Review Article Open Access
Functionalized Biomaterials - Oxygen Releasing Scaffolds
Jeong Ok Lim1,2*, Jeung Soo Huh1, Syed Izhar Haider Abdi1,2, Sing Muk Ng3 and James J Yoo41Kyungpook National University, Daegu 700-412, Republic of Korea
2Biomedical Research Institute, Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu 700-412, Republic of Korea
3School of Engineering, Computing and Science, Swinburne University of Technology Sarawak Campus, Jalan Simpang Tiga, 93350, Kuching, Malaysia
4Institute for Regenerative Medicine, Wake Forest University Health Sciences, Winston Salem, NC USA
- Corresponding Author:
- Jeong Ok Lim
Department of Biomedical Science
Kyungpook National University, Daegu 700-412, Republic of Korea
Tel: 82534205447
E-mail: jolim@knu.ac.kr
Received date:: April 25, 2015; Accepted date:: May 28, 2015; Published date:: June 04, 2015
Citation: Lim JO, Huh JS, Abdi SIH, Ng SM, Yoo JJ (2015) Functionalized Biomaterials - Oxygen Releasing Scaffolds. J Biotechnol Biomater 5:182. doi:10.4172/2155-952X.1000182
Copyright: © 2015 Lim JO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The advancement in tissue engineering has reached a considerably high level with major achievements, especially in mimicking the nature in terms of morphology, structure, functionality and mechanical strength. Nonetheless, the current technology still fails to deliver the urgent need in producing construct of larger volume such as organs, which will be more effective in tackling chronic diseases related to organ failure. One of the main causes identified is due to the serious necrosis that occurs as a result of the deficient of oxygen due to its low dissolution and diffusion in thick tissue matrices. The rate of vascularization is far too low compared to the differentiation rate of the cells. In order to sustain the survival of cells before the establishment of blood vessel, an alternative supply of external oxygen to the cells will be of advantage. Current trend has seen to be moving towards this direction, and the external supply can be obtained from tissue scaffolds. This approach is made possible by functionalizing biomaterials with well controlled oxygen producing mechanism. This review concentrates on such efforts and discusses some of the insights that are related in developing functionalized biomaterial scaffolds with the intention to adequately supply oxygen for tissue engineering purpose.
Keywords
Oxygen supplying scaffold; Drug delivery system; Tissue engineering; Hypoxia; Polymeric microsphere; Regenerative medicine
Introduction
Millions of people in the world suffer from a variety of diseases that could be aided from therapies such as organ or tissue transplantation. Despite the widespread need for transplantable organ, the current availability still far below the demand causing many patients dies while waiting for donor organs. This is basically due to lack of donor and the issue of bio-suitability since not all organs are readily accepted by the host of the acceptor. Thus, this initiated tissue engineering; an interdisciplinary field that involves the use of biological sciences and engineering to develop tissues to custom restore, maintain, or enhance tissue functions [1]. In this approach, cells are grown on biomaterial scaffolds designed to guide cell differentiation in forming tissue structure that can be later implanted into a host acceptor. The engineered tissues must have immediate functionality and the ability to be remodeled and integrated into the body.
In the last years have marked a substantial paradigm shift about design strategies for scaffolds, which includes the need for cellular and tissue patterning [2], microcirculation development [3], and the use of suitable stimuli [4] to ensure the appropriate bio-functional and mechanical properties are restored within the newly formed tissue. More advanced stage involves the design of artificial implant materials for functional tissue regeneration that mimics the natural extracellular matrix (ECM) [5]. The artificial ECM provides optimum needs such as nutrients, oxygen, and growth factors, besides specific functionality for cell survival. Thus, it is critical to recreate conditions that mimic the natural ECM environment for a particular cell type in order to support their viability until reaching a full functional organ. When cells are in contact with the interface of the biomaterials, the surface property will be significantly influencing the behavior and performance of the cell. As so, trends tend to progress toward designing and functionalizing the biomaterials that are able to modulate and control the behavior of the cell.
In real practical needs, the issues of cell survival and functionality are often the major concern since dealing with more complex system of large volume implant [6]. The main problem is the inability to supply sufficient oxygen to the cells in the initial phase after implantation yet oxygen is one of the most important elements for cell survival [7]. As a result, hypoxia often occurs within the deeper regions of the tissue-engineered construct and falling the effort of organ transplant [8,9]. Although this reason has been identified and well accepted, it remains a tough challenge in designing suitable scaffold to supply sufficient amount of oxygen to cells. Numerous efforts have been made to overcome this limitation over the years which include the use of synthetic carriers [10], enhance the vascularization process [7], fabricating oxygen generating scaffold [11], and designing oxygen producing micro-system [12,13]. However, these efforts have yet to demonstrate a full success in achieving survival of a clinical acceptable large tissue mass. In acknowledging the need of greater research effort and development in this area, this review plans to give some of the overviews and insights on those currently available efforts that might be related and able to generate the new ideas in overcoming the problem of oxygen deficiency in tissue engineering. The review first discusses on the influence of hypoxia on engineered construct, followed by the current approaches in minimizing necrosis, effort in developing oxygen releasing micro-system, quantitative and qualitative evaluation of the micro-system, and discussing the possible challenges in tackling oxygen deficiency via functionalizing the biomaterials.
Influence of Hypoxia on Tissues
In natural biological system, blood vessels are part of the circulatory system. Blood is used to transport oxygen, nutrients and waste products, to and from almost every part of the body. Three distinct structures can be distinguished in the vascular system, which are (i) macro vessels (arteries and veins) that branch out into (ii) micro vessels (arterioles and venules) and finally into (iii) capillaries. The capillaries facilitate the actual distribution of nutrients to the tissues in the body.
New blood-vessel formation is required for tissues to grow beyond 100–200 μm since the oxygen will not be able to penetrate through due to the diffusion limit of oxygen. This also applies for tissue-engineered constructs [14,15]. During in vitro culture, larger tissue-engineered constructs are commonly supplied with nutrients using perfusion bioreactors. However, after the implantation, such optimum conditions are no longer controllable in the in vivo system. The supply of oxygen and nutrients to the implant is often limited by diffusion processes and can only reach cells in proximity of 100–200 μm from the nearest capillary [14]. In order for implanted tissues of greater size to survive, the tissue has to be vascularized in forming new capillary network within the tissue. After implantation, blood vessels from the host generally invade the tissue to form such a network, in part in response to signals that are secreted by the implanted cells as a reaction to hypoxia [16]. However, this spontaneous vascular in-growth is often limited to several tenths of micrometers per day, meaning that the time needed for complete vascularization of an implant of several millimeters is in the order of weeks [17]. During this time, insufficient vascularization can lead to nutrient deficiencies and/or hypoxia deeper in the tissue. These changes have been associated with the increase in cell density over time, resulting in a higher overall consumption of oxygen. Besides the pores of the scaffolds will be filled with the cells [18]. This blocks any potential convection through the scaffold, thus potentially hampering the diffusion of nutrients into the construct [19]. Moreover, nutrient and oxygen gradients will be presented in the outer regions of the tissue, which could result in non-uniform cell differentiation and integration that decrease tissue functionality [20].
Since the slow rate of vascularization after implantation is a major problem in tissue engineering, the successful use of tissue-engineered constructs is currently limited only to thin or a vascular tissues such as skin or cartilage [21]. Post-implantation neo-vascularization from the host is sufficient for these thin tissues to meet the demand for oxygen and nutrients. Majority of cells in the mammalian body are highly dependent on oxygen. The exceptions are possibly being chondrocytes that originate from a non-vascularised tissue and cartilage, which are adapted to survive under low-oxygen conditions [22]. Oxygen is normally delivered through erythrocytes, where oxygen is taken up in the lungs and distributed along the journey through the vascular network in exchange for carbon dioxide. In order to succeed in engineering larger tissue volume such as bone and muscle, the problem of slow vascularization within the new tissue-construct has to be solved [9].
