Research Article Open Access
Frontal Metabolite Concentration Deficits in Opiate Dependence Relate to Substance Use, Cognition, and Self-Regulation
Donna E Murray1,2*, Timothy C Durazzo3,4, Thomas P Schmidt1,2, Christoph Abé5, Joseph Guydish6 and Dieter J Meyerhoff1,2
1Center for Imaging of Neurodegenerative Diseases (CIND), San Francisco VA Medical Center, San Francisco, CA, USA
2Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, USA
3Department of Psychiatry and Behavioural Sciences, Stanford University School of Medicine, Stanford, CA, USA
4Mental Illness Research Mental Illness Research and Education Clinical Centers; Sierra-Pacific War Related Illness and Injury Study Center, VA Palo Alto Health Care System, Palo Alto CA, USA
5Department of Clinical Neuroscience, Osher Center, Karolinska Institutet, Nobelsväg 9, 17177 Stockholm, Sweden
6Philip R. Lee Institute for Health Policy Studies, University of California San Francisco, San Francisco, CA, USA
- *Corresponding Author:
- Donna E Murraya
Center for Imaging of Neurodegenerative Diseases (CIND)
San Francisco VA Medical Center
San Francisco, CA, USA
Tel: 415-221-4810 x2-2553
E-mail: donna.murray@ucsf.edu
Received date May 27, 2016; Accepted date July 8, 2016; Published date July 15, 2016
Citation: Murray DE, Durazzo TC, Schmidt TP, Abé C, Guydish J, et al. (2016) Frontal Metabolite Concentration Deficits in Opiate Dependence Relate to Substance Use, Cognition, and Self-Regulation. J Addict Res Ther 7:286. doi:10.4172/2155-6105.1000286
Copyright: © 2016 Murray DE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objective: Proton magnetic resonance spectroscopy (1H MRS) in opiate dependence showed abnormalities in neuronal viability and glutamate concentration in the anterior cingulate cortex (ACC). Metabolite levels in dorsolateral prefrontal cortex (DLPFC) or orbitofrontal cortex (OFC) and their neuropsychological correlates have not been investigated in opiate dependence.
Methods: Single-volume proton MRS at 4 Tesla and neuropsychological testing were conducted in 21 opiatedependent individuals (OD) on buprenorphine maintenance therapy. Results were compared to 28 controls (CON) and 35 alcohol-dependent individuals (ALC), commonly investigated treatment-seekers providing context for OD evaluation. Metabolite concentrations were measured from ACC, DLPFC, OFC and parieto-occipital cortical (POC) regions.
Results: Compared to CON, OD had lower concentrations of N-acetylaspartate (NAA), glutamate (Glu), creatine +phosphocreatine (Cr) and myo-Inositol (mI) in the DLPFC and lower NAA, Cr, and mI in the ACC. OD, ALC, and CON were equivalent on metabolite levels in the POC and γ-aminobutyric acid (GABA) concentration did not differ between groups in any region. In OD, prefrontal metabolite deficits in ACC Glu as well as DLPFC NAA and choline containing metabolites (Cho) correlated with poorer working memory, executive and visuospatial functioning; metabolite deficits in DLPFC Glu and ACC GABA and Cr correlated with substance use measures. In the OFC of OD, Glu and choline-containing metabolites were elevated and lower Cr concentration related to higher nonplanning impulsivity. Compared to 3 week abstinent ALC, OD had significant DLPFC metabolite deficits.
Conclusion: The anterior frontal metabolite profile of OD differed significantly from that of CON and ALC. The frontal lobe metabolite abnormalities in OD and their neuropsychological correlates may play a role in treatment outcome and could be explored as specific targets for improved OD treatment.
Keywords
Alcoholism; Brain; Cognition; Opiate; Proton magnetic resonance spectroscopy; Smoking
Introduction
The misuse of opiates is a serious problem worldwide, is increasing in young adults [1-3], and has substantial individual and societal consequences. In 2014 in the United States alone, approximately 1.9 million people had an opiate use disorder, including 586,000 heroin users [2]. Neuroimaging in opiate dependence indicates both altered brain structure, particularly in the anterior cingulate cortex (ACC; [4-7]), and brain function involving dorsolateral prefrontal cortex (DLPFC) and ACC [8,9]. Magnetic resonance spectroscopy (1H MRS) allows the non-invasive quantitation of brain metabolites that provide information on the neurophysiologic integrity of brain tissue [10]. The few 1H MRS studies in opiate dependence to date revealed lower concentration of N-acetylaspartate (NAA), a marker of neuronal integrity, in the medial frontal cortex, including the ACC [11-13], as well as lower glutamate (Glu), a primary excitatory neurotransmitter, or glutamate+glutamine concentration in some [11,13,14] but not all studies [15]. The discrepant MRS findings may relate to differences among study participants regarding the prevalence and severity of comorbid substance use (i.e., alcohol, tobacco, illicit drugs), the type, dose and duration of replacement therapy for heroin users (buprenorphine, methadone), and/or participant age.
The ACC, DLPFC and orbitofrontal cortex (OFC) are important components of the brain reward/executive oversight system, a neural network critically involved in the development and maintenance of addictive disorders [16,17]. Structural brain imaging in opiate dependence revealed generally lower gray matter volume or density in (pre)frontal regions [5-7,9,18], including the DLPFC [9,19], with thinner frontal cortices related to longer duration of opiate misuse [4]. Functional MR imaging showed that the DLPFC, OFC and ACC are involved in decision making [20-22], and in opiate dependent individuals, lower task-based fMRI activity in the ACC [8] related to compromised behavioural control of cognitive interference [8,11]. Furthermore, smaller frontal grey matter volume in opiate dependence related to higher impulsivity on the Barratt Impulsivity Scale (BIS-11; [19,23]). Correspondingly, opiate dependence is associated with cognitive deficits [24-28], primarily in executive functioning and selfregulation (impulsivity, decision-making, risk taking [19,29]). Thus, the neuroimaging literature in opiate dependence suggests altered frontal brain structure as well as compromised neuronal integrity and glutamatergic metabolism. Few if any studies however investigated their relationships to opioid and other substance use behaviour or cognition. Further research into specific regional brain effects and their potential cognitive and behavioural correlates may inform better targeted treatment of individuals with opioid use disorders.
