Review Article Open Access
Functional Electrical Stimulation (FES): Transforming Clinical Trials to Neuro-Rehabilitation Clinical Practice- A Forward Perspective
| Gad Alon* | |
| Department of Physical Therapy & Rehabilitation Sciences, School of Medicine, University of Maryland, USA | |
| Corresponding Author : | Gad Alon Associate Professor Department of Physical Therapy & Rehabilitation Sciences School of Medicine, University of Maryland 100 Penn Street Baltimore, MD 21201, USA E-mail: galon@som.umaryland.edu |
| Received June 20, 2013; Accepted August 20, 2013; Published August 23, 2013 | |
| Citation: Alon G (2013) Functional Electrical Stimulation (FES): Transforming Clinical Trials to Neuro-Rehabilitation Clinical Practice- A Forward Perspective. J Nov Physiother 3:176. doi:10.4172/2165-7025.1000176 | |
| Copyright: © 2013 Alon G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Presenting a forward perspective on the topic of personalized functional electrical stimulation (FES) and discussing its critical role in clinical practice is a challenge, particularly when the goal is to provide biomedical engineers and clinicians with a guide to bridge the gap between laboratory, clinical research, and clinical practice. There are several dimensions to the complexity of the topic. First, is the prevailing and misleading terminology, inadequate evidencebased training of physicians and rehabilitation therapists, and the recognition that until recently most existing FES systems were not designed as wearable systems and are “not patient or therapist friendly”. Most importantly, is the wellestablished phenomenon, that following damage to the musculo-skeletal system or brain, patients’ profile of functional recovery and thus utilization FES as part of the recovery is highly variable, prolonged and largely unpredictable. As a result, legacy research and training methods that depend on interpretation of statistically significant and clinically meaningful findings are inherently limited addressing the needs of most patients. This monograph will focus on: 1) identifying the specific deficits and recovery profiles that each patient presents, 2) providing examples of the diverse modes of actions (mechanisms) that govern wearable FES utilization, 3) the latest developments and shortcoming of wearable FES technologies, and 4) the recognition that FES has limited value if applied in isolation. Finally, an example of personalized training paradigm, centered on individual patient’s needs and measureable progress in functional outcomes will be presented.
| Keywords |
| Electrical stimulation; Biomedical engineering; Neuromuscular electrical stimulation; Therapeutic electrical stimulation |
| FES: Evolution of Terminology |
| The “Name game” |
| Applying electrical stimulation to manage countless physical and emotional ailments can be traced to the beginning of clinical medicine. In more modern forms, electrical stimulation has been promoted under various generic and commercially-driven names. Promoted acronyms around the world include transcutaneous electrical nerve stimulation ( TENS), neuromuscular electrical stimulation (NMES), electrical muscle stimulation (EMS), and therapeutic electrical stimulation (TES), as well as poorly understood names such as “High or Low-Volt”, “High or low frequency”, Interferential current (IC) and “Micro-current”. Regrettably, the proliferation of names in the absence of corollary objective clinical evidence or sound electrophysiological modes of action has been a major barrier to clinical acceptance. The prevailing on-going confusion among physician, therapists, and researchers could be eliminated rather quickly by referring to reproducible, outcome measure-based, and less commercially motivated published clinical literature. Much has been written on the need to disregard meaningless names or vague terms and focus instead on understanding when, under what condition, why, and how best to utilize electrical stimulation in clinical practice [1]. |
| Common to the vast majority of non-invasive electrical stimulators is the delivery of small amounts of electrical current in the form of pulses. When delivered, these pulses depolarize peripheral sensory and motor nerves and indirectly lead to muscle contraction, joint motion, augmentation of peripheral blood flow, and connective tissue mobilization. Concurrently, the depolarization of peripheral nerves indirectly leads to alteration in spinal cord and brain activation. All these basic responses have nothing to do with the name of the stimulator or whether it is powered by a battery or by an electrical outlet in the clinic. |
| In principle and regardless of name, the vast majority of biologically and physiologically efficacious electrical currents of similar basic parameters have indistinguishable direct and indirect effects on the periphery and on the central nervous system (CNS). Data supporting this principle can be found in numerous publications, whether the effects are on skeletal muscles, blood flow, internal organs or the brain [2-13]. The origin of the misconception that TENS is only indicated in managing pain and is not efficacious in managing slow to heal wounds is untraceable. Similarly, the fallacy that NMES or FES is ineffective in pain management is contradicted by abundance of clinical data and experience [14,15]. The principle difference between TENS and NMES is that the latter can be set (or programmed) to interrupt the train of pulses every second or few seconds. As a result, NMES can induce intermittent tetanic (or twitch) contractions of skeletal muscles while TENS can only induce continuous (and mostly purposeless) tetanic (or twitch) contractions. The fact that both TENS and NMES appear equally effective in eliciting sensory stimulation provides credence to expect equal clinical outcomes in managing pain [16-18]. |