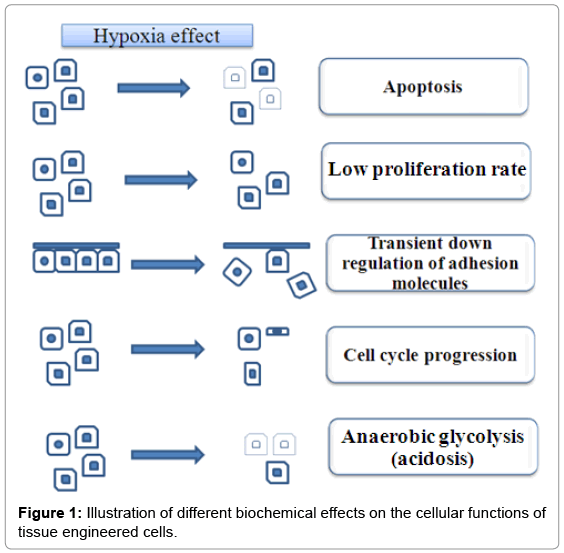
Under the well-controlled cell culture conditions, the concentration of oxygen for the cells to survival is set at optimum in the presence of all required nutrients. Thus, this generally avoids necrosis for the cell constructed on the scaffold in vitro. The problem arises only when the cell (tissue)-scaffolds are implanted onto a tissue lesion, as cells (tissue) are exposed suddenly to an in vivo environment with both low concentrations of oxygen and nutrients. Surgical intervention often provides the surrounding with the variety of cytokines and growth factors aiming to heal the wound, besides providing inflammatory reaction with activated granulocytes and later macrophages. However, the intracellular pathways responding to hypoxia condition remains problematic and requires further exploration for innovative solutions. Without solving this, the problem of oxygen deficiency will clearly influence the proliferation rate, cell cycle progression, apoptosis, adhesion of the cells, etc. Research are ongoing and focus should aims on the biomolecular mechanisms involved [23,24] (Figure 1).
Approaches to Overcome Hypoxia
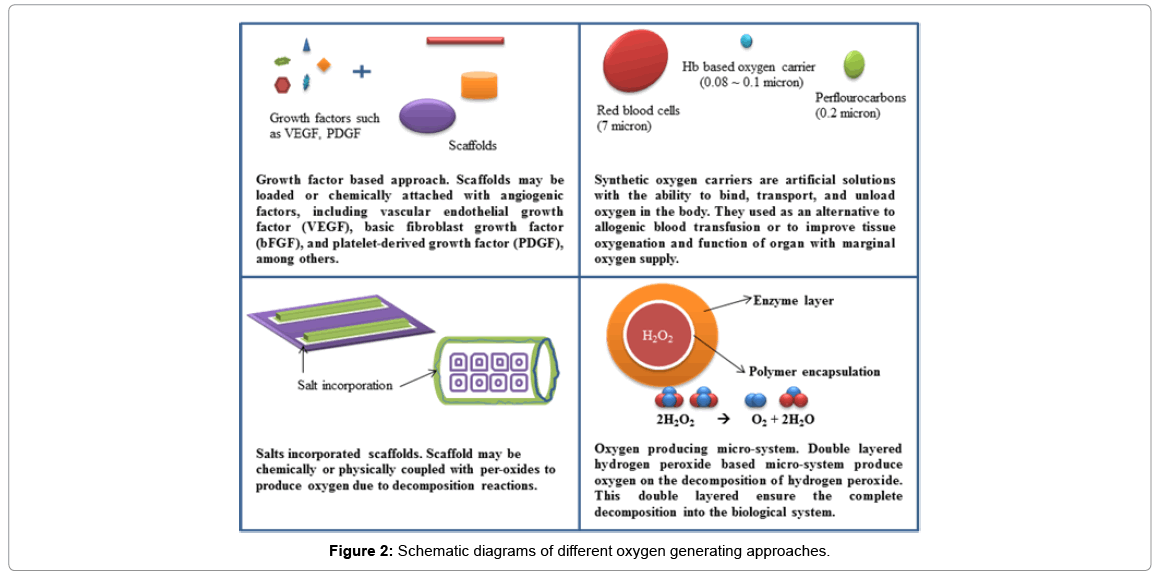
Since oxygen deficiency has been recognized as major failure reason of large volume implant effort, it has attracted increasing attention especially in tackling problem due to slow diffusion of oxygen through tissue, lower solubility of oxygen under in vivo condition, and a higher consumption rate of oxygen compared to other major nutrients such as glucose [25-29]. Methods have been used to accelerate and facilitate the formation of blood vessel by using growth factors or endothelial cells. Various efforts have been made to overcome this inadequacy over the years, which include the use of enhance vascularization through growth factors [30-33] and synthetic oxygen carriers [34-38]. Alternatively suggestions have been made to temporarily providing oxygen and nutrients through scaffolds [11,39] and using of oxygen producing micro-system [12,13] before permanent vascularization occurs to mitigate the deleterious consequences associated with immediate lack of blood supply after implantation. All these efforts enhanced the supply efficiency of oxygen within the engineered system. The following section will discuss some current overviews and insights on the strategies in promoting the concentration of oxygen to tissues implants. The summary of the approaches are given in Figure 2.
Enrichment of vascularization
In biological system, tissues rely on blood vessels for the supply of oxygen and nutrients. The formation of new blood vessels is required when tissue grows beyond the diffusion limit of oxygen [40]. Having this understanding, approach has been taken to induce and speed-up the formation of new blood vessels in the implant and the merging process of blood vessels in the construct with the host’s respiratory system. One of the classical approaches to overcome this issue is to enhanced scaffolds with pro-angiogenic factors such as VEGF, bFGF, or PDGF [41-43]. Endothelial (progenitor) cells are activated and stimulated to migrate towards the factor gradient besides promoting cell assembly, vessel formation and maturation. It is important to choose the suitable specific growth factor and delivery it at an optimum rate and amount in order to coordinate both vascularization and tissue regeneration at a balance level.
Growth factors are usually demonstrated in vivo to be very unstable and therefore the bolus injection of growth factors was increasingly popular replacing the area-restricted and long-time delivery strategy [44,45]. Alternative approach to overcome the high degradation rate of the expensive growth factors is to design new natural, synthetic or composite biomaterials with controlled-release property. Some findings have demonstrated biomaterials with degradable porous structures or pre-encapsulated microspheres have been used to control effective targeting [46-48]. For an effective long-term delivery, growth factors can be encapsulated in biodegradable polymers such as poly (lactic-coglycolic acid) (PLGA) [49,50]. The dosage of these growth factors must be well-controlled as excess release has shown causing severe vascular leakage and hypotension [51]. Furthermore, newly formed vessels require stabilization and this is usually performed by a recruitment of smooth muscle cells and pericytes to the vessels that can subsequently produce the extracellular matrix to support the vessels structure.
PDGF is responsible for the recruitment of smooth muscle cells and pericytes, transforming growth factor (TGF-β) for the production of extracellular matrix, besides ensuring optimum interaction between endothelial cells and mural cells [52-54]. Both the formation and subsequent stabilization of new vessels are important for the creation of a functional vascular network within a tissue-engineered graft. The delivery of two or more sets of factors that are able to stimulate new blood-vessel formation and maturation might be necessary for optimal blood perfusion [55-58]. For instance, the delivery of both VEGF and PDGF has proven to result in the formation of a highly dense mature vessels in implanted [54,59]. Freeman et al.demonstrated that by sequentially delivering of three angiogenic factors i.e. VEGF, PDGFBB and transforming growth factor-β1 (TGF-β1) has resulted in greater vessel density compared to the use of bFGF alone [60]. While success has been achieved with these different types of multi bulk-loading and surface-coupling approaches, the remaining problem falls on the restriction in delivering of these factors to the region of need as well as controlling the temporal release profile [40,51].