We measured in opiate dependent individuals’ metabolite concentrations from the ACC and previously unexplored DLPFC and OFC and related them to quantitative measures of neurocognition, self-regulation, and substance use. Specifically, we compared opiate dependent individuals (OD) on buprenorphine maintenance to controls (CON). We also included another control group, a substancedependent ‘control’ group of 3 week abstinent alcohol dependent individuals (ALC), a commonly investigated treatment-seeking group to differentiate opiate dependence from not only control individuals but also individuals with a substance dependence (here, alcohol dependence). Our primary hypotheses were that: (1) OD have lower NAA and Glu concentrations than CON in ACC, DLPFC, and OFC, (2) these frontal cortical NAA and Glu deficits are associated with the level of opiate use and cigarette-smoking severity, (3) the frontal NAA and Glu deficits in OD relate to higher impulsivity, poorer executive function, and lower decision making skills, and (4) OD have more pronounced metabolite concentration deficits than ALC. The results of this study will contribute to a better understanding of the neurobiology and neuropsychology in OD, helping to identify novel targets for the treatment of opiate dependence.
Materials and Method
Participant characterization
All participants provided informed consent according to the Declaration of Helsinki and underwent procedures approved by the University of California, San Francisco and San Francisco VA Medical Center (institutional review board number 10-01042). Twenty-one chronic cigarette smoking OD, stable on buprenorphine maintenance therapy for at least 3 months, met DSM-IV criteria for dependence on opiates; they were allowed to meet DSM-IV criteria for current abuse or dependence on cocaine, amphetamines, and/or cannabis, but dependence on alcohol or benzodiazepines was exclusionary. OD was part of a buprenorphine treatment program focusing on smoking cessation and they were studied before smoking cessation. For group comparisons of metabolite concentrations specifically in the ACC, DLPFC, and POC and when correlated with neuropsychological variables, there were data from thirty-five cigarette smoking ALC recruited from local treatment programs of the VA and Kaiser Permanente and 28 cigarette smoking CON recruited from the community. The ALC group met DSM-IV criteria for alcohol dependence and was abstinent from alcohol (not tobacco) for 21 ± 11 days at time of study. For group comparisons of metabolite concentrations in the OFC and when correlated with neuropsychological variables (the OFC VOI only), there were smokers and non-smokers included in the ALC and CON groups: 21 ALC (9 non-smokers, 12 smokers) and 19 CON (14 non-smokers, 5 smokers) due to a lack of sufficient data in smokers. All participants were studied with structural MRI, 1H MRS, and neuropsychological testing, all were fluent in English and they were allowed to smoke ad libitum before assessment and during breaks. Table 1 contains demographics, tobacco and alcohol use variables, mood measures, and laboratory variables for the three groups.
| Variable | OD | ALC | CON | p (OD vs. CON) | p (OD vs. ALC) | p (ALC vs. CON) |
|---|---|---|---|---|---|---|
| Total n [male, female] | 21 (13,8) | 35 (29,6) | 28 (24,4) | |||
| Demographics | ||||||
| Age [years] | 41.1 ± 11.7 | 46.6 ± 8.8 | 44.2 ± 8.5 | NS | 0.053 | NS |
| Education [years] | 14.4 ± 1.5 | 13.3 ± 1.6 | 14.6 ± 2.0 | NS | 0.013 | 0.004 |
| Body Mass Index | 26.2 ± 5.3 | 25.9 ± 5.4 | 26.2 ± 3.4 | NS | NS | NS |
| Cigarette and alcohol use, mood measures | ||||||
| FTND total | 4.8 ± 1.4 | 3.6 ± 1.5 | 4.5 ± 1.8 | NS | 0.009 | 0.044 |
| Cigarette Pack Years | 16 ± 16 | 15 ± 12 | 18 ± 18 | NS | NS | NS |
| Total Cigarettes per day | 19 ± 8 | 12 ± 8 | 17 ± 7 | NS | 0.003 | 0.006 |
| Years Smoking | 23 ± 11 | 24 ± 11 | 23 ± 10 | NS | NS | NS |
| 1year avg. alcohol [drinks/mo] | 47 ± 101 | 307 ± 180 | 23 ± 20 | NS | 0.000 | 0.000 |
| 3year avg. alcohol [drinks/mo] | 58 ± 108 | 307 ± 173 | 23 ± 20 | NS | 0.000 | 0.000 |
| Lifetime avg. alcohol [drinks/mo] | 57 ± 52 | 204 ± 131 | 26 ± 13 | 0.001 | 0.000 | 0.000 |
| Years Drinking any alcohol | 23 ± 12 | 28 ± 11 | 8 ± 14 | 0.000 | NS | 0.000 |
| Beck Depression Inventory | 11.6 ± 8.2 | 13.6 ± 8.7 | 5.7 ± 4.5 | 0.002 | NS | 0.000 |
| STAI State | 33.0 ± 9.8 | 43.3 ± 12.2 | 30.3 ± 6.2 | NS | 0.002 | 0.000 |
| STAI Trait | 39.0 ± 11.6 | 37.9 ± 12.6 | 34.3 ± 9.6 | NS | NS | NS |
| Cognitive domains (z-scores) | ||||||
| Cognitive Efficiency | -0.15 ± 0.40 | -0.12 ± 0.54 | -0.21 ± 0.50 | NS | NS | NS |
| Executive Functioning | -0.12 ± 0.60 | -0.34 ± 0.62 | -0.64 ± 0.70 | 0.01 | NS | NS |
| Processing Speed | -0.21 ± 0.50 | -0.19 ± 0.64 | -0.29 ± 0.51 | NS | NS | NS |