| Comparison between NMES, EMS, and TES on one hand and FES on the other involves equally troubling misconception. NMES, EMS and TES are typically recognized as useful in managing peripheral impairments collectively known as therapeutic effects, including strengthening of weak muscles, improving joints’ range of motion, augmenting motor control (classically but ambiguously termed “muscle re-education”), reducing limb edema, improving peripheral blood flow, and minimizing spasticity [2,8,14,17,19]. When the same NMES or TES device is used during ambulation, sit-stand activities, or upper extremity functions, the name of the stimulator “converts” to FES [1]. In fact, the major electronic difference between NMES and FES is the provision built in most FES systems to synchronize the activation of specific muscles with the functional activity. But improving functional ability does not negate the therapeutic benefits of FES. The critical difference is not in the name, but in how the stimulator is being used by the patients and clinicians. For recent reviews see references [20-27]. |
| The examples given above should be sufficient to convince the reader not to fall into the trap of names and ambiguous claims, but rather to direct all energies to the substantive but admittedly complex topic of FES utilization. |
| Scope and limits of non-invasive FES |
| By definition, “non-invasive” dictates the use of surface electrodes. The obvious advantage of non-invasive FES is the ability to stop the stimulation at any time, thus minimizing the risk of adverse events and medical complications. What may be less obvious is that because the electrodes interface with the skin, their quality (uniform conductivity, conformity to irregular body parts, durability, and hydration) is a prerequisite determinant of successful stimulation. Poor electrode quality is the primary determinant of uncomfortable, ineffective stimulation, and in fact the most likely cause of skin irritation and even skin burn regardless of the FES system used [28]. To maintain good quality, surface electrodes should be replaced often, thus adding to the cost of FES utilization. All clinicians involved with FES must be aware that maintaining good quality electrodes is vital to assure successful outcomes. |
| The position and size of the surface electrodes contribute to the perceived comfort of stimulation. The size should not be too large or too small and the position must be correct to assure contraction only in the target muscle group. While non-invasive FES can benefit many muscle groups, including the abdominals and torso extensors [29], some muscles (most notably the ilio-psoas, sub-scapularis, and serratus anterior) cannot be reached effectively with non-invasive FES. Moreover, electrically induced contraction of wrist/fingers or elbow flexors or extensors can result in full flexion and extension of the respective joints. Similar results are expected when stimulating the dorsi/plantar flexors and quadriceps. Stimulation of the shoulder flexors or abductors, however, is expected to result in only partial ROM, a clinical knowledge that also includes achieving limited ROM of knee flexion, hip abduction or hip extension during stimulation of the hamstrings, hip abductors and extensors respectively. |
| The main reasons for the inability to move the shoulder joint through full range are because most FES systems only offer 2 channels thus enabling concurrent stimulation of only 2-3 muscles out of at least a dozen needed for full flexion or abduction [30]. Even if 5-6 channels were available, the inaccessibility of muscles covered by the scapula (subscapularis, serratus anterior) that are critical to shoulder girdle movements, would make it impossible to achieve full ROM even in a healthy individual. Involuntary muscle activation because of spasticity, unstable gleno-humeral and thoraco-scapular joints as a result of damage to the brain further contribute to the limit of achievable shoulder ROM by FES. Similar difficulties are encountered around the hip joint where the ilio-psoas is located very deep and is covered by the abdominal muscles making it impossible to get strong hip flexion. The hip abductors are readily accessible to surface stimulation but require very strong contraction that most patients could not tolerate particularly if the electrodes are small relative to the size of the muscle mass. The primary hip extensors are the Gluteus maximus and the Hamstrings. Again, overlooking the critical role of electrode size, relative to very large muscles, particularly the Hamstrings, which are elongated muscles intermixing muscle fibers with connective tissue (semi tendinosus and semi membranosus) may explain the difficulty of getting full hip extension. Collectively, the limited ROM FES can generate is closely associated with subject inability to tolerate the inevitable sensation that accompanies the contraction. Whereas most patients can be conditioned to tolerate the stimulation, individual patients’ tolerances vary considerably, and all patients can only tolerate so much [31]. Another reason for limited ROM is the basic knowledge that full range of normal movements involves coordinated activation of several muscle groups, yet FES typically activates only 1-2 muscles groups. Finally, FES appears minimally effective in generating movements along longitudinal axis such as pronation-supination of the forearm, external-internal rotation at the gleno-humeral joint and at the hip. |
| FES can be applied safely to the majority of patients in the rehabilitation field. However, the safety of using FES is not known during pregnancy and thus should not be used, and FES is contraindicated if the patient also has an implanted electronic device. Noninvasive FES has the potential of interfering with implantable electronic devices (such as pacemaker, defibrillator, bladder stimulator) [32], and the inability to ascertain no conflict between devices is the reason for contra-indication.FES should not be applied over irritated or open skin lesions as it is likely to increase irritation and further damage the lesion. Epileptic seizures, congestive heart failure, bypass or heart transplant, chronic obstructive pulmonary disease, and diabetes are typical examples where FES is not contra-indicated; in these instances, FES has shown clinical efficacy and effectiveness [33-35], but it requires caution during application to ascertain that no adverse reaction results. Monitoring vital signs is a good clinical practice particularly, when initiating FES program and the patient’s response to the stimulation is not known. In frequently, patients may become dizzy, have shortness of breath, or develop temporary headaches. Clinicians must be aware of these rare episodes and stop the stimulation if the patient reports any unwanted response. |
| Finally, the one area in which FES is not likely to be helpful is managing patients with peripheral nerve damage. This includes damage to anterior horn (Poliomyelitis), traumatic severance of peripheral nerves or their roots in the upper and lower extremities, degenerative disease of peripheral nerves, and axonotmesis associated with prolonged compression on the nerve, such as in degenerative changes of the vertebral structures or prolonged compression of the peroneal nerve. The primary reason for ineffectiveness is that currently available FES devices cannot induce therapeutically viable muscle contractions in the absence of peripheral innervation. |
| FES utilization framework |
| FES utilization as part of a comprehensive rehabilitation intervention can be organized into three categories: 1) FES dependent, meaning that applying the FES enables the patient to perform specific tasks or functions that she/he cannot perform at all, or not as well without FES, 2) FES independent (re-learned), where FES is being applied for a finite time period to minimize impairments and practice new tasks or functions, and 3) the so-called neuro-modulation. As related to FES independent, by gradually reducing the stimulation’s intensity over time, it is hypothesized that the brain will become less dependent on the FES, enabling the patient to eventually re-learn to perform specific tasks and functions without FES. The third category, the so-called neuro-modulation, is out of scope of this paper. |
| Fundamental Mechanism of Action |
| Functional deficits of the upper and lower extremities |
| The literature does not clearly resolve or address what constitutes functional deficits following damage to the brain. For example, data concerning people who survived a stroke permit prediction that only 10-12% will recover pre-stroke, full use of the upper extremity [36,37]. Such prediction means that the paretic upper extremity in the vast majority of stroke survivors can be expected to assume a new role of assisting hand in the best scenario, notwithstanding being the dominant hand prior to the stroke [38]. How much assistance in activities of daily living (ADL) the paretic upper extremity can provide appears to depend in part on the severity of motor loss, and in part on which bimanual activities contribute to functional independence [39,40]. The dogma of traditional rehabilitation professionals (and government or private insurance companies worldwide) has been that after the initial treatment intervention and spontaneous recovery plateau, typically within 3 months, the best option to the patient is to accept reality and learn to be independent with one upper extremity. This dogma is now being challenged with new data that FES can enable patients to perform bimanual ADL not possible without FES [41-44]. |
| Severity of motor loss, recovery of motor control of all joints of the upper extremity, and the time course of recovery appear the primary clinical markers of upper extremity functional gains [36,37,45,46]. The challenge to most clinicians is to selecta test battery that is functionally relevant, objectively measured, and reproducible. There have been at least 28 different tests in the English language for the upper extremity, many of which depended on each other to establish their own validity; selecting one test appears highly associated with the comfort of the investigators with tests that they or their co-investigators developed [37]. It is equally critical for clinicians to recognize that a valid repeatable test of bimanual daily functions where the paretic upper extremity is the assistive hand is rarely published [47]. The anticipation that for many patients the paretic upper extremity will play an assistive role underscores the need to develop such a test. To wait until such a test is developed, tested and accepted by researchers and clinicians may provide little comfort for current and near future stroke survivors. Moreover, the well-established, highly variable recovery profile of the upper extremity [36,43-45] most likely will further delay the development of a more inclusive test. A pragmatic alternative is to develop an individual patient’s test of motor loss and motor recovery. A template for such test is offered later in this paper. |
| Functional deficits of the lower extremity appear to have more consensus regarding classification as well as markers of progress. Measurements of distance walked, walking speed, and to a lesser degree the amount of human assistance and dependence on assistive devices are repeatedly reported in rehabilitation literature [48-56]. Measurements of stairs climbing, successful crossing of neighborhood streets, number of falls, or returning to drive a car (the vast majority of published research in stroke rehabilitation enrolled subjects in their mid-late 50 s, long before most people stop driving) are inconsistently and subjectively reported. Data documenting dependence on assistive devices and patient satisfaction with the devices (canes/quadcanes, hemi or full walker, ankle-foot-knee orthosis), number of falls, and quality of life measures are particularly relevant to FES, but they are infrequently reported [53,57,58]. Most importantly, an individual patient’s profile of recovery is highly variable, mainly for going information regarding use of assistive devices, gaining walking speed and distance as well as elimination of falls. As with the upper extremity, developing a patient’s specific locomotion test battery testing only those measures that are relevant to the individual patient, and designing task-specific training programs to improve the specific outcome measures of relevance should help clinician transform clinical research to clinical practice. |
| Effects of FES on peripheral systems |
| The ability of FES to excite directly peripheral sensory and motor nerves is the principal physiological event leading to bidirectional propagation of action potentials. The excitation of sensory nerves results in the perception of tingling. The excitation of motor nerves leads to muscle contraction. The direct excitatory responses expand into numerous indirect responses affecting the peripheral neuromuscular, vascular, and articular (joint) systems while concurrently affecting the central nervous system (CNS). |
| Neuromuscular system: When using surface electrodes, excitation of sensory nerve fibers typically occurs first. As the amount of the pulse’s electric charge increases (clinicians commonly term it increase stimulation intensity), more sensory fibers are depolarized, and the perception of tingling increase. When the pulse charge is sufficient, motor nerve fibers are also excited, and the muscle fibers innervated by the excited nerve fibers (motor units) contract [26]. Thus the perception of tingling and the muscle contraction are indirect responses to the excitation of the sensory and motor nerves respectively. As the intensity of stimulation (pulse charge) continues to increase, more nerve fibers are excited; the perception of tingling increases further and muscle contraction grows stronger, leading to joint motion. Thus the higher the intensity, the less comfortable is the perception of stimulation [31]. It is critical to recognize that the correlation between stimulation intensity and force of muscle contraction among patients is poor: some patients require low intensity to elicit very strong contraction, while others require high stimulus intensity that only elicits very weak contraction. Accordingly, the clinician must focus on the ability to achieve the desired contraction (and comfort perception) and not on the stimulator visual display of milliamps (or dial). From an electrophysiological perspective, the objective is to induce sufficient muscle contraction required to perform the task while keeping the sensory perception at a tolerable level. |
| Vascular system: The peripheral vascular system is comprised of arterial, venous and lymphatic vessels, and the flow in all three is amenable to change with the application of FES. The electrically induced contraction increases interstitial pressure, resulting in augmented lymphatic and venous flow; this leads to a reduction of edema, if present [59]. The FES induced contraction also augments the well-known energetic-metabolic cycle associated with skeletal muscle contraction, including but not limited to enhancement of calcium ion concentration in the sarcoplasmic reticulum (SR),and relative changes in phosphocreatine (PCr), inorganic phosphate (Pi), intracellular pH (pHi), and glycogen metabolism [13]. The known response to these metabolic costs is to increase arterial blood flow and replenish the muscular system with oxygen and other nutrients. The reader must be alerted that these and other vascular mechanisms are integral parts of normal muscle activation by healthy individuals; they do not depend on electrically induced contractions. The value of applying electrical stimulation to augment vascular response is realized only when the vascular system is compromised due to damage to the neuromuscular or vascular or both systems. |
| Articular (peripheral joints) system: Loss of joint range of motion (ROM) is a common impairment of the musculo-skeletal system consequent to immobilization. The restriction of normal range is typically brought about by shortening and adhesion formation in soft tissues surrounding the joint, and in the case of damage to the brain by uncontrolled activation of muscles. Maintaining or restoring ROM has been achieved with the use of FES [5,14,41-43,48] The mechanism is a simple biomechanical proposal. The electrically induced contraction force translates at the joint into torque (moment of force), and the joint moves, overcoming the opposite toque generated by the shortened soft, connective tissues. |
| Effects of FES on the Central Nervous System (CNS) |
| The preceding few paragraphs highlight the basic and extensively investigated effects of electrical stimulation on the primary peripheral systems related to rehabilitation medicine. The last decade of investigations now provides considerable insight into the diverse pathways by which FES can affect the CNS. The principle that governs these effects originates from the fact that direct excitation of peripheral sensory nerves leads to action potential propagation viaspino-thalamic tracts reaching the sensory cortex. Concurrently, motor nerve stimulation and the resulting muscle contraction activate Golgi tendon organs, proprioceptors and other mechano-metabolic biological sensors, all of which generate multi-modal afferent inputs that reach and modify the activity in multiple regions within the brain. Credible, reproducible data obtained by functional magnetic resonance imaging (fMRI) clearly demonstrate that all major regions associated with sensory-motor control of movements, including the cerebellum, have been hemodynamically enhanced by stimulation of the upper or lower extremities [8,10,11]. |
| fMRI is one of several advanced technologies being used to study brain plasticity and the adaptive changes associated with learning or re-learning new tasks. Another well understood aspect of effective learning is the need to repeatedly practice various tasks or functions and in so doing re-direct, strengthen, and fine-tune brain connectivity. That is where FES may prove a most valuable intervention, particularly with patients who lost control of their movements following damage to the CNS after stroke, traumatic brain injury, cerebral palsy, or sclerotic disease of the nervous system. |
| Technological Advancements in FES |
| Meaningful technological advancements in the newest FES systems evolved from recognizing the critical shortcomings of existing devices and addressing major issues related to electrodes, stimulator size, ineffective and time consuming evaluation of patient’s candidacy, and inability to synchronize with functional training (not in the clinic and not at home). To date, the most advanced FES are wearable systems that incorporate the latest in bio-compatible materials interfacing with the body, micro-electronic infra-structure, small and fast recharging batteries, and software-driven selection of parameters as well as functional and task-specific training programs. Few FES systems also incorporate “intelligent” software that monitors patient performance and automatically adjusts the stimulation as the task or functional activity changes, or automatically adjust to account for changes in terrain while walking [60,61]. |
| These technological advancements introduce new names or terms with whose meanings clinicians should become familiar. Biocompatible materials refer mostly to the electrodes, and the material used to secure them to the body. The typical self-adhesive electrodes may soon be replaced with water-soaked, highly absorbent fabric to further minimize skin irritation and improve the comfort of stimulation. The wearable system’s material should also be easy to clean to minimize bacterial and other contamination. This is particularly important for patients who depend on the FES for several hours every day. Wireless FES means that the typical lead wires have been eliminated- a major practical and cosmetic improvement. Wireless may also mean that communication between the stimulator, the sensors that control the stimulation, and the programming of the stimulator to set the individual patient’s treatment and functional activities are all done wirelessly. Wireless communication makes the patients’ evaluation and training considerably faster, saving the clinicians’ precious time. It likewise dramatically improves the ease of at-home use for the patient. “Intelligent software” could have diverse meanings, and the astute clinician should learn the specific meaning from the product’s manufacturer. |
| Today’s advanced FES systems have noteworthy limitations. The first limitation is offering only one channel to control foot drop (Bioness, L300 and Walk Aid™) [60,61]; these systems are unlikely to adequately train and control the remaining lower extremity [62]. Another limitation is the restricted area for electrodes placement, which may cause exclusion of some patients with foot drop because of their inability to obtain adequate dorsiflexion. In fact, these systems depend on well-defined inclusion criteria that require testing by a certified clinician. Having such criteria assures quality control, reduces the chance of inappropriate prescription or misuse of the system, and dramatically improves compliance. Finally, the cost of advanced FES limits the number of users to those who can afford such a system. To date, no commercially available advanced FES system addresses the needs of the upper extremity. The closest is a 2-channel FES (Bioness, H-200™). |
| For those patients (and clinicians) who cannot wait until such an advanced system becomes commercially available, a custom-made system may be constructed by the clinician converting a standard NMES to a “self-administered” home-based FES system (Figure 3). |
| Barriers to Successful Physical Rehabilitation |
| In this section, a patient who survived a stroke will be used as a model to identify the foremost barriers to successful physical rehabilitation. The focus is on barriers that can be removed or minimized by the utilization of FES. The model is considered inclusive of patients with other neurological presentations, including TBI, CP, and MS provided that the primary barriers are identified. The model considers 3categories of barriers: the severity of CNS damage, impairments as primary barriers, and currently published training programs. |
| Severity of CNS damage |
| Damage to the CNS can be documented anatomically by the size and location of the lesion(s) within the brain and clinically by the severity of motor loss. The relationships between the lesion’s size or location and the patient’s motor loss appear complex, and neither size nor location is as strong predictor of functional recovery as the time-dependent motor recovery profile of individual patients [36,37]. Motor loss and recovery have been reported in many well controlled clinical trials typically using clinical scales and sub-scales. A frequently published test, the Fugl-Meyer scale for the upper and lower extremities, includes many items reflecting the subject’s ability to volitionally move various joints of the extremities in specific patterns. The test appears to predict functional recovery of the paretic upper and lower extremities in about 70% of cases [36,37]. Lang and Beebe [46] recently demonstrated that measuring, in degrees, volitional ROM at the shoulder, elbow, wrist and fingers also predicted functional use of the upper extremity about 70% of the time. Conceivably, simple active ROM of large joints in both upper and lower extremities and the ability to grasp, hold, move, and release objects (hand function) can serve as an effective and not labor intensive test to document the severity of motor loss and recovery profile over time. |
| Impairments as primary barriers |
| Clinicians who work with patients following damage to the CNS should recognize without difficulty the following barriers: 1) decreased ability to generate adequate muscle force; 2) spasticity; 3) limited joint (s) range of motions due to contracture/adhesion; 4) shoulder subluxation, 5) knee hyper extension during stance phase; and 6) ankle instability. However, from the perspective of FES utilization, a number of comments are warranted. Decreased ability to generate adequate volitional muscle force should be assessed clinically, regardless of hypo or hyper-tonicity/spasticity. Muscle force should be considered inadequate if the patient is unable to fully extend or flex the target joint. If applying FES enables more extension or flexion of a joint than the volitional effort, the patient is likely to benefit from FES training to minimize the muscle force impairment. |
| Managing spasticity with electrical stimulation has been documented extensively [63-65]. Whereas statistically significant reduction has been reported [15,63], the lasting effect of spasticity reduction is short lived (only minutes). In fact, clinically meaningful reduction can be expected only during the application of FES, typically by inducing contraction in the muscle group opposite to the spastic group. The clinical message should be clear: if the clinical objective is to manage spasticity, the patient most likely needs to apply the FES several times daily for many months, if not years. Similar statements can be extended to the treatment objectives of maintaining or improving joints ROM, manage shoulder subluxation, or controlling knee hyper extension and ankle instability during ambulation. Joint contracture and shoulder subluxation are more likely to develop shortly after stroke [66,67], and the sooner the FES program begins, the better the chance of minimizing these impairments [38]. Furthermore, if either subluxation or contracture is already present, and regaining volitional movements fails to occur, the patient should apply the FES several times daily for many months. |
| Barriers imposed by published training programs |
| The third group of barriers may have been an unintended consequence of the current rehabilitation delivery system’s reliance on evidence-based studies and the principal requirement to judge functional outcome against rehabilitation cost. The dependence on studies published in refereed scientific journals, and particularly on randomized clinical trials, resulted in a major barrier to transforming research to clinical practice because of several shortcomings common to most studies. The foremost shortcomings include1) arbitrary and most likely insufficient treatment dose; 2) selection of studies’ end point (termination) based on undisclosed rationale, conceivably unrelated to patients’ profiles of recovery; 3) studies’ inclusion criteria that favor subjects with less motor loss, thus excluding many patients; 4) isolating rather than combining FES with task-specific or functional training; and 5) measuring post-intervention outcomes for the upper extremity almost exclusively without the FES (FES independent, meaning the effect on re-learning the task or function). Documenting functional improvement with the FES (FES dependent) as done with FES for foot drop is essential to clinical practice as more patients with moderatesevere motor loss are likely to improve performance of daily functions with the FES on. More details of these shortcomings can be found in other publications [38]. |
| The focus of this essay is to help clinicians determine to what extent an FES training program can be based on published programs and to what extent the program should be modified to address the individual patient’s clinical profile of motor loss and recovery profile. In order to set an individual program, it is critical to contrast the findings of a newly admitted patient with published studies. This contrast must address many factors: 1) severity of sensory-motor loss; 2) how early “activity depended training” was initiated; 3) how long the intervention continued; 4) whether the training was constructed specifically to address the primary barriers to progress; and 5) whether the training was modified according to progress. |
| It is highly probable that for many patients seeking benefits from FES, the published studies were too short, the primary outcome measures were selected by the researchers but may not have been of priority or meaningful to the patient, and the specificity of training was not related to the individual patient’s primary barriers to progress or modified according to patient’s progress. In summary, published clinical trials provide an overall framework for FES training, but they will have to be transformed into an individual patient’s program if the objective is to maximize the functional recovery of each patient. |
| The New Paradigm: Transforming Existing Research to Clinical Practice, a Patient-Driven FES Intervention |
| The move toward patient-driven, or personalized intervention, began to gain momentum in medicine when technological advancements made it possible to target testing to the specific location of the malfunction, whether the location is at the sub-molecular, molecular, or cellular level of each individual patient. Examples include gene therapy based on the patient’s own identified malfunctioning gene, or prescribing a specific antibiotic only after the specific bacteria is identified. While the technologies needed to implement daily practice of gene therapy or automated dosages of insulin infusion are developing at a rather slow rate, personalized intervention with FES is already available for clinical implementation. |
| Advantages over evidence-based medicine (EBM) or practice (EBP) |
| In principle, EBM or EBP is a statistically based paradigm where a systematic and universally accepted approach enables collection of data from a rather small representative sample of patients and infers the findings to the population. However, the generalization should be confined to the methods and procedures used in the study. Accordingly, if the study’s treatment dose was limited to 10 minute session, 3 times per week, or if the treatment only lasted 4-6 weeks, or if the FES training did not include task-specific functional exercises or was not included in the outcome measure, the published study is of little value to many patients post-stroke who are referred to physical rehabilitation. This is particularly important if the clinicians determine that the patients are candidates for a much higher dosage of training, much longer treatment duration, or different task-specific training from what was done during the study. Clinicians should recognize that there are many constraints inherent in EBM/EBP randomized clinical trials, including strict inclusion/exclusion criteria and attempts to minimize the heterogeneity (variability) of study subjects. In addition, the very high cost of conducting such studies forces researchers to limit treatment dose and duration irrespective of patient progress. |
| The realization of the limits inherent in EBM, combined with a growing body of knowledge that prolonging task-specific training enables many patients post-stroke, TBI, MS, and CP to improve performance months and even years after the initial damage to the brain, provides the foundation to individual patient’s driven intervention. Moreover, the clinical presentation, including impairments and functional deficits, are likely to vary considerably among patients regardless of the time since stroke onset. As a result, selecting outcome measures that the FES is likely to improve, constructing specific training programs to maximize functional improvement, and assuring that the intervention objectives are relevant and important to the individual patient, all constitute the framework for personalized, patient-driven FES training. |
| FES as part of the “Enabling Intervention Paradigm” |
| The enabling intervention paradigm encompasses a number of technologies, some simple and others more advanced. In addition to FES that lately has become very advanced, the most published technologies include tread mill (with or without partial body weight support), various perturbation systems, robotic enhanced training, virtual reality training, dynamic bracing, and training shoes. Due to space limit, the main message in this paper is to highlight the concept that no single intervention option alone can maximize functional outcome. At a minimum, FES should be combined with task-specific training. Combining FES with treadmill training and adding dynamic bracing and training shoes are particularly useful because of the complementary effects that each can add to the FES training. Examples of these combinations are presented in the patient-driven programs. |
| Patient-driven FES programs |
| This last section provides a few examples of setting up FES programs to manage stroke survivors’ upper and lower extremities. All examples are relevant whether the time post stroke onset is 2-3 weeks or 2-3 years. Common to all programs is the need to evaluate each patient and identify primary impairments and functional deficits. Equally critical is to be thoroughly familiar with available FES systems and to select the system capable of minimizing the specific impairments and functional deficits of the individual patient. The simplest example of correct matching is a stroke survivor whose primary concerns include loss of motor control and weakness of the wrist and finger extensors and flexors and the inability to open, grasp, hold and release objects. At this writing, the Bioness H-200™ is a commercially available system in the USA and few other countries that matches the patient’s needs. But what if the H-200™ does not fit the patient’s forearm, or does not elicit adequate opening of the hand, or there is no financial support to obtain the system? A similar situation is the case of a stroke survivor having a foot drop and gait deviations that are caused primarily by the inability to control the ankle. The Bioness L-300™ and the Walk Aide™ are two commercially advanced FES systems specifically designed to activate the dorsiflexors at the correct timing of the gait cycle. But what if one of the two systems is more comfortable or easier to apply, leading to more likely compliance with long term use? What if the patient’s plantar flexors are also weak or the primary gait deviations are caused mostly by loss of control of the hamstrings or quadriceps? |
| All these questions represent non-matching and require the clinician to consider adaptation of existing FES devices and modifications in the training programs. An additional but equally important non-matching to consider is the patient’s perception of meaningful improvement vs. the clinician’s objective outcome measures. A typical example is that while using the FES, the patient improves walking speed from 0.40 to 0.55 meters/sec but feels no difference in terms of need to continued dependence on assistive device (cane or quadcane), or perceives no difference in confidence and ease of walking. Conversely, with FES, the patient demonstrates no change in gait velocity or walking distance, but is now able to walk without dependence on hand support. The mismatch between patient goals and clinician goals is clear. Taken together, reality dictates that matching patient’s goals with FES combined with task or functional-specific training require patient’s commitment to long-term training and intermittent but continued support from the clinician. The following are few examples of patientdriven FES programs: |
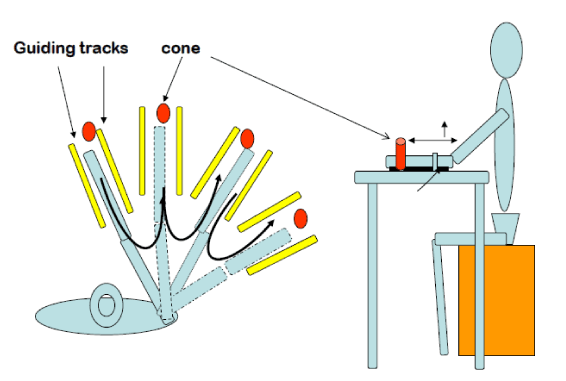
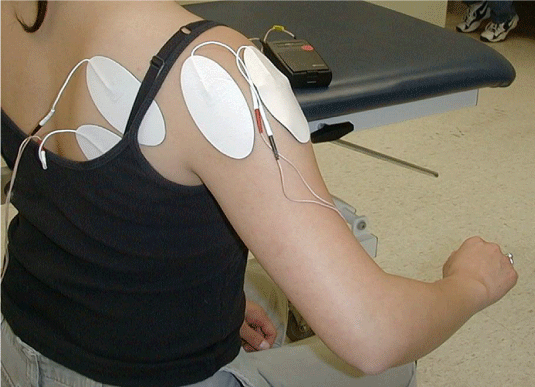
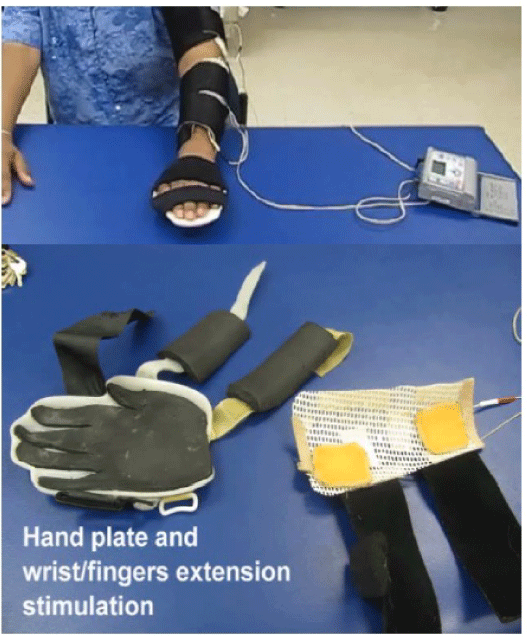
| FES for the upper extremity: A simple way to document loss and recovery of motor control can be done using the task-specific template illustrated in Figure 1. If the patient is unable to open the hand, one channel is placed over the wrist/finger extensors. If unable to extend the elbow during reach, a second channel is placed over the triceps brachii. If unable to lift the extremity of the table, another channel is placed over the deltoid/infraspinatus or scapular adductors (Figure 2). The training begins on the table with reaching, grasping an object, moving and releasing it in all directions with the knowledge that the most difficult task is reaching out laterally to the paretic side. Learning to reach quickly must be incorporated into the program, and so must bimanual reaching. Reaching while the upper extremity is supported (on the table or manually) is much easier than reaching unsupported. Accordingly, lifting the upper extremity off the table should be attempted routinely, and the patient’s ability to demonstrate such lift while reaching out represents a major improvement in recovery of motor control. Both clinician and patient must follow the definition of FES dependent vs. FES independent to determine improvement in performing the reaching tasks. In cases where stimulating the wrist/finger extensors results in wrist extension but concurrent finger flexion and inability to open the hand, a custom hand plate can be made out of readily available thermoplastic material (Figure 3). The hand plate assures better fingers position while strengthening the wrist/finger extensors and stretching the wrist/finger flexors. Progress is documented when opening the hand can be achieved with FES but without the hand plate. Finally, reaching training can be done in different positions; these include supine and standing and should include both uni and bimanual reaching, keeping in mind that the paretic upper extremity may be restored functionally as an assistive hand only in the majority of stroke survivors. |
| FES for the lower extremity: Following a stroke, the first clinical question should be: Is the patient able to stand and walk? If so, the clinician must identify the major deviations in performing the tasks of level ground ambulation, curb or stair negotiation, and sit-to-stand transition. In each of these three locomotion functions clinician must consider: a) If the patient uses cane/quad-cane/walker (hand support) and if she/he would like not to use it; B) If the patient uses AFO or KAFO, would he/she like ambulate without such assistance. If the patient is satisfied with the assistive devices the FES training would differ dramatically compared to training designed to help the patient become less dependent on assistive devices. The simplest example is a patient ambulating with AFO and is very satisfied. The Bioness L-300™ or WalkAid™ should not be offered to this patient. Similarly, a patient who is happy ambulating with a cane (or quadcane) and FES to control the foot, should not be offered task-specific training to improve walking speed, distance, and balance control leading to elimination of dependence on hand support. |
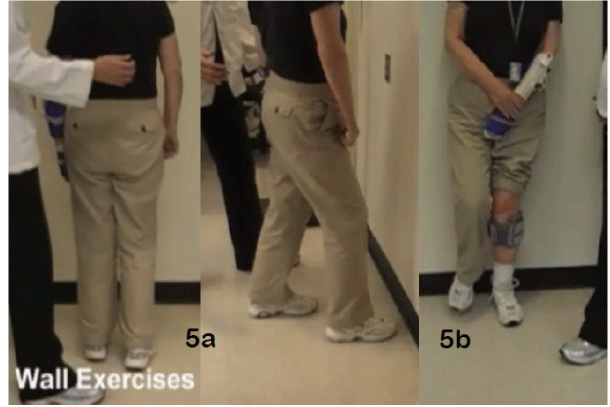
| For patients interested in becoming less dependent on hand support in daily locomotion, a task-specific training should be offered. In these cases, the FES is combined with other technologies and specific activities directed to minimize the primary barriers to independent locomotion. One example is the use of treadmill to improve walking speed. Figure 4 presents a patient 2-years post-stroke, learning to walk on a treadmill without hand support at a patient-driven ability to increase speed. Five months later the patient no longer depended on hand support (cane) in daily ambulation. A series of “wall exercises” are very effective in minimizing a number of primary barriers to independent locomotion. Figure 5a presents stepping toward the wall with the non- paretic foot, maintaining balance in this position for few seconds and stepping back. As the patient improves, the distance from the wall increases. Figure 5b presents a controlled squat-like training. The patient is standing against the wall, shifting weight onto the paretic lower extremity, then bending the knees and dorsiflexing the ankles, then lifting off the non-paretic foot, and then volitionally extending the paretic knee. As the patient improves his ability to perform the task on a single limb, the paretic foot, the task difficulty can increase by bending the paretic knee into more flexion, increasing the speed and repetitions of single limb partial squat. These wall exercises should be combined with FES applied to the dorsiflexors, plantar flexors, hamstrings, and quadriceps, depending on which “wall exercise” task is being practiced. In a single limb squat, all 4 muscles contract concurrently; a 4-channel FES would be helpful. The FES augments both eccentric and concentric control during wall squat training. |
| One final example of the “Enabling Intervention Paradigm” is the concept of training shoes. A patient with moderate to severe spasticity is likely to experience involuntary inversion-pronation of the foot overcoming the dorsiflextion-eversion generated by the FES. In this case, the shoe should be modified by increasing its lateral border as seen in Figure 6. How much to extend the lateral border depends on clinical verification that the foot is forced into eversion-supination at the instant of contact with the ground. With the modified shoe, the patient is able to ambulate independently and practice the above listed task-specific training programs. As the patient gains control over the spasticity and the inversion-pronation diminish, the lateral border of the shoe is made smaller and hopefully eventually returned to normal shoe. Without providing such training shoe, the FES may not control the ankle and may negate the potential for improving functional independence. |
References |
|
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 21378
- [From(publication date):
October-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 16381
- PDF downloads : 4997