Ultimately, the ability to control the release of growth factors will influence the degree of vascularization within the tissue. To direct angiogenesis, it is critical to determine the release kinetics of the angiogenic factors from the scaffold [52,53,61]. In some cases, the vascularization rate may not be compatible with the growth rate of the cell throughout the construct, causing cells at the center of the tissue undergo apoptosis before vascularization is complete [62]. Due to this cell response dependency to the vascularization, the technique is not highly controllable. The prohibitive cost and complexity of maintaining high level of recombinant growth factor over a sufficient time frame are also some of the major obstructs to bring this alternative into clinical trial stage [44,56]. Various strategies for generating oxygen are summarized as shown in the Table 1.
| Strategy | Achievements | Problems | References |
|---|---|---|---|
| Growth factor based approaches | Proven to be effective Control vascularization |
Very slow vascularization Prolong storage of these growth factors |
[40,44,63] |
| Synthetic oxygen carrier | Blood substitute Increased tissue oxygenation |
Biologically inert Molecules are seize in the reticuloendothelial system |
[10,35,38,44,64] |
| Scaffolds for oxygen release | Versatile strategy Used for multiple cells |
Limited results to be expected Cell seeding problems |
[11,39] |
| Oxygen release micro-system | Versatile applications Used for multiple cells Only oxygen and water were produced as by products |
Size (micro) | [12,13] |
Table 1: Comparison of different strategies used for generating oxygen.
Synthetic oxygen carrier
Hemoglobin (Hb) is the functional component in the blood that is responsible for the binding oxygen in the lung, delivers, and releases it to cells over the entire body. Nevertheless, the mimic of such elegant system for implant engineering is still not achievable due to the current technological limitations. However, efforts are still being put in this direction especially to develop artificial oxygen carrier system. Two that are most establish and successful liquid artificial oxygen carriers are based on synthetic Hb and perflurochemicals (PFC).
In 1957 Chang et al, have first demonstrated that free Hb could be encapsulated in a polymer membrane. Later, Hb encapsulated in phospholipid vesicles (liposomes) was developed as a potential red cell substitute [65]. Similar concept has been extended to imbed totally synthetic heme between two lipid bilayers (lipid-heme vesicles) [35]. In a different approach, stable fat mircrospheres suspension have been achieved by emulsifying triglycerides with lipid-heme as a surfactant [34,66,67]. These lipid-heme products are reported to have heme concentration and reversible oxygen binding nature close to that of normal blood. However, free Hb itself tends dissociate into individual dimmers that can cause directly functional effect on kidney and also reducing its effective lifetime of usage [68,69]. In addition, cell-free Hb is lacking of its essential affectors; 2,3-diphosphoglycerate that prevents the release of oxygen to the tissue [70]. Therefore modifications are required to increase the stability of the carrier besides optimizing its performance towards the delivery of oxygen. Possible modifications are such as direct cross-linking between Hb or the encapsulation of Hb into a secondary matrix [71,72]. However, these products did not manage to pass clinical trials for real application due toxicity issue [73,74]. Harmful side-products can be released causing deleterious effects on cells. To overcome this issue, Centis et al. suggested the use of liposomes encapsulated with human Hb that is further dispersed in a fibrin gel to deliver oxygen to cells [74]. They demonstrated that the available oxygen provided by the liposomes containing Hb promoted cell growth in 3D scaffold.
Perfluorocarbons (PFCs) are another kind of commonly used oxygen carriers [36]. PFCs are chemically inert synthetic molecules consist of primarily carbon and fluorine atoms with low toxicity toward biological system [75]. Uniquely, solubility co-efficient of oxygen in PFCs is at most 20 times higher than blood [76]. They also have the ability to physically dissolve significant quantities of many other gases including carbon dioxide. PFCs are hydrophobic and since not miscible with water [77], PFCs have to be emulsified for intravenous use. Using advanced technology, it is possible to produce stable emulsion with exceptionally small particles (median diameter<0.2 μm) [75] that could be employed as oxygen carrier for regenerative applications. Optimization of the protocol and formulation are required to obtain good emulsions and the maximum concentration of PFCs is usually around 15% (v/v). Such PFC-containing physiological saline or culture medium can produce an overall oxygen solubility of is at most four times higher than those of culture medium. This is still not enough to meet the demand of large tissue regeneration [73]. Although PFCs are biologically inert, the emulsified form that are infused to humans can cause complement activation and reduced platelet functions [72]. In addition, “Oxygent”, the only commercially available product reported so far has been terminated from human clinical trials. This seems to make PFC-based oxygen carrier less popular [78]. To overcome this issue, Radisic et al. have proposed an idea to combine channeled 3D scaffolds with perfusion of oxygen carrier-containing culture medium [79]. The channels were perfused with 5% (v/v) of PFCs to increase the overall oxygen solubility in the perfused culture medium. Having this, oxygen gradient occurring along the direction of the perfusion can be minimized. Besides, the formed tissue is showing higher DNA contents and enhanced expression of cardiac marker genes.
Another way to utilize PFCs is by increasing the local oxygen delivery flux to cells immobilized in hydrogels-based matrices [80]. However, these matrices produced non-consistent results. Maillard et al. demonstrated that culturing islets in a fibrin-based matrix supplemented with emulsified perfluorodecalin (PFC family member) can prevent islet hypoxia [81]. They also concluded that such system is beneficial for promoting islet viability, functionality, and morphology. In a similar study, Chin et al. have demonstrated an improvement in the cellular function in model system consisted of alginate immobilized with PFCs and human hepatocellular carcinoma (HepG2) cells [80]. The results show that PFCs manage to increase the oxygen environment at the hydrogels interface. Conversely, Goh et al. found no statistically significant improvement on the cell growth, metabolic activity or to the induced insulin secretion from the cells after the addition of PFCs emulsion (10 vol%) to encapsulated system of βTC-tet cells in calcium alginate [82].
It is clear that system incorporated with PFCs can increase oxygenation by enhancing dissolved oxygen effective diffusivity through the matrix, but not serving as an oxygen reservoir. PFCs in this system have only limited capacity to supply oxygen and did not have any re-oxygenation mechanism to introduce back the reservoir in the PFCs. However, it remains unclear whether the increase in effective diffusivity is sufficient to produce consistent and experimentally measurable positive effects on the encapsulated cells. Furthermore, during the design of such systems, it is important to incorporate PFCs at a concentration that will not compromise mechanical integrity and immune-protective characteristics of the original matrix selected.
Scaffold for oxygen release
Nature offers pattern of systems having smart materials and functioning mechanism, which can be used as learning model in efforts to make improvements that fulfill the need of daily life. The mimicking efforts no doubt is demanding but given the most promising potential of accomplishment in various areas especially those tissue engineering related. It is well known that peroxide salts will get dissociated into hydrogen peroxide upon dissolved in water and subsequently the hydrogen peroxide further decomposes into water and oxygen. There are attempts to supply sufficient amount of oxygen for tissue regeneration via direct method using chemical generated oxygen that is incorporated within scaffolds.