| Visuospatial Skills | -0.08 ± 0.96 | 0.15 ± 0.93 | 0.19 ± 0.80 | NS | NS | NS |
| Working Memory | -0.10 ± 0.47 | -0.04 ± 0.70 | 0.14 ± 0.66 | NS | NS | NS |
| Global Cognition | -0.02 ± 0.45 | -0.02 ± 0.49 | 0.11 ± 0.45 | NS | NS | NS |
| Self-regulation | ||||||
| BIS-11 Total Impulsivity | 67.3 ± 11.2 | 66.3 ± 10.5 | 65.8 ± 12.0 | NS | NS | NS |
| BIS-11 Attention | 17.3 ± 4.1 | 16.7 ± 4.0 | 16.0 ± 4.3 | NS | NS | NS |
| BIS-11 Motor | 24.4 ± 5.1 | 23.3 ± 5.1 | 24.5 ± 4.3 | NS | NS | NS |
| BIS-11 Nonplanning | 25.6 ± 4.8 | 26.3 ± 4.6 | 25.4 ± 5.3 | NS | NS | NS |
| BART [adjusted avg pumps] | 33.1 ± 12.3 | 25.7 ± 11.7 | 35.3 ± 14.8 | NS | 0.071 | 0.029 |
| IGT [net total] | 13.2 ± 35.6 | 15.9 ± 26.7 | 6.0 ± 27.8 | NS | NS | NS |
| Laboratory variables | ||||||
| MCV [fl] | 89.7 ± 5.6 | 94.6 ± 6.4 | 93.1 ± 3.8 | NS | 0.008 | NS |
| γ-GTP [U/l] | 18.5 ± 10.8 | 59.8 ± 44.5 | 17.0 ± 4.5 | NS | 0.000 | 0.042 |
| Anion Gap [mmol/L] | 7.9 ± 1.7 | 8.6 ± 1.9 | 6.9 ± 1.8 | NS | NS | 0.006 |
| Potassium [mmol/L] | 4.0 ± 0.3 | 3.8 ± 0.3 | 4.3 ± 0.6 | NS | NS | 0.019 |
| Blood Urea Nitrogen [mg/dL] | 14.6 ± 4.4 | 13.3 ± 4.3 | 18.5 ± 4.8 | NS | NS | 0.012 |
| Hemoglobin [g/dl] | 13.3 ± 1.3 | 14.6 ± 1.3 | 14.8 ± 1.5 | 0.036 | 0.002 | NS |
| Hematocrit [%] | 38.9 ± 3.7 | 42.6 ± 4.1 | 43.2 ± 4.0 | 0.024 | 0.003 | NS |
Table 1: Demographics, alcohol and tobacco use, mood symptomatology, cognitive domains, self-regulation, and laboratory variables for OD, ALC and CON (mean ± standard deviation), NS: Non-Significant (p>0.05); FTND: Fagerstrom Tolerance Test for Nicotine Dependence; BIS-11: Barratt Impulsivity Scale version 11; BART: Balloon Analogue Risk Task; IGT: Iowa Gambling Task. Blood tests with non-significant differences between groups: Albumin, Aspartate Aminotransferase, Alanine Aminotransferase, Alkaline Phosphatase, Chloride, Cortisol, Carbon Dioxide, Glucose, Osmolality, Prealbumin, Protein, Red and White Blood Cell count and Sodium.
Further exclusion criteria for ALC and CON are described elsewhere [30]. In brief, ALC and CON participants were excluded for neurological disorders (e.g. seizures, neurodegenerative disorder, traumatic brain injury with loss of consciousness >5 min), psychiatric disorders (e.g. history of schizophrenia spectrum, bipolar and panic disorders, posttraumatic stress disorder), and medical and vascular risk factors (e.g. endocrine diseases, chronic obstructive pulmonary disease, type-1 diabetes, myocardial infarction, cerebrovascular accident, migraine headaches), known to affect neurobiology or cognition as well as for MRI contraindications. In OD and ALC, hepatitis C, type-2 diabetes, hypertension, unipolar mood disorder, or generalized anxiety disorders were not exclusionary due to their high prevalence in addiction [31-35]. Six OD, 4 ALC and 1 CON had hepatitis C (by self-report and medical chart review), while 4 OD and 13 ALC had medically-controlled hypertension.
All OD were on buprenorphine maintenance therapy averaging 15 ± 9 mg/day. Table 2 depicts their recent and lifetime substance use histories. Overall, OD as a group were all cigarette smokers (by design) and had comorbid stimulant and marijuana use over lifetime, which they reduced during the year before study. Only a few OD individuals had drug use within the last 30 days: 3 used opiates and/or cocaine but only 1 used opiates for 20 days, 1 other OD used amphetamines daily, and about one-third of the sample used marijuana. The majority of OD individuals were moderate alcohol drinkers over their lifetime, but they reduced their alcohol consumption during the last year before study; only 3 had consumed alcohol on more than 10 days within the last 30 days. The ALC group for the ACC, DLPFC, and POC VOI analyses were cigarette smokers abstinent from alcohol for about 3 weeks and used other drugs occasionally (5 ALC used marijuana and 1 ALC used cocaine within the last 30 days). Thus, the ALC group for the majority of the analyses (3 of 4 VOIs) and the entire OD group were cigarette-smoking treatment seekers, abstinent from their main drug of abuse for several weeks and they had similarly low levels of drug use within the last month before study.
| Substance | % | Duration in yrs | Lifetime g/mo | Previous yr g/mo |
|---|---|---|---|---|
| Opiates | 100 | 11 ± 7 (2-22) | 16 ± 14 (0.04-43) | 6 ± 13 (0.5-44; n=7) |
| Tobacco | 100 | 23 ± 11 (7- 45) | 554 ± 242 (180-1200)^ | not available |
| Alcohol* | 90 | 23 ±12 (6- 44) | 57 ± 52 (1-94) | 47 ± 101 (1-330) |
| Cocaine | 71 | 5 ± 7 (1-25) | 24 ± 52 (2-240) | 3 ± 8 (0.25-32) |
| Methadone | 62 | 2 ± 3 (1-10) | not available | not available |
| Marijuana | 62 | 18 ± 13 (3-41) | 17 ± 24 (2-90) | 8 ± 23 (2-90) |
| Amphetamines | 38 | 2 ± 3 (3-10) | 4 ± 9 (0.5-38) | 0.2 ± 1 (0.5-4; n=2) |
Table 2: Substance use histories of the OD group. Mean ± standard deviation; range in parentheses.
Clinical, neurocognitive and behavioural assessment
OD and ALC completed the Structured Clinical Interview for DSMIV Axis I disorders Patient Edition, v2.0 [36], CON were administered the corresponding screening module. The clinical and neurocognitive assessments of ALC and CON are detailed elsewhere [30]. In all groups, alcohol consumption was estimated with the lifetime drinking history interview [37,38], nicotine dependence was assessed with the Fagerstrom Tolerance Test for Nicotine Dependence [39], and lifetime substance use history (other than alcohol) was assessed with an inhouse questionnaire [40]. All participants completed the Beck Depression Inventory (BDI; [41]) and the State-Trait Anxiety Inventory (STAI; [42]).
A neurocognitive battery assessed the major domains affected by opioid and alcohol use disorders and Z-scores were calculated based on corresponding normative data. Cognitive domains were formed from specific neurocognitive tasks (see [30] for details and Table 3). The cognitive domain scores in ALC and CON were calculated according to the shortened neurocognitive battery of tests administered to the OD group and therefore, the constituent measures for cognitive domains in this study are different from our previous publications. All participants completed self-regulation measures, which included the BIS to assess self-reported impulsivity, the Balloon Analogue Risk Task (BART; [43]) to assess risk taking, and the Iowa Gambling Task (IGT; [44]) to assess decision making. Laboratory tests within 2-3 days of the MR scan evaluated the nutritional status and alcohol-related or other hepatocellular injury in OD and ALC. See Table 1 for laboratory variables, cognitive domain and self-regulation measures for the three groups.
| Cognitive Domain | Constituent Measures |
|---|---|
| Executive functions | •Short Categories Test [75] |
| •Stroop Test, color-word subtest [76] | |
| •Trail Making Test B [77] | |
| •Wisconsin Card Sorting Test-64 (WCST-64): Computer Version 2-Research Edition non-perseverative errors, perseverative errors, perseverative responses [78] | |
| Visuospatial skills | •Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III) Block Design [79] |
| Processing speed | •WAIS-III Digit Symbol [79] |
| •WAIS-III Symbol Search [79] | |
| •Stroop, colour-word subtests [76] | |
| •Trail Making Test A [77] | |
| Working memory | •WAIS-III Arithmetic [79] |
| •WAIS-III Digit Span [79] | |
| Global cognition | •The arithmetic average of z-scores for all of the individual cognitive domains |
| Cognitive efficiency | •The arithmetic average z-scores for tests that were timed, or where the time to complete the task influenced the score obtained |
| •Stroop Test, colour-word subtest [76] | |
| •Trail Making Test A and B [77] | |
| •WAIS-III Arithmetic, Block Design, Digit Symbol, Symbol Search [79] |
Table 3: Cognitive domains formed and constituent measures.
Magnetic resonance methods
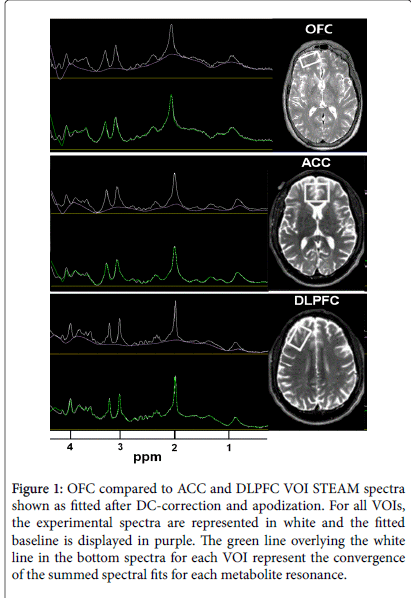
MR data were acquired on a 4 T Bruker MedSpec system with a Siemens Trio console (Siemens, Erlangen, Germany) using an 8- channel transmits-receive head coil. 3D sagittal T-1-weighted and 2D axial T2-weighted images were acquired using Magnetization Prepared Rapid Gradient imaging (TR/TE/TI=2300/3/950 ms, 7º flip angle, 1 × 1 × 1 mm3 resolution) and turbo spin-echo (TR/TE=8400/70 ms, 150º flip angle, 0.9 × 0.9 × 3 mm3 resolution) sequences, respectively. NAA, creatine+phosphocreatine (Cr), choline containing metabolites (Cho), myo-Inositol (mI) and Glu signals in MRS volumes-of-interest (VOIs) were acquired with a Stimulated Echo Acquisition Mode (STEAM) sequence (TR/TE/TM=2000/12/10 ms, 90º flip angle, 2000 Hz spectral bandwidth, 2.5 min) ([45]) and placed over the ACC (35 × 25 × 20 mm3), right DLPFC (20 × 40 × 20 mm3), right OFC (40 × 20 × 10 mm3) at the base of the inferior prefrontal cortex, and medial parietooccipital region (POC; 40 × 20 × 20 mm3) to maximize the corresponding cortical gray matter (GM) content. See Figure 1 for VOI placements and example MR spectra. γ-aminobutyric acid (GABA) signals from ACC, DLPFC and POC were acquired from the exact same VOIs with a modified J-editing sequence (MEGA PRESS: TR/ TE=2000/71, 90º flip angle, 2000 Hz spectral bandwidth, 12.5 min) [46]). STEAM and GABA spectra were not always acquired from all VOIs in all participants and the numbers of VOI-specific spectra analysed are shown in Table 4. The corresponding structural MR images were segmented into GM, white matter (WM), and cerebrospinal fluid (CSF; [47]) to estimate tissue fraction and CSF contributions to each VOI for calculation of metabolite concentrations in institutional units (i.u). Quantitated metabolite concentrations were corrected for CSF contribution and scaled to the water level from the corresponding VOI (Table 4). For full methods details see [48]. Twelve percent of CON and 47% of ALC participants of the current study were included in our previous reports on metabolite concentrations in individuals with alcohol and poly-substance dependence [48,49].
| VOI | Group | Number of smokers/non-smokers | WM Fraction | CSF Fraction | GM Fraction |
|---|---|---|---|---|---|
| ACC * | OD | 21/0 | 36.0 ± .2 | 17.1 ± 2.3 | 46.1 ± 3.7 |
| ALC | 28/0 | 34.7 ± 5.0 | 18.4 ± 3.4 | 45.9 ± 3.0 | |
| CON | 27/0 | 32.8 ± 3.8 | 19.2 ± 3.6 | 47.0 ± 2.6 | |
| DLPFC | OD | 20/0 | 53.2 ± 7.4 | 6.3 ± 3.0 | 39.6 ± 5.4 |
| ALC | 23/0 | 51.7 ± 6.8 | 7.0 ± 3.1 | 40.4 ± 4.6 | |
| CON | 27/0 | 51.3 ± 6.9 | 7.3 ± 3 | 40.5 ± 4.7 | |
| OFC # | OD | 14/0 | 58.8 ± 3.4 | 3.3 ± 1.2 | 37.1 ± 3.0 |
| ALC | 12/9 | 55.6 ± 5.5 | 4.7 ± 2.3 | 38.9 ± 4.6 | |
| CON | 5/14 | 58.8 ± 6.1 | 4.9 ± 2.7 | 35.5 ± 4.3 | |
| POC | OD | 18/0 | 29.2 ± 4.6 | 7.3 ± 2.0 | 62.7 ± 4.5 |
| ALC | 29/0 | 27.5 ± 5.0 | 10.3 ± 5.4 | 61.3 ± 4.1 | |
| CON | 6/2 | 28.8 ± 5.2 | 9.5 ± 3.1 | 60.9 ± 4.4 |
Table 4:Percent grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) contributions to the four volumes of interest (VOIs) (mean ± standard deviation), * WM or # GM tissue fraction differed in pairwise group comparisons (p<0.05).
Statistical analyses
Univariate analyses of covariance (ANCOVA) tested for group differences on demographic and clinical variables. All statistical analyses were performed with SPSS version 22. Separate ANCOVAs were performed for the four VOIs and each metabolite, followed by planned pairwise comparisons to test for group differences in metabolite concentrations between OD, ALC and CON. Given the participants’ wide age range (23-60 years) and as age correlates with metabolite concentrations (e.g., [50]), age was used as a covariate in group comparisons. As GM, WM, and CSF contributions to the VOIs affect brain metabolite levels [51] and as tissue content in ACC and OFC VOIs differed between groups (see Table 4), we included these variables as predictors in the ANCOVAs.
Figure 1: OFC compared to ACC and DLPFC VOI STEAM spectra shown as fitted after DC-correction and apodization. For all VOIs, the experimental spectra are represented in white and the fitted baseline is displayed in purple. The green line overlying the white line in the bottom spectra for each VOI represent the convergence of the summed spectral fits for each metabolite resonance.
Each a priori hypothesis was tested with an alpha level of 0.05. In pairwise group comparisons of metabolite levels without a specific a priori hypothesis, we used corrected alpha levels to account for the multiplicity of metabolites in each VOI via a modified Bonferroni procedure [52], which yielded adjusted alpha levels for each VOI separately by using the number of metabolites under investigation and their average inter-correlation coefficients (ACC: r=0.26; DLPFC: r=0.42; OFC: r=0.62; POC: r=0.44). The adjusted alpha levels for statistical significance were p=0.018 for ACC, 0.022 for DLPFC, 0.038 for OFC, and 0.020 for POC. OFC spectra often did not have a welldefined mI resonance (overlap with residual water) and therefore, OFC mI was not analysed. Effect sizes were calculated via Cohen’s d [53]. Correlations between outcome measures were corrected for age (i.e., partial correlations), except for correlations with cognitive domains (based on age-adjusted normative data), and reported as Pearson coefficients.
Results
Participant characterization
Age and years of education did not differ between OD and CON (Table 1). ALC were equivalent on age to OD and CON, but had fewer years of education. OD had lower hemoglobin and hematocrit than both CON and ALC. There were no significant differences in blood tests of liver function (γ-GTP, Albumin, Aspartate Aminotransferase, Alanine Aminotransferase and Alkaline Phosphatase) in individuals with and without Hepatitis C within the ALC group and also within the OD group. In addition, none of the individuals with Hepatitis C were taking medications at the time of study for Hepatitis C. Furthermore, the individuals taking hypertension medication did have controlled blood pressure by self-report but blood pressure levels at time of study were not measured. Nicotine dependence scores were higher in OD than ALC; OD and CON also smoked significantly more cigarettes per day than ALC, but all groups were equivalent on cigarette smoking duration. Gender did not contribute to any group difference or correlation. See Table 1 for drinking severity measures in OD, CON and ALC.
Group comparisons of metabolite concentrations
Significant main effects of group were observed for NAA, Cr, and mI in the ACC and for NAA, Cr, mI, and Glu in the DLPFC (Table 5). In pairwise comparisons of OD and CON, the DLPFC showed the greatest magnitude metabolite concentration differences, with effect sizes up to 1.64. Specifically, NAA, Glu, Cr, and mI were all significantly lower in OD (all p<0.01) than CON and ALC. OD also had lower NAA, Cr, and mI concentrations in the ACC (all p<0.001), while ACC Glu tended to be lower than in CON (p=0.06). GABA concentrations did not differ between OD and CON in any region. In the OFC, metabolite concentrations were not different between OD and CON, while Cho tended to be higher in OD (p=0.09). The CON group for OFC comparisons comprised both smokers and nonsmokers; in previous MRS research, smoking CON revealed metabolite deficits compared to non-smoking CON in DLPFC NAA, Cr, mI and Glu [54]. Here, we found lower OFC Cho and Glu in smoking versus non-smoking CON (effect size 1.55). Correspondingly, OFC Glu and Cho were significantly higher in OD than smoking CON (effect sizes 0.6-1.4), with no group differences for OFC NAA and Cr. In contrast to frontal VOI metabolite concentrations, POC NAA, Cr, Cho, mI and Glu concentrations did not differ significantly between OD and CON. The 3 week abstinent ALC did not differ significantly from CON in DLPFC metabolite concentrations, however, ALC had NAA and Cr reductions in the ACC similar to those of OD. In the OFC, ALC (comprised of both smokers and non-smokers) had significantly higher Glu and Cho than CON (potentially driven by the smaller proportion of smokers among CON).
| ROIs & Metabolites | Mean ± SD | Effect Sizes | Group Significance | ||||
|---|---|---|---|---|---|---|---|
| OD | ALC | CON | OD vs. CON | OD vs. ALC | ALC vs. CON | ||
| DLPFC NAA | 4.0 ± 0.6 | 4.7 ± 0.6 | 5.0 ± 0.6 | 1.59* | 1.09* | 0.5 | F(2,68)=14.82, p<0.001 |
| DLPFC CR | 3.3 ± 0.6 | 4.3 ± 0.6 | 4.2 ± 0.6 | 1.64* | 1.67* | 0.03 | F(2,70)=19.22, p<0.001 |
| DLPFC CHO | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.46 | 0.71* | 0.25 | NS |
| DLPFC MI | 2.3 ± 0.7 | 3.0 ± 0.7 | 3.0 ± 0.7 | 0.94* | 0.93* | 0.01 | F(2, 69)=6.12, p=0.004 |
| DLPFC GLU | 2.7 ± 0.5 | 3.0 ± 0.5 | 3.2 ± 0.5 | 1.00* | 0.65 | 0.36 | F(2, 70)=5.74, p=0.005 |
| DLPFC GABA | 1.4 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.23 | 0.11 | 0.12 | NS |
| ACC NAA | 4.8 ± 0.8 | 4.8 ± 0.8 | 5.4 ± 0.8 | 0.78* | 0.01 | 0.79* | F(2,76)=5.22, p=0.009 |
| ACC CR | 3.7 ± 0.7 | 3.8 ± 0.7 | 4.5 ± 0.7 | 1.18* | 0.21 | 0.96* | F(2,74)=12.46, p<0.001 |
| ACC CHO | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 | 0.45 | 0.12 | 0.57 | NS |
| ACC MI | 2.3 ± 1.0 | 3.6 ± 1.0 | 3.4 ± 1.0 | 1.15* | 1.31* | 0.17 | F(2,74)=10.96, p<0.001 |
| ACC GLU | 3.3 ± 0.9 | 3.4 ± 0.8 | 3.8 ± 0.9 | 0.59 | 0.15 | 0.44 | NS |
| ACC GABA | 1.5 ± 0.5 | 1.4 ± 0.5 | 1.6 ± 0.5 | 0.11 | 0.21 | 0.32 | NS |
| OFC NAA | 4.9 ± 1.0 | 4.7 ± 0.9 | 4.8 ± 0.9 | -0.12 | -0.24 | -0.12 | NS |
| OFC CR | 3.6 ± 1.0 | 3.9 ± 1.0 | 3.7 ± 1.0 | 0.11 | 0.3 | 0.19 | NS |
| OFC CHO | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.2 | -0.61 | -0.54 | -1.14* | F(2,51)=5.84, p=0.005 |
| OFC GLU | 2.6 ± 0.7 | 3.0 ± 0.7 | 2.4 ± 0.7 | -0.24 | -0.62 | -0.86* | F(2, 50)=3.76, p=0.031 |
| POC NAA | 5.3 ± 0.8 | 5.1 ± 0.8 | 4.9 ± 0.8 | -0.49 | -0.25 | -0.24 | NS |
| POC CR | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.1 ± 0.6 | -0.34 | 0.01 | -0.33 | NS |
| POC CHO | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | -0.86 | 0.41 | -0.45 | NS |
| POC MI | 2.9 ± 0.9 | 2.3 ± 0.9 | 2.8 ± 0.9 | -0.14 | -0.67 | -0.53 | NS |
| POC GLU | 3.7 ± 0.6 | 3.6 ± 0.6 | 3.7 ± 0.6 | 0.09 | -0.14 | -0.23 | NS |
| POC GABA | 1.7 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.3 | -0.62 | -0.48 | 0.13 | NS |
Table 5: Region specific metabolite concentrations for OD, ALC, CON, (mean ± standard deviation) effect sizes (Cohen’s d), and group statistics (ANCOVA), Reported values are estimated mean and standard deviation in institutional units from a 3-group ANCOVA with age and tissue contribution as covariates as needed, NS: Not significant; Significant group effects in bold, * Significant pairwise group comparison after Bonferroni adjustment.
Associations between metabolite concentrations and cigarette smoking measures
Cigarette smoking measures did not correlate significantly with metabolite concentrations in OD, but trends emerged: ACC NAA and Cr tended to correlate negatively with more cigarettes/day (both r>-0.39, p<0.08). In sCON, ACC Glu was negatively associated with pack-years (r=-0.41, p=0.04, statistical trend), and in sALC, FTND score and cigarettes/day was positively related to OFC mI (both r>0.70, both p<0.02).
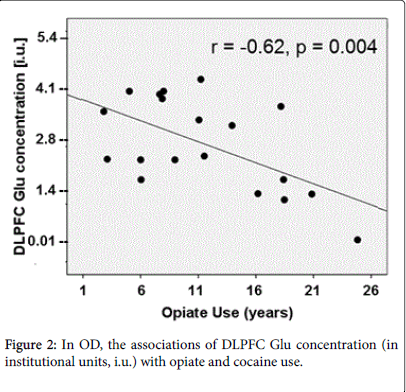
Associations between regional metabolite concentrations and substance use in OD
Greater substance use in OD related to altered metabolite concentrations, after adjusting for age: DLPFC Glu was negatively associated with lifetime duration of opiate (r=-0.62, p=0.004) and cocaine use (r=-0.45, p=0.02) (Figure 2), whereas DLPFC NAA did not correlate with any substance use measure. ACC GABA correlated negatively with monthly opiate use in the previous year and with monthly cocaine use over lifetime (both r>-0.47, both p<0.043, trends after multiple comparison correction). In addition, ACC Cr correlated negatively with monthly marijuana use in the previous year (r=-0.54, p=0.016) and over lifetime (r=-0.47, p=0.03, statistical trend) and positively with amphetamine use in the previous month (r=0.49, p=0.03, statistical trend). Finally, POC Cr correlated negatively with longer duration of opiate use (r=-0.58, p=0.014) and mI correlated negatively with monthly opiate use in the previous year (r=-0.65, p=0.003).
Cognitive domains and self-regulation in OD
OD had better executive functioning scores than (smoking) CON (p=0.01), but did not differ on any other cognitive domain, decision making, or risk taking measure (Table 1). Also, OD did not differ significantly from abstinent ALC on cognitive domain scores, decision making or risk-taking. In OD, working memory related to lifetime years of opiate use (r=-0.53, p=0.01). OD performed in the average range of functioning across all domains based on the domain z-scores derived from normative data.
Associations of metabolite concentrations with cognition and self-regulation measures
In OD, DLPFC NAA concentration correlated with executive function (r=0.54, p=0.024, uncorrected), and NAA and Cho correlated with visuospatial skills and global cognition (all r>0.51, all p<0.031, uncorrected). Also in CON, DLPFC NAA correlated with visuospatial skills (r=0.47, p=0.01). In OD, ACC Glu correlated with working memory (r=0.50, p=0.02); the low NAA and Glu concentrations in DLPFC and ACC did not correlate with any of our measures of selfregulation; only OFC Cr was negatively related to non-planning impulsivity (r=-0.65, p=0.021).
Discussion
This study compared cortical metabolite concentrations, neurocognition, and self-regulation between cigarette-smoking opiate dependent individuals on buprenorphine maintenance therapy, treatment-seeking alcohol dependent smokers, and smoking controls. OD had significant metabolite alterations in markers of neuronal integrity (NAA), cell membrane turnover/synthesis (Cho), glutamate concentration (Glu), cellular bioenergetics (Cr), and astrocyte integrity (mI) in frontal lobe regions implicated in the development and maintenance of addictive disorders. OD had lower NAA, Glu, Cr and mI concentrations than CON in the DLPFC and lower NAA, Cr and mI in the ACC. The metabolite concentration deficits in OD were most pronounced in the DLPFC, were associated with various substance use measures, and correlated with worse performance on measures of global cognition, executive and visuospatial functioning. However, OD and CON were equivalent in regional GABA concentrations, most cognitive domains, and self-regulation measures. Relative to 3 week abstinent ALC, OD had significantly lower NAA, Cr, Cho and mI concentrations in the DLPFC, with NAA and Cho deficits having cognitive ramifications.
Consistent with most previous reports [11-14], we found metabolite deficits in the ACC of OD. In addition, OD had similar deficits in NAA, Cr, and Glu reductions in the DLPFC. This suggests reduced neuronal and astrocyte viability and cellular bioenergetics in both the ACC and DLPFC, with additional glutamatergic injury in the DLPFC. ACC Glu and also DLPFC NAA and Cho metabolite abnormalities related to poorer cognitive function, which, however, did not differ significantly from CON. Of note, GABA concentrations in ACC and DLPFC of OD were equivalent to those in smoking CON, similar to findings in 3-week abstinent ALC versus smoking CON (this study) and 1 week abstinent ALC vs. mostly non-smoking CON [48]. However, ACC GABA reductions were reported in abstinent individuals with cocaine- [55] and polysubstance-dependence [49]. The POC and occipital region have been used as control regions in MRS studies as they are typically not altered in addiction [56,57]. This appears to be true also for OD, who showed the most pronounced metabolite deficits in anterior frontal brain regions.
We also assessed the OFC region previously not investigated in OD. The lateral OFC subserves motivation, drive, reward valuation, and aspects of social executive skills, is affected in opiate dependence [58] and other drug abuse [59], and the OFC has altered brain activity in decision making task-based fMRI studies of individuals with substance use disorders [60]. OFC metabolite concentrations did not differ between OD and CON, the latter including mostly non-smokers. However, and in contrast to DLPFC and ACC findings, OD showed elevated Glu and Cho concentrations in the OFC when compared to a subset of CON, the small group of smoking CON. Although the small group size warrants caution when interpreting results, our finding of lower OFC Cho concentration in smoking vs. non-smoking CON is consistent with lower Cho measured in frontal, midbrain and vermis regions of smoking vs. non-smoking controls [61].
In OD, lower DLPFC Glu and strong trends for lower ACC GABA correlated with greater severity and duration of opiate use. These findings are congruent with other neuroimaging studies that reported lower DLPFC GM density [9,18] and poorer functional connectivity between DLPFC and parietal regions associated with greater duration of opiate use [9]. ACC Glu and NAA were not related to opiate use, consistent with previous reports [11]. However, greater cocaine and marijuana misuse in our OD group was associated with significantly lower metabolite concentrations, commensurate with findings in other substance using/dependent populations [62-64].
Metabolite concentrations in the DLPFC and ACC of OD related to executive function, visuospatial skills, global cognition and working memory, but not to self-regulation measures. Previous 1H MRS studies in opiate dependence did not report on such relationships, but studies in marijuana-dependent and recreational ecstasy users reported relationships between altered frontal metabolite levels and impaired cognition or higher impulsivity [56,65,66]. Although previous research in opiate addicts reported neuropsychological deficits [24,25], our OD group performed in the average range across various cognitive domains and self-regulation measures. There is some evidence that buprenorphine maintenance is associated with better cognition compared to other maintenance drugs [67-70], and buprenorphine has been shown to improve brain perfusion in cocaine dependence [71,72]; correspondingly, buprenorphine may have had an effect on cognitive performance in OD in this study. Future studies on the effects of buprenorphine on brain function and cognition in OD may be useful to inform effective treatment.
Our study showed that OD on maintenance therapy had greater anterior frontal brain metabolite abnormalities than 3 week abstinent ALC, and we found previously that even 1 week abstinent ALC did not show metabolite abnormalities in the DLPFC [48]. The greater DLPFC metabolite abnormalities in OD may relate to the greater relapse rate in opiate than alcohol dependence [73], which may require differently tailored approaches for treatment of OD and ALC. Metabolite deficits in the DLPFC of OD are more reminiscent of 1H MRS results in polysubstance users [49,64], recreational cannabis users [62], and methamphetamine dependent individuals [63]. The DLPFC is critically involved in executive functions, such as working memory, cognitive flexibility, planning, inhibition, and abstract reasoning. As such, DLPFC brain metabolite abnormalities, in addition to those in ACC, may be promising targets to monitor the efficacy of cognitive behaviour therapy in OD treatment, especially as they correlate with cognition and substance use behaviour.
This study has limitations. Drug use histories were based on selfreport and gender effects across groups could not be assessed due to the small number of females (21%). Menstrual cycle appears to affect brain GABA levels [74], but data on the time since last menstrual cycle was not collected. However, excluding the female participants from statistical analyses did not alter the finding of no significant GABA differences between groups. The number of analysed spectra for some comparisons was relatively small, especially those involving smoking CON with OFC and POC VOIs; therefore, these analyses need to be considered hypothesis generating rather than definitive. Further, differences to previous metabolite and neuropsychological research in OD may relate to differences in comorbid tobacco, alcohol, marijuana and stimulant abuse as pointed out previously [28]. Of note in this context is the relatively low lifetime and current alcohol use in our OD sample. An additional limitation is that the duration of buprenorphine maintenance therapy was not assessed, although OD had to be on therapy for at least 3 months. Furthermore, the results may not be generalizable to OD who is not on buprenorphine therapy. Finally, we cannot rule out the possible contributions of premorbid, developmental, and dietary/nutritional factors to the neurobiological group effects reported [75-79].
Conclusion
Our findings of regional metabolite concentration abnormalities in the absence of frank neuropsychological impairment in OD are of clinical significance. They extend previous reports of ACC metabolite abnormalities in OD to DLPFC and OFC, all important components of brain circuitry relevant to relapse risk, and they include comparisons with smoking CON. While the findings are largely consistent with the broader literature on prefrontal brain deficits in substance users, they also expose differences of the frontal metabolite profile between OD and ALC, revealing metabolic abnormalities more similar to those of polysubstance, cannabis and methamphetamine users and related to cognitive performance, opiate, and comorbid substance use. In efforts to facilitate endogenous neuroplasticity, these metabolite abnormalities and comorbid substance use should be explored as important targets in the treatment of opiate dependence including heroin addiction. From a methodological point-of-view and because MRS measures are related to cognition, quantitative 1H MRS may be useful for monitoring both pharmacological and cognitive behavioural therapy intended to facilitate abstinence in OD.
Acknowledgement
We extend our appreciation to all participants who volunteered for this study. For patient recruitment we thank Dr. Sharon Hall, Gary Humfleet, Kevin Delucchi, and colleagues of the San Francisco Department of Public Health, Mary Rebecca Young, Bill Clift, Kathleen Altieri, Ricky Chen, and Drs. Peter Banys, Steven Batki, and Ellen Herbst of the Veterans Administration Substance Abuse Day Hospital, and Dr. David Pating, Karen Moise, and colleagues from the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco.
Role of Funding
This work was supported by grants R01 AA10788 (DJM), P50 DA009253 (JG), and DA24136 (TCD) from the National Institutes of Health and by the use of resources at the SFVA Medical Center. The research has been administered by the Northern California Institute for Research and Education. Apart from funding and use of resources, the center did not contribute to this research or to the manuscript preparation.
Contributors
Dr. Dieter Meyerhoff had central oversight and overall responsibility for this research. He designed the study and wrote the study protocol. Dr. Joseph Guydish and his clinical team facilitated access to the OD individuals who participated in a smoking cessation study. Drs. Donna Murray and Christoph Abé were responsible for MR data acquisition. Thomas Schmidt was responsible for assessment of demographic, behavioural and cognitive data, supervised by Dr. Timothy Durazzo. Dr. Donna Murray was responsible for data processing and preparation, statistical analyses, literature review and she wrote the first draft of the manuscript. Dr. Dieter Meyerhoff processed the GABA data and contributed to the literature review. Dr. Meyerhoff supervised the data processing while Dr. Durazzo supervised the statistical analyses. All authors approved the final version of the manuscript.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Tang YL, Zhao D, Zhao C, Cubells JF (2006) Opiate addