In one of our previous studies, we reported that PANI can epitaxially grow onto rGO surfaces due to lattice matching between the d-values [~2.48 Å] of graphene and {120} planes of PANI as shown in Scheme 2, showing high quality p-n junction properties [50]. Thus optimization of reaction condition with doped-PANI and graphene is very much necessary to achieve superior electrochemical signatures. We have also shown in another work that growth of PANI chains along a surface (graphene) is very ordered up to certain thickness, beyond which they start getting entangled and poor junction results! This feature is again highly controlled by rate of polymerization; the rate being faster in electrochemical polymerization compared to chemical polymerization, where monomers rapidly combine as soon as come in contact with electrode leading to formation of straight chains and thus, high interfaces are obtained. That may be one reason for obtaining such wide range of specific capacitance values of the PANI-G composites. Moreover, the extent of doping in PANI is very crucial to observe charge storing properties. PANI can exist in different oxidation states: fully reduced leuco-emeraldine (LE) (y=1), half oxidized emeraldine base (EB) (y=0.5) and fully oxidized pernigraniline (PE) (y=0), as illustrated in Scheme 3 [54]. The emeraldine form (EB) is reported to exhibit greater stability and higher charge transport properties after protonation. On the contrary, both LE and PE even after the protonation are insulators. Usually, the PANI used in electrodes is a mixture of its three states and we expect highest portion of PANI-EB form in the composite to contribute to better performances! However, one of the major disadvantages of using conjugated polymers systems is their electrochemical degradation that occurs far rapidly than inorganic materials. As for PANI, on repeated cycling, it slowly gets decomposed and oxidized to p-benzoquinone and no alteration other than replacement of the electrode material is required [54]. Thus electrodes of such materials are required to take special precautions to avoid or improvise such difficulties!
Innumerable efforts are being accomplished daily to improve the electrode materials including the combination of EDL capacitance with reversible pseudocapacitance active materials. Various modified synthetic procedures, reaction conditions as well as utilizing organic electrolyte or ionic liquids are being employed to achieve higher stability potential window or employing hybrid asymmetric system to optimize the overall working potential [55,56]. Pioneer works on implication of flexible supercapacitors with other electronic and energy devices (i.e., solar cells, Li-ion batteries, electrochromic devices and nanogenerators) are well in progress [57,58]. Polymeric gel electrolyte are now often employed in deriving a high flexibility device, complimenting to the ability of gel structure to penetrate efficiently to the porous electrode to the nearest contact, while providing a stable structure in maintaining the dimension that is not susceptible to the physical bending force [59]. Further research is still needed to explore the role of the nano-hybrid morphologies and also on the control/tunability of the interfacial interaction between graphene and pseudocapacitive materials in order to improve the overall Faradic processes across the interface, which is yet to be achieved. Besides, multifunctional or self-powered hybrid systems are currently of considerable interests for future technological development. Despite all of the achievements and excellent progress, one of the main obstacles in supercapacitor technology is the high production cost when compared with other energy-storage devices. Therefore, the future tasks rightly focus toward the performance in a cost-effective route. One-step synthesis without any additional treatment would be ideal towards practicality on an industrial scale. Moreover, the ability of the fabricated supercapacitors to perform at elevated temperature/stringent environment remains doubted. Nonetheless, the superior properties so far obtained have paved a way which introduces the lime light that focus it as proficient material in energy storage devices while the effort in the development of a higher energy systems is in continuous progress!
Acknowledgements
DM acknowledges Barasat Govt. College, Barasat; IACS and Jadavpur University, Kolkata, WB, India for the necessary infrastructural facilities. Figure 2 has been taken from A nanostructured graphene/polyaniline hybrid material for supercapacitors. Nanoscale 2 (2010) 2164 Reproduced by permission of The Royal Society of Chemistry. DM also acknowledges ACS as well as Wiley publishers for granting copyright permission regarding incorporation of figures in the current manuscript.
References
- Burke A (2000) Ultracapacitors: why, how, and where is the technology. J Pow Sour 91: 37-50.
- Wang G, Zhang L, Zhangm J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41: 797-828.
- Nayak PK, Munichandraiah N (2012) Mesoporous MnO2 synthesized by hydro-thermal route for electrochemical supercapacitor studies. J Solid State Electrochem 16: 2739-2749.
- Wang K, Wu H, Meng Y, Wei Z (2014) Conducting polymer nanowire arrays for high performance supercapacitors. Small 10: 14-31.
- Yang XH, Wang YG, Xiong HM, Xia YY (2007) Interfacial synthesis of porous MnO2 and its application in electrochemical capacitor. ElectrochimicaActa 53: 752-757.
- Babu VJ, Vempati S, Ramakrishna S (2013) Conducting polyaniline-electrical charge transportation
- Mi H, Zhang X, Yang S, Ye X, Luo J (2008) Polyaniline nanofibers as the electrode material for supercapacitors. Mater Chem Phys 112: 127-131.