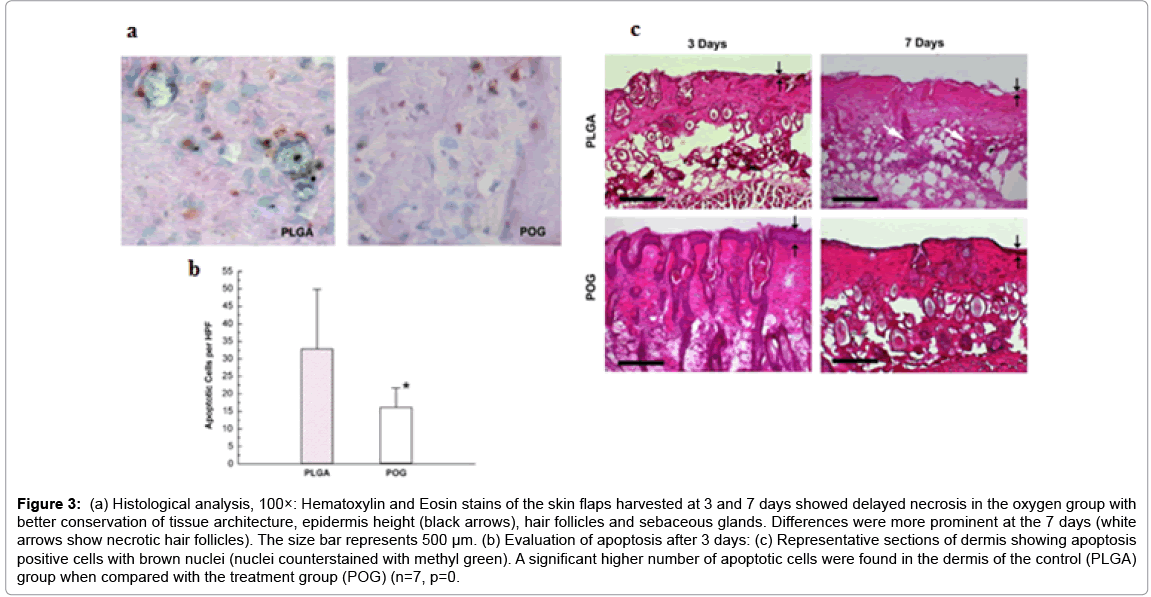
Researchers at Wake Forest University have been working with peroxide related compounds that generate oxygen through chemical decomposition mechanism [11,39]. These peroxides incorporated scaffolds represent a novel class of biomaterials that provide oxygen in situ for a temporary period up to 2 weeks. These scaffolds begin to generate oxygen upon the contact with aqueous solution. Harrison et al. have used sodium percarbonate incorporated scaffolds for in vivo tissue salvage with an implantable film form in an ischemic skin flap model in mice [39]. It managed to delay the onset of necrosis via early supplementation of oxygen (Figure 3). In the system employed, solid sodium percarbonate was used to produce oxygen along with sodium and bicarbonate ions. The study shows that typically degradation processes associated with hypoxia, such as apoptosis, discoloration, and lactate increase seem to be slowed down with the supplementation of oxygen. In a similar work, Oh et al. incorporated calcium peroxide in a 3D porous poly (lactic-co-glycolic acid) scaffold through a porogen leeching process. The oxygen-generating biomaterials were seeded with cells and cultured in a hypoxic environment, where majority of the oxygen supply was from the scaffold. The result shows that the metabolic rate remained steady for 5 days and only dropped dramatically (~80%) by day 10. This lack of proliferation followed by a continuous decrease in cell number is likely due to oxygen deficiency. In contrast, the metabolic rate in the calcium peroxide containing scaffolds doubled over that same time period. Scaffolds containing calcium peroxide are found to maintain better cell viability over time using histological analysis. After 2 weeks of incubation under 1% oxygen, almost all cells had completely disappeared from the control scaffolds. However, in the oxygen generating scaffolds, there was an increase in the number of cells with homogenous distribution by day 10.
Figure 3: (a) Histological analysis, 100×: Hematoxylin and Eosin stains of the skin flaps harvested at 3 and 7 days showed delayed necrosis in the oxygen group with better conservation of tissue architecture, epidermis height (black arrows), hair follicles and sebaceous glands. Differences were more prominent at the 7 days (white arrows show necrotic hair follicles). The size bar represents 500 μm. (b) Evaluation of apoptosis after 3 days: (c) Representative sections of dermis showing apoptosis positive cells with brown nuclei (nuclei counterstained with methyl green). A significant higher number of apoptotic cells were found in the dermis of the control (PLGA) group when compared with the treatment group (POG) (n=7, p=0.
These results illustrate the potential of these novel oxygenincorporating biomaterials used in extending cell viability and tissue preservation both in vitro and in vivo until vascularization can be established. However the byproducts generated from these decomposition mechanisms is still remain major concern (Table 2). Basically when calcium peroxide decomposes, it also forms hydroxide ions as byproducts in addition to oxygen, which resulted in pH increase and too alkaline for cell survival. Similarly, the decomposition of sodium percarbonate release sodium and bicarbonate ions as well.
| Decomposition mechanism | Chemical reaction | By products |
|---|---|---|
| Decomposition through calcium peroxide | CaO2 + 2H2O → Ca (OH)2 + H2O2 2H2O2 → O2 + 2H2O |
|
| Decomposition through sodium bicarbonate | [Na2CO3]2 • 3H2O2 → 2Na++2CO3−2+3H2O2 2H2O2→O2+2H2O |
|
| Direct decomposition of hydrogen peroxide | 2H2O2→O2+2H2O |
|
Table 2: Decomposition mechanisms used to produce oxygen for tissue engineering applications and their byproducts which may produce toxic effect in the microenvironment of the scaffolds.
Oxygen Releasing Functionalized Systems
The use of oxygen releasing functionalized systems might be a good solution in overcoming the limitation in tissue engineering caused by necrosis. Some of the insight of such systems will be discussed in the following section. It basically focuses on the development aspect such as formulation and methodology, and the evaluation assay for such systems.
Formulation and methodology
Several main factors are required to be considered in developing of a functionalized oxygen releasing microsystem (Figure 4). First criteria will be the identification of a suitable oxygen releasing source that can release oxygen upon activation. This can be of chemical compounds or biological substances. In nature, photosynthesis, chlorate respiration, decompositions of salts or gases and the detoxification of reactive oxygen species are the pathways that used to produce oxygen and can be selected from to be utilized in this system. However, by-products generated from these pathways need to be identified since it might cause major concerns once interfering with the tissue construct. Table 2 summarizes the advantages and disadvantages of some of the decomposition mechanism used to produce oxygen for tissue engineering applications. Even byproducts may not be directly toxic such as metal cations in some cases, but its accumulation to higher concentration might interfere with the biological system. After the oxygen source has been identified, the next factor to be considered is the materials that can be used to form the shell of the microspheres. The materials are regardless synthetic or natural must be biocompatible and with added merit if biodegradable. Recent literature shows that suspensions of degradable microspheres can be employed for sustained drug release at desirable doses and by implantation without surgical procedures [83,84]. Biocompatibility is usually being achieved by using of natural polymers such as alginate, chitin, and chitosan or by the employment of polymers made from naturally occurring monomers such as lactic and glycolic acids.
Besides the type of polymers shell used, processing conditions employed during preparation very much determine the release profile of oxygen. Properties such as the size and density, polymer ratio, structure properties that include surface and internal areas and encapsulation efficiency will all influence the release of oxygen from the system. L. Du et al. demonstrated that release properties of the microspheres were influenced by different LA/GA ratio of PLGA [85]. Drug release from PLGA microspheres was governed by an asymptotic profile in which up to 87% (40/60, mol/mol), 64% (50/50, mol/mol), 60% (60/40, mol/ mol) of the peptide was released within 30 h. The work concludes that the initial release profile of the drug was mainly dominated by permeation factor. Along with the introducing of the GA content, the hydrophobicity of the materials was decreased markedly. It makes the drug diffusion through the microsphere’s wall much easier.
Having the main ingredients ready, then an appropriate method can be chosen based on its suitability in forming microspheres and the capability to have high loading efficiency of the oxygen source. Different encapsulation methods have their own advantages and disadvantages towards a particular drug delivery system and such information have been well reported and in review papers [83,84,86,87]. For instance, Kristin et al., shown that three different microencapsulation techniques solid-in-oil-in-water (s/o/w), water-in-oil-in-water (w/o/w), and oilin- oil-in-water (o/o/w) are applicable for the preparation of PLGA microspheres as sustained release devices for human insulin [84]. However, due to having the highest insulin loading efficiency and the lowest initial insulin burst release, the w/o/w emulsification technique is considered to be most appropriate. They concluded that these microspheres showed sustained release of structurally intact and biologically active insulin that promoted the formation of cartilagespecific ECM. However in this case, the ideal technique should be rapid, simple, highly reproducible, and uses simple instrumentations. Final stage of the development will be identifying a suitable storage method for the microspheres to avoid loss of oxygen when not in use.
Based on the mentioned criteria, Lim and co-workers [12,13] have developed oxygen producing micro-system using H2O2 as oxygen source. The main concept behinds the system is the production of oxygen upon the chemical decomposition of H2O2 encapsulated in double layered microspheres. Although microencapsulation of water soluble compounds is not something new and has been commonly reported [88], it is not commonly done on molecule like H2O2 that has molecular weight less that 100 g/mol. Major reason is related to the high diffusion coefficient of H2O2 within polymeric matrices due to its small size. This leads to low encapsulation efficiency as leaching occurs seriously during the production of microspheres. SM Ng, et al. used a backward concentration gradient method to increase the loading efficiency, where H2O2 was added in high concentration into the continuous phase during the production that employed the double emulsions solvent evaporation method [12]. Under such condition, H2O2 will diffuse from high concentration region of the continuous phase into the core of the microspheres in achieving effective encapsulation.
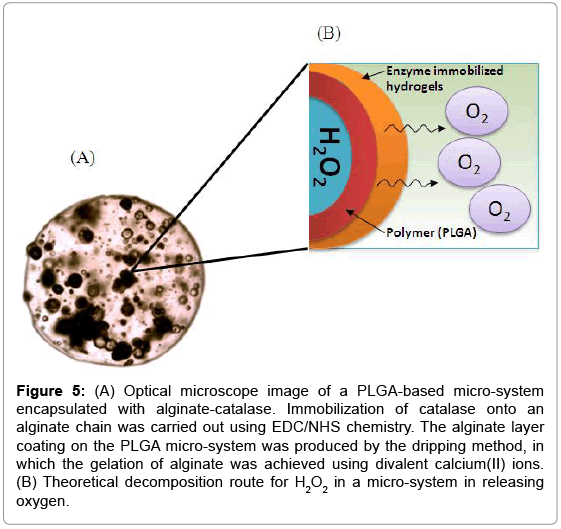
Since H2O2 are toxic for cell [12,13], the micro-system was designed for having polymeric shells as boundary to avoid the possible direct contact of H2O2 with the cells (Figure 5). Besides, the double layered walls acted as a controlled release mechanism in prolonging the release of oxygen and sustaining oxygen at the optimum level for cell survival. As an added security feature, catalase has been immobilized in the secondary alginate layer to ensure complete decomposition of H2O2. The mobility of catalase need to be confined and should not be released into the surrounding that may cause serious contamination. The decomposition process of H2O2 is rather simple, consisting of a single step reaction and produces only oxygen and water as products. This eliminates the possibility of having harmful by products that will contaminate the biological environment.
Figure 5: (A) Optical microscope image of a PLGA-based micro-system encapsulated with alginate-catalase. Immobilization of catalase onto an alginate chain was carried out using EDC/NHS chemistry. The alginate layer coating on the PLGA micro-system was produced by the dripping method, in which the gelation of alginate was achieved using divalent calcium(II) ions. (B) Theoretical decomposition route for H2O2 in a micro-system in releasing oxygen.
Evaluation of oxygen releasing profile
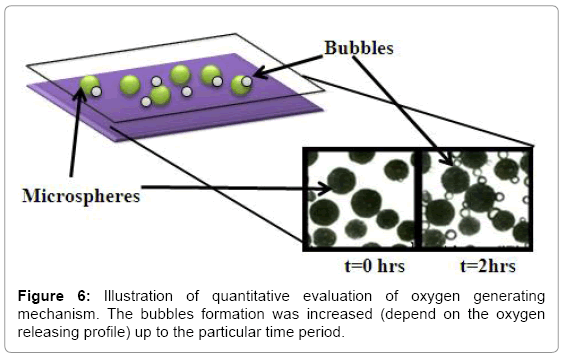
Qualitative observation: The evaluation way on the release of oxygen from scaffold or micro-system is via qualitative observation by naked eye under an optical microscope. Choi, et al. describe this kind of observation in evaluating their oxygen micropump [89]. They observed the various sizes of oxygen bubbles during the decomposition of H2O2. To observe bubble, the system can sandwiched between two glass slides and added with water through the edge. The formation of oxygen if any can be directly observed under microscope. The release rate can be correlated semi-quantitatively by taking the images at a periodical period, which continuous release will cause the bubble size to grow. Example of such image is shown in Figure 6. Although this method is semi-quantitative, the direct observation can give very convincing evidence in proving the oxygen release.
Quantitative analysis: Besides having qualitative observation, precise analytical measurements are required in evaluating the release profile of oxygen from the releasing system. This approach generates quantitative concrete values that can be used directly to do analysis and comparison in term of the performance of batches prepared using either similar or not in materials, methods, or producing settings. In addition, the releasing system can be categorized accordingly to the oxygen releasing amount and rate, which is useful for dosage control purpose. Practically, analytical measurement of oxygen level can be performed via different standard conventional laboratory techniques, optical spectroscopy, or with the use of commercially available portable oxygen meter as shown in the Table 3.
| Oxygen releasing strategy | Instrumentation/Measuring method | References |
|---|---|---|
| PLGA films with sodium per carbonate | Observed the volume of water displaced by the oxygen gas generated from the films. Used chemical equation for decomposition of sodium per carbonate and the ideal gas law. | [39] |
| PLGA/Calcium peroxide based scaffold | BloodGas Analyzer (EasyBloodGas, Medica) | [11] |
| Hydrogen peroxide base functionalized micro-system | Concentration was recorded using a portable oxygen meter (Thermo Orion Series, 3 Star) through electrode of the meter (Thermo, ORION 081010 MD). | [12,13] |
Table 3: Instrumentation used to measure the concentration of oxygen generating systems.
In conventional measuring method, oxygen releasing system will be placed in dry or incubated in solution inside an air tight reaction vessel. The vessel is having an outlet connected with tubing, which the other end tip will be inserted into a burette inlet pre-filled with water and placed backward in a water reservoir. The oxygen produced from the microspheres will replace the water inside the burette and the amount can be recorded directly from the granulated mark of the burette. Harrison et al. used the same method to measure the oxygen generated from biomaterials [39]. In addition, maximum amount of oxygen that could be generated theoretically can be calculated using chemical stoichiometry of decomposition of sodium percarbonate that has been loaded into the system. This method is simple and cheap, but however subjected with higher errors and lower accuracy since it is difficult to evaluate the average encapsulation efficiency. It will be also problematic if the amount of oxygen produced is too low and not detectable.
Another option for a sensitive and accurate measurement will be via the use of standard optical spectroscopy. Zhao et al.utilized luminescence-based imaging-fiber oxygen sensors first time for the in situ measurement of oxygen consumption from intact perfused mouse hearts [90]. These luminescence materials based on ruthenium complexes immobilized on silica gel particles. The work concluded that these materials are very stable, although extremely sensitive to molecular oxygen. Therefore, measurement recorded using luminescence materials are very reliable but required the handling of trained personal and the use of expensive optical instrumentation [91-93].
In contrast, the use of commercially available oxygen sensors is the simplest and cost effective approach for oxygen measurement. These sensors are very common nowadays and can be purchased with comparatively low price [92]. Oxygen concentration is recorded directly without the need of conversion, calibration curve or extra chemical [12,13,94,95]. The handling of such sensors is also often simple and efforts are only needed on setting-up a good controlled study set to validate and evaluate the oxygen releasing profile.
Biological evaluation: Although the quantitative and qualitative evaluations can give good indication of the oxygen release profile, biological studies are still required since the micro-system will be ended up in biological system during the application. Therefore, the microspheres not only should release oxygen as planned but also should be biocompatible in biological environment. Thus, the oxygen releasing system need to be tested with series of studies in ensuring the safety aspect is fulfilled.
Commonly, cell culture study will be used as initial assessment before upgraded into more complex system including in vivo transplant if necessary. Cell assay can be planned accordingly to be used as another potent approach to evaluate and verify the release of oxygen from the oxygen releasing system. Modarressi et al.investigated the switching from high (21%) to low (2%) oxygen levels effects on cultured skin myofibroblasts, essential cells for the normal wound repair process [96]. The work demonstrated that culture for 5 days at 2% oxygen (hypoxia) significantly reduced myofibroblast differentiation and contraction. They concluded that these effects were reversible by restoring high oxygen conditions to improve ischemic and chronic wound healing. Usually these assays determine the ability of cells or tissues to maintain or recover its viability and allow sensitive colorimetric evaluation for the determination of cell viability. For instance, comparison of cell density for culture seeded under normoxic and hypoxic conditions in the presence and absence of oxygen releasing system over a period of time can be made. As shown in Table 4, different approaches were used to evaluate cells conditions under normoxic and hypoxic environment.
| Oxygen producing approach | Cell type | Hypoxic parameters | Effect | References |
|---|---|---|---|---|
| Oxygen producing scaffold | NIH 3T3 fibroblasts | hypoxicglovebox system (1% O2, 5% CO2) (BioSpherix, USA) |
Higher cells numbers found as compare to normoxic condition | [11] |
| Oxygen releasing microspheres | Clone Neuro-2a (N2a, ATCC No. CCL-131) | Artificially by closing the air passage of the culture dish | Viability of the cells increased | [12] |
| Oxygen producing micro-system | Skeletal muscle cells (L6) | Billups–Rothenberg modular incubation chamber, USA (1% O2, 5% CO2, and 94% N2) |
Increase viability in the skeletal muscle cells | [13] |
Table 4: Hypoxic parameters and instrumentations used for the in-vitro experiments.
Theoretically, cell fails to survive under long hypoxic condition. Based on this, if an oxygen releasing system is integrated into a hypoxia condition and the cells show sign of surviving longer compared to the control set (without oxygen releasing system), this gives an indication that oxygen is present. This must be from the system. In contrast, if cells fail to survive and showing the same result as those controls, this indicates a failure of the oxygen releasing system. Mostly, tissue cultures are maintained in vitro at oxygen levels of approximately 20%. Ironically, natural cell microenvironments appear to contain much lower oxygen tensions; the mean oxygen concentration of arterial blood is approximately 12%, and that of tissue is 3% [97]. It varies based on the body location and thus the type of oxygen releasing system (having specific release rate) need to be chosen carefully based on the in vivo micro-environmental condition. Similarly, one needs to be mindful of oxygen tension when interpreting the observations from experiments. Usually, in this type of comparison experiments, all conditions set between control and sample need to be identical and well controlled. Such kind of evaluation method has been practically used in the work by Oh, et al. [11]. In more advanced stage, Harrison et al. used skin flap model in nude mice to observe the effect of critically/marginal perfused tissues in vivo [39]. This model is well accepted and serves as a good standard for research on ischemic tissue survival. They analyzed the tissue flaps on tissue, cellular and biochemical levels. For this purpose, u-shaped flap will be placed in the back of the mice, which causing ischemia in the flap. On a tissue level, it becomes apparent for the nonoxygen producing system that the tissue because necrotic in appearance as compared to oxygen producing system. Mice with film containing oxygen producing system had a significantly reduced amount of visible necrosis at days 2 and 3. By day 7, the tissue became necrotic in appearance similar to the control mice. In addition, the tissue in contact with the oxygen producing system showed less tissue architecture decay at day 7 compared to the control films with remaining defined layers and intact hair follicles. In this study they found lower level of lactate in the oxygen’s sample, this is suggestive that the oxygen generating biomaterials can produce sufficient oxygen to prevent anaerobic metabolism from occurring.
Potential application of oxygen producing functionalized micro-system
The capability to integrate and utilize of the oxygen producing micro-system for prolonging cell survival effectively under oxygen deficient condition is an important aspect. The micro-system should be able to release the oxygen to cell at the targeted place and time, without targeting on a wrong area. This, this reflects the need in designing of elegant delivery mechanism, integrated on a suitable platform that acts as bridge to merge the micro-system into real biological purpose. This section will discuss some of the potential applications with implication based on the author’s opinion.
Oxygen producing micro-system incorporated scaffold: The incorporation of microspheres into scaffolds for tissue engineering purpose has been widely reported [98-101]. The motivation is to combine the strengths and advantages, both the conventional scaffold and drug encapsulated microspheres into a new class of composite biomaterial. Xiaoqin et al.incorporated growth factors (bone morphogenetic protein 2, rhBMP-2 and insulin-like growth factor I, rhIGF-I) encapsulated PLGA microspheres in alginate and silk scaffolds to form concentration gradient [100]. Silk sponge-like scaffolds were superior to alginate gel scaffolds in forming deep linear grow factor gradients. The osteogenic and chondrogenic differentiation of hMSCs seeded in these silk scaffolds corresponded to the gradient distribution of rhBMP-2 and reverse distribution of rhBMP-2/rhIGF-I, showing a trend of gradient increase. rhIGF-I enhanced the effect of rhBMP-2 but it alone did not induce hMSC differentiation, probably due to its limited loading and fast release. They concluded, control of specific tissue formation can be achieved by controlling spatial distribution of growth factors in a polymer scaffold via microsphere incorporation. In this sense, the concept of composite scaffold utilizing of oxygen producing micro-system in tissue engineering can be adopted. The micro-system can be integrated uniformly as part of the scaffold within the main matrix. The micro-system act as active oxygen generator system, while cells can attach, proliferate, differentiate, and form an ECM on the specially designed scaffold.
Oxygen as a drug: The failure in supplying sufficient oxygen to organs cause damage to its functionality and subsequently can paralyze a normal operating biological system. One of the well-known examples is stroke, caused by the blockage of the cerebral microvasculature and results in decreased blood and nutrients supply to post-occlusion brain tissue. This ischemic and hypoxic environment leads to several necrotic tissue events including loss of ATP production, elevated levels of intracellular calcium, etc. that ultimately lead to neuronal and cerebrovascular cell death. Under such condition, it will be useful to employ the oxygen producing micro-system as a drug to oxygenate tissue. Due to its small size, the micro-system can be directly injected to the affected area and act as temporary generator for oxygen supply before the problem of vessel blockage is overcome via surgical or other methods.
Future challenges and prospect
In the view of the authors, there are still huge rooms for the technology to grow and improve from the current level of achievement. This review has gathered a number of works that have demonstrated the relevancy of the currently available technologies that can contribute toward this area. However, it should be clear that the idea of using oxygen generating scaffolds to supply external oxygen to tissue construct is still at the initial stage. There are still some important aspects and challenges that need to be addressed and investigated before such technology can reach clinical trial stage and eventually being used in real application. Three key challenges have been identified, which are size, safety, and release profile.
The size of the system producing oxygen should be ideally small for its ease blending into the tissue scaffolds that are currently used in tissue engineering area. The size might need to go down to nanosized scale from the current micro-sized scale in order to minimize major alteration on the initial property of the scaffold. This is such as the reduction of mechanical strength on the initial scaffold due to the formation of clear and wide boundary between the system and the matric of the scaffold if the size of the system is too huge. Once the size is reduced, this will directly introduce fabrication limitations and difficulties. This is more obvious if the architecture consists of several coated layers like the one suggested by Abdi, et al. [13].
Another major concern of such system is on safety issue. Since the system is dealing with oxygen, it is very common to encounter free radical of oxygen that can cause serious effect to cells. The scavenge of this radical from the system is not easy, while even there is a method, it will definitely increase the complexity and reduce the practicality in adopting this system in real applications. Besides, some of the oxygen sources might be highly toxic to the cell. It can reach the cell if happen the designed system has some faulty flows or there is no specific safety feature incorporated to avoid this when the system is first designed.
Next challenge to be handled will be on the controlled release profile of this system. Although the release rate can be monitored as reviewed in the early section, quantitative measurement can only be achieved in vitro, while not in vivo. This will be problematic as the system is intended to work in vivo and the biological environment might alter the release rate. Any amount of oxygen deviated from optimum level will bring serious damage to the cells in contact. Thus, a correlation between the release rate under in vitro and in vivo conditions should be established to ensure correct dosage is given for different cell types; however such correlation is not possible using current technology since the evaluation study using biological system is semi-quantitative and based on trial and error approach. Correct dosage still might be able to be achieved, but definitely this will be a time consuming and high cost study.
The use of functionalized biomaterials scaffolds that are able to supply oxygen while vascularization is taking place within a tissue construct seems to be a potential solution to overcome necrosis caused by oxygen deficient problem. The prospect for continuation development is certainly bright since the oxygen will be supplied in a more independent manner and can be easily fine tune to the required dosage via controlled released technology. Besides, the state of art to blend the oxygen reservoir within the tissue scaffolds will reduce the physical obstacle that might be induced to the biological environment if it is blended in the tissue but outside of the scaffold. In another word, there will be no extra physically matrices need to be added although the functionality of providing oxygen has been introduced.
Acknowledgement
This work was supported by ETRI R&D Program (The title of research project: "Local-based medical device/robot development & medical IT convergence for small and medium enterprises revitalization project", 15ZC3100) funded By the Government of Korea and Regenerative Medicine Project by Daegu Metropolitan City.
References
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920-926.
- Bryant SJ, Cuy JL, Hauch KD, Ratner BD (2007) Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 28: 2978-2986.
- Bhatia SN, Chen CS (2000) Tissue Engineering at the Micro-Scale. Tissue Eng 2: 131-144.
- Anderson DG, Burdick JA, Langer R (2004) Materials science. Smart biomaterials. Science 305: 1923-1924.
- Gilbert TW, Stolz DB, Biancaniello F, Simmons-Byrd A, Badylak SF (2005) Production and characterization of ECM powder: implications for tissue engineering applications. Biomaterials 26: 1431-1435.
- Place ES, Evans ND, Stevens MM (2009) Complexity in biomaterials for tissue engineering. Nat Mater 8: 457-470.
- Phelps EA, García AJ (2010) Engineering more than a cell: vascularization strategies in tissue engineering. CurrOpinBiotechnol 21: 704-709.
- Malda J, Woodfield TBF, Van Der Vloodt F, Kooy FK, Martens DE, et al.(2004)The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 25: 5773-5780.
- Volkmer E, Drosse I, Otto S, Stangelmayer A, Stengele M, et al. (2008) Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A 14: 1331-1340.
- Iyer RK, Radisic M, Cannizzaro C, Vunjak-Novakovic G (2007) Synthetic oxygen carriers in cardiac tissue engineering. Artif Cells Blood SubstitImmobilBiotechnol 35: 135-148.
- Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS (2009) Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30: 757-762.
- Ng SM, Choi JY, Han HS, Huh JS, Lim JO (2010) Novel microencapsulation of potential drugs with low molecular weight and high hydrophilicity: hydrogen peroxide as a candidate compound. Int J Pharm 384: 120-127.
- Abdi SI, Ng SM, Lim JO (2011) An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. Int J Pharm 409: 203-205.
- Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, et al. (2015) Fibroblast-endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A 21: 1055-1065.
- Carfì Pavia F, Carrubba V, Ghersi G, Brucato V (2010) A Composite PLLA Scaffold for Regeneration of Complex Tissues. Int J Mater Form 3: 571-574.
- Nomi M, Atala A, Coppi PD, Soker S (2002) Principals of neovascularization for tissue engineering. Mol Aspects Med 23: 463-483.
- Carletti E, Motta A, Migliaresi C (2011) Scaffolds for tissue engineering and 3D cell culture. Methods MolBiol 695: 17-39.
- Alvarez-Barreto JF, Linehan SM, Shambaugh RL, Sikavitsas VI (2007) Flow perfusion improves seeding of tissue engineering scaffolds with different architectures. Ann Biomed Eng 35: 429-442.
- Du D, Furukawa K, Ushida T (2008) Oscillatory perfusion seeding and culturing of osteoblast-like cells on porous beta-tricalcium phosphate scaffolds. J Biomed Mater Res A 86: 796-803.
- Malda J, Klein TJ, Upton Z (2007) The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng 13: 2153-2162.
- Tian L, George SC (2011) Biomaterials to prevascularize engineered tissues. J CardiovascTransl Res 4: 685-698.
- Bijlsma JW, Berenbaum F, Lafeber FP (2011) Osteoarthritis: an update with relevance for clinical practice. Lancet 377: 2115-2126.
- Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, et al.(2002) Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. PlastReconstrSurg 109: 2384-2397.
- Wollenick K, Hu J, Kristiansen G, Schraml P, Rehrauer H, et al.(2012) Synthetic transactivation screening reveals ETV4 as broad coactivator of hypoxia-inducible factor signaling. Nucleic Acids Res 40: 1928-1943.
- Seal B (2001) Polymeric biomaterials for tissue and organ regeneration Materials Science and Engineering: R: Reports 34: 147-230.
- Pathi P, Ma T, Locke BR (2005) Role of nutrient supply on cell growth in bioreactor design for tissue engineering of hematopoietic cells. BiotechnolBioeng 89: 743-758.
- Moon JJ, West JL (2008) Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr Top Med Chem 8: 300-310.
- Kaully T, Kaufman-Francis K, Lesman A, Levenberg S (2009) Vascularization--the conduit to viable engineered tissues. Tissue Eng Part B Rev 15: 159-169.
- Ravi S, Chaikof EL (2010) Biomaterials for vascular tissue engineering. Regen Med 5: 107-120.
- Supp DM, Supp AP, Bell SM, Boyce ST (2000) Enhanced vascularization of cultured skin substitutes genetically modified to overexpress vascular endothelial growth factor. J Invest Dermatol 114: 5-13.
- Yau TM, Li G, Weisel RD, Reheman A, Jia ZQ, et al. (2004) Vascular endothelial growth factor transgene expression in cell-transplanted hearts. J ThoracCardiovascSurg 127: 1180-1187.
- Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, et al. (2011) Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A 17: 349-359.
- Takikawa M, Nakamura SI, Nakamura S, Nambu M, Ishihara M, et al.(2011) Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J Biomed Mater Res B ApplBiomater 97: 373-380.
- Tsuchida E, Sou K, Sakai H, Komatsu K, Takeoka S, et al.(2003) Safety of artificial oxygen carrier (synthetic erythrocytes) and the ability to supply oxygen to tissues Masui 52: S55-66.
- Komatsu T, Oguro Y, Teramura Y, Takeoka S, Okai J, et al.(2004) Physicochemical characterization of cross-linked human serum albumin dimer and its synthetic heme hybrid as an oxygen carrier. BiochimBiophysActa 1675: 21-31.
- Khattak SF, Chin KS, Bhatia SR, Roberts SC (2007) Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. BiotechnolBioeng 96: 156-166.
- Pelled G, Kimelman-Bleich N, Zilberman Y, Gazit Z, Gazit D (2009) Synthetic Oxygen Carriers Enhance Bone Defect Regeneration Induced by BMP-Expressing Mesenchymal Stem Cells. Transactions of the Orthopaedic research society 34: 93.
- Fitzgerald MC, Chan JY, Ross AW, Liew SM, Butt WW, et al.(2011) A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anaemia following trauma The Medical journal of Australia 194: 471-473.
- Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ (2007) Oxygen producing biomaterials for tissue regeneration. Biomaterials 28: 4628-4634.
- Lee K, Silva EA, Mooney DJ (2011) Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8: 153-170.
- Aiello LP (1997) Vascular endothelial growth factor. 20th-century mechanisms, 21st-century therapies. Invest Ophthalmol Vis Sci 38: 1647-1652.
- Zhang J, Hu M (2005) [Advance in research of vascularization of bone tissue engineering]. Zhonghua Kou Qiang Yi XueZaZhi 40: 349-350.
- Yarlagadda PK, Chandrasekharan M, Shyan JY (2005) Recent advances and current developments in tissue scaffolding. Biomed Mater Eng 15: 159-177.
- Lovett M, Lee K, Edwards A, Kaplan DL (2009) Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 15: 353-370.
- Krishnan L, Willett NJ, Guldberg RE (2014) Vascularization strategies for bone regeneration. Ann Biomed Eng 42: 432-444.
- Hao X, Silva EA, Månsson-Broberg A, Grinnemo KH, Siddiqui AJ, et al. (2007)Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction Cardiovasc Res 75: 178-185.
- Chen RR, Silva EA, Yuen WW, Brock AA, Fischbach C, et al. (2007) Integrated approach to designing growth factor delivery systems. FASEB J 21: 3896-3903.
- Davis HE, Leach JK (2011) Designing bioactive delivery systems for tissue regeneration. Ann Biomed Eng 39: 1-13.
- Laschke MW, Rücker M, Jensen G, Carvalho C, Mülhaupt R, et al. (2008) Improvement of vascularization of PLGA scaffolds by inosculation of in situ-preformed functional blood vessels with the host microvasculature. Ann Surg 248: 939-948.
- Laschke MW, Rücker M, Jensen G, Carvalho C, Mülhaupt R, et al. (2008) Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds. J Biomed Mater Res A 85: 397-407.
- Zisch AH, Lutolf MP, Hubbell JA (2003) Biopolymeric delivery matrices for angiogenic growth factors. CardiovascPathol 12: 295-310.
- Risau W (1997) Mechanisms of angiogenesis. Nature 386: 671-674.
- Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389-395.
- Hirschi KK, Skalak TC, Peirce SM, Little CD (2002) Vascular assembly in natural and engineered tissues. Ann N Y AcadSci 961: 223-242.
- Schmidmaier G, Lucke M, Schwabe P, Raschke M, Haas NP,et al.(2006) Collective review: bioactive implants coated with poly(D,L-lactide) and growth factors IGF-I, TGF-beta1, or BMP-2 for stimulation of fracture healing. Journal of longterm effects of medical implants 16: 61-69.
- Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, et al.(2014) Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling.MolTher 22: 1243-1253.
- Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, et al. (2008) Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials 29: 1518-1525.
- Silva EA, Mooney DJ (2010) Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 31: 1235-1241.
- Cool J, DeFalco TJ, Capel B (2011) Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. ProcNatlAcadSci U S A 108: 167-172.
- Freeman I, Cohen S (2009) The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials 30: 2122-2131.
- Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9: 685-693.
- Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, et al. (2006) Engineering complex tissues. Tissue Eng 12: 3307-3339.
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13: 9-22.
- Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23: 47-55.
- Gaber BP, Yager P, Sheridan JP, Chang EL (1983) Encapsulation of hemoglobin in phospholipid vesicles. FEBS Lett 153: 285-288.
- Vaska L (1963) Oxygen-Carrying Properties of a Simple Synthetic System. Science 140: 809-810.
- Rabinovici R, Rudolph AS, Ligler FS, Yue TL, Feuerstein G (1990) Liposome-encapsulated hemoglobin: an oxygen-carrying fluid. Circ Shock 32: 1-17.
- Singel DJ, Stamler JS (2005) Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99-145.
- Speicher PJ, Beasley GM, Jiang B, Lidsky ME, Palmer GM, et al.(2014) Hypoxia in melanoma: using optical spectroscopy and EF5 to assess tumor oxygenation before and during regional chemotherapy for melanoma. Ann SurgOncol 211: 435-440.
- Oski FA, Gottlieb AJ, Miller WW, Delivoria-Papadopoulos M (1970) The effects of deoxygenation of adult and fetal hemoglobin on the synthesis of red cell 2,3-diphosphoglycerate and its in vivo consequences. J Clin Invest 49: 400-407.
- Riess JG, Krafft MP (1998) Fluorinated materials for in vivo oxygen transport (blood substitutes), diagnosis and drug delivery. Biomaterials 19: 1529-1539.
- Centis V, Doillon CJ, Vermette P (2007) Perfluorocarbon emulsions cytotoxic effects on human fibroblasts and effect of aging on particle size distribution. Artif Organs 31: 649-653.
- Riess JG (2001) Oxygen carriers ("blood substitutes")--raison d'etre, chemistry, and some physiology. Chem Rev 101: 2797-2920.
- Centis V, Vermette P (2009) Enhancing oxygen solubility using hemoglobin- and perfluorocarbon-based carriers. Front Biosci (Landmark Ed) 14: 665-688.
- Goodman RL, Moore RE, Davis ME, Stokes D, Yuhas JM (1984)Perfluorocarbon emulsions in cancer therapy: preliminary observations on presently available formulations.Int J RadiatOncolBiolPhys 10: 1421-1424.
- Lowe KC, Davey MR, Power JB (1998) Perfluorochemicals: their applications and benefits to cell culture. Trends Biotechnol 16: 272-277.
- Spahn DR (1999) Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care 3: R93-97.
- Alayash AI (2004) Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov 3: 152-159.
- Hsieh A, Feric NT, Radisic M (2015) Combined hypoxia and sodium nitrite pretreatment for cardiomyocyte protection in vitro. BiotechnolProg 31: 482-492.
- Chin K, Khattak SF, Bhatia SR, Roberts SC (2008) Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. BiotechnolProg 24: 358-366.
- Maillard E, Juszczak MT, Clark A, Hughes SJ, Gray DR, et al. (2011) Perfluorodecalin-enriched fibrin matrix for human islet culture. Biomaterials 32: 9282-9289.
- Goh F, Gross JD, Simpson NE, Sambanis A (2010)Limited beneficial effects of perfluorocarbon emulsions on encapsulated cells in culture: experimental and modeling studies. Journal of Biotechnology 150: 232-239.
- Tan H, Wu J, Lao L, Gao C (2009) Gelatin/chitosan/hyaluronan scaffold integrated with PLGA microspheres for cartilage tissue engineering. ActaBiomater 5: 328-337.
- Andreas K, Zehbe R, Kazubek M, Grzeschik K, Sternberg N, et al. (2011) Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: investigation for cartilage tissue engineering. ActaBiomater 7: 1485-1495.
- Du L, Cheng J, Chi Q, Qie J, Liu Y, et al. (2006) Biodegradable PLGA microspheres as a sustained release system for a new luteinizing hormone-releasing hormone (LHRH) antagonist. Chem Pharm Bull (Tokyo) 54: 1259-1265.
- Liu R, Huang SS, Wan YH, Ma GH, Su ZG (2006) Preparation of insulin-loaded PLA/PLGA microcapsules by a novel membrane emulsification method and its release in vitro. Colloids Surf B Biointerfaces 51: 30-38.
- Wu G, Chen L, Li H, Wang YJ (2014) Hyaluronic acid as an internal phase additive to obtain ofloxacin/PLGA microsphere by double emulsion method. Biomed Mater Eng 24: 751-756.
- Fernández-Carballido A, Herrero-Vanrell R, Molina-Martínez IT, Pastoriza P (2004) Biodegradable ibuprofen-loaded PLGA microspheres for intraarticular administration. Effect of Labrafil addition on release in vitro. Int J Pharm 279: 33-41.
- Choi YH, Sang Uk Son SSL (2004) A micropump operating with chemically produced oxygen gas. Sensors and Actuators A: Physical 111: 8-13.
- Zhao Y, Richman A, Storey C, -adford NB, Pantano P (1999) In situ fiber-optic oxygen consumption measurements from a working mouse heart. Anal Chem 71: 3887-3893.
- Preininger C, Klimant I, Wolfbeis OS (1994) Optical Fiber Sensor for Biological Oxygen Demand. Anal Chem 66: 1841- 1846.
- Xiao D, Mo Y, Choi MMF (2003) A hand-held optical sensor for dissolved oxygen measurement.MeasSciTechnol 14: 862-867.
- Cai H, Chu F, Qu R, Fang Z (2008) U-shaped plastic optical fiber dissolved oxygen sensor. Proceedings of SPIE 7004: 4.
- Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, et al. (2014) Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508: 269-273.
- Liu L, Shang L, Liu C, Liu C, Zhang B, et al. (2010) A new mediator method for BOD measurement under non-deaerated condition. Talanta 81: 1170-1175.
- Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, et al. (2010) Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol 130: 2818-2827.
- Csete M (2005) Oxygen in the cultivation of stem cells. Ann N Y AcadSci 1049: 1-8.
- Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, et al. (2012) Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A 100: 162-170.
- Lv Q, Nair L, Laurencin CT (2009) Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. J Biomed Mater Res A 91: 679-691.
- Dinis TM, Vidal G, Jose RR, Vigneron P, Bresson D, et al. (2014) Complementary effects of two growth factors in multifunctionalized silk nanofibers for nerve reconstruction. PLoS One 9: e109770.
- Singh M, Sandhu B, Scurto A, Berkland C, Detamore MS (2010) Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent. ActaBiomater 6: 137-143.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16734
- [From(publication date):
August-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11909
- PDF downloads : 4825