- Channu VSR, Holze R, Rambabu B, Kalluru RR (2012) Synthesis and characterization of PANI nanostructures for supercapacitors and photoluminescence. Iran Polym J 21: 457-462.
- Shown I, Ganguly A, Chen LC, Chen KH (2015) Conducting polymer?based flexible supercapacitor. EnerSciEng 3: 2-26.
- Moussa M, El-Kady MF, Wang H, Michimore A, Zhou Q, et al. (2015) High-performance supercapacitors using graphene/polyaniline composites deposited on kitchen sponge. Nanotechnology 26: 075702.
- Zhang H, Zhao Q, Zhou S, Liu N, Wang X, et al. (2011) Aqueous dispersed conducting polyaniline nanofibers: Promising high specific capacity electrode materials for supercapacitor. JPow Source 196: 10484-10489.
- Sumboja A, Wang X, Yan J, Lee PS (2012) Nanoarchitectured current collector for high rate capability of polyaniline based supercapacitor electrode. ElectrochimicaActa 65: 190-195.
- Coskun E, Zaragoza-Contreras EA, Salavagione HJ (2012) Synthesis of sulfonated graphene/polyaniline composites with improved electroactivity. Carbon 50: 2235-2243.
- Li F, Shi J, Qin X (2010) Synthesis and supercapacitor characteristics of PANI/CNTs composites. Chinese science bulletin 55: 1100-1106.
- Liu H, Xu B, Jia M, Zhang M, Cao B, et al. (2015) Polyaniline nanofiber/large mesoporous carbon composites as electrode materials for supercapacitors. Carbon 332: 40-46.
- Lee SY, Kim JI, Park SJ (2014) Activated carbon nanotubes/polyaniline composites as supercapacitor electrodes. Energy 78: 298-303.
- Zhang K, Zhang LL, Zhao XS, Wu J (2010) Graphene/polyaniline nanofiber composites as supercapacitorelectrodes. Chem Mater 22: 1392-1401.
- Ramakrishna Matte HSS, Subrahmanyam KS, Rao CNR (2011) Synthetic aspects and selected properties of graphene. Nanomater Nanotechnol 1: 5.
- Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, et al. (2008) Superior thermal conductivity of single-layer graphene. Nano lett 8: 902-907.
- Guo S, Dong S (2011) Graphene nanosheet: synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem Soc Rev 40: 2644-2672.
- Geim AK, Novoselov KS (2007) The rise of graphene. Nature materials 6: 183-191.
- Rao CEE, Sood AE, Subrahmanyam KE, Govindaraj A (2009) Graphene: the new two?dimensional nanomaterial. Ange Chem IntEdn 48: 7752-7777.
- Wang X, Liu X, Chen K (2016) Effect of Different Conductive Additives on the Electrochemical Properties of Mesoporous MnO2 Nanotubes. Int J ElectrochemSci 11: 6808-6818.
- Kim H, Park KY, Hong J, Kang K (2014) All-graphene-battery: bridging the gap between supercapacitors and lithium ion batteries. Scientific Reports 4.
- Gao L, Guest JR, Guisinger NP (2010) Epitaxial graphene on Cu (111). Nano lett 10: 3512-3516.
- Guo HL, Wang XF, Qian QY, Wang FB, Xia XH (2009) A green approach to the synthesis of graphene nanosheets. ACS Nano 3: 2653-2659.
- Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, et al. (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45: 1558-1565.
- Berger C, Song Z, Li T, Li X, Ogbazghi AY, et al. (2004) Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J Phys Chem 108: 19912-19916.
- Basirun WJ, Sookhakian M, Baradaran S, Mahmoudian MR, Ebadi M (2013) Solid-phase electrochemical reduction of graphene oxide films in alkaline solution. NanoscaleRes lett 8: 397.
- Toh SY, Loh KS, Kamarudin SK, Daud WRW (2014) Graphene production via electrochemical reduction of graphene oxide: synthesis and characterisation. ChemEng J251: 422-434.
- Xia J, Chen F, Li J, Tao N (2009) Measurement of the quantum capacitance of graphene. Nature Nanotechnol4: 505-509.
- Brownson DA, Varey SA, Hussain F, Haigh SJ, Banks CE (2014) Electrochemical properties of CVD grown pristine graphene: monolayer-vs. quasi-graphene. Nanoscale 6: 1607-1621.
- Wang DW, Li F, Zhao J, Ren W, Chen ZG, et al. (2009) Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 3: 1745-1752.
- Zhang Q, Li Y, Feng Y, Feng W (2013) Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor. ElectrochimicaActa 90: 95-100.
- Xu J, Wang K, Zu SZ, Han BH, Wei Z (2010) Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 4: 5019-5026.
- Wang H, Hao Q, Yang X, Lu L, Wang X (2010) Effect of graphene oxide on the properties of its composite with polyaniline. ACS Appl Mater Inter 2: 821-828.
- Wang H, Hao Q, Yang X, Lu L, Wang X (2009) Graphene oxide doped polyaniline for supercapacitors. ElectrochemComm 11: 1158-1161.
- Feng W, Zhang Q, Li Y, Feng Y (2014) Preparation of sulfonated graphene/polyaniline composites in neutral solution for high-performance supercapacitors. JSolid State Electrochem 18: 1127-1135.
- Wang Q, Yan J, Fan Z, Wei T, Zhang M, et al. (2014) Mesoporous polyaniline film on ultra-thin graphene sheets for high performance supercapacitors. JPow Source 247: 197-203.
- Mitchell E, Candler J, De Souza F, Gupta RK, Gupta BK, et al. (2015) High performance supercapacitor based on multilayer of polyaniline and graphene oxide. Synthetic Metals 199: 214-218.
- Yan J, Wei T, Shao B, Fan Z, Qian W, et al. (2010) Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 48: 487-493.
- Gómez H, Ram MK, Alvi F, Villalba P, Stefanakos EL, et al. (2011) Graphene-conducting polymer nanocomposite as novel electrode for supercapacitors. J Pow Source 196: 4102-4108.
- Wang H, Hao Q, Yang X, Lu L, Wang X (2010) A nanostructured graphene/polyaniline hybrid material for supercapacitors. Nanoscale 2: 2164-2170.
- Hu L, Tu J, Jiao S, Hou J, Zhu H, et al. (2012) In situ electrochemical polymerization of a nanorod-PANI–Graphene composite in a reverse micelle electrolyte and its application in a supercapacitor. Phys Chem ChemPhys 14: 15652-15656.
- Luo Z, Zhu L, Zhang H, Tang H (2013) Polyaniline uniformly coated on graphene oxide sheets as supercapacitor material with improved capacitive properties. Mater ChemPhys 139: 572-579.
- Sarker AK, Hong JD (2012) Layer-by-layer self-assembled multilayer films composed of graphene/polyaniline bilayers: high-energy electrode materials for supercapacitors. Langmuir 28: 12637-12646.
- Zhang LL, Zhao S, Tian XN, Zhao XS (2010) Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir 26: 17624-17628.
- Li Y, Peng H, Li G, Chen K (2012) Synthesis and electrochemical performance of sandwich-like polyaniline/graphene composite nanosheets. Eur Poly J 48: 1406-1412.
- Parveen N, Mahato N, Ansari MO, Cho MH (2016) Enhanced electrochemical behavior and hydrophobicity of crystalline [email protected] graphene nanocomposite synthesized at elevated temperature. Composites Part B: Engineering 87: 281-290.
- Majumdar D, Baskey M, Saha SK (2011) Epitaxial growth of crystalline polyaniline on reduced graphene oxide. Macromol Rapid Comm32: 1277-1283.
- Jin Y, Fang M, Jia M (2014) In situ one-pot synthesis of graphene–polyaniline nanofiber composite for high-performance electrochemical capacitors. Appl Surface Sci 308: 333-340.
- Fan W, Zhang C, Tjiu WW, Pramoda KP, He C, et al. (2013) Graphene-wrapped polyaniline hollow spheres as novel hybrid electrode materials for supercapacitor applications. ACS Appl Mater Inter5: 3382-3391.
- Xu G, Wang N, Wei J, Lv L, Zhang J, et al. (2012) Preparation of graphene oxide/polyaniline nanocomposite with assistance of supercritical carbon dioxide for supercapacitor electrodes. Indus Eng Chem Res 51: 14390-14398.
- Khdary NH, Abdesalam ME, Enany GE (2014) Mesoporous polyaniline films for high performance supercapacitors. J Electrochem Soc 161: G63-G68.
- Hsieh CT, Hsu SM, Lin JY, Teng H (2011) Electrochemical capacitors based on graphene oxide sheets using different aqueous electrolytes. J Phys Chem 115: 12367-12374.
- Iessa KHS, Zhang Y, Zhang G, Xiao F, Wang S (2016) Conductive porous sponge-like ionic liquid-graphene assembly decorated with nanosized polyaniline as active electrode material for supercapacitor. J Pow Sourc 302: 92-97.
- Chee WK, Lim HN, Zainal Z, Huang NM, Harrison I, et al. (2016) Flexible graphene-basedsupercapacitors: a review. JPhys Chem 120: 4153-4172.
- Cong HP, Ren XC, Wang P, Yu SH (2013) Flexible graphene–polyaniline composite paper for high-performance supercapacitor. Ener EnvironSci 6: 1185-1191.
- Yu H, Wu J, Fan L, Lin Y, Xu K, et al. (2012) A novel redox-mediated gel polymer electrolyte for high-performance supercapacitor. J Pow Sourc 198: 402-407.
Citation: Majumdar D (2016) Functionalized-Graphene/Polyaniline Nanocomposites as Proficient Energy Storage Material: An Overview. Innov Ener Res 5: 145.
Copyright: ©2016 Majumdar D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 12580
- [From(publication date): 12-2016 - Dec 18, 2024]
- Breakdown by view type
- HTML page views: 11616
- PDF downloads: 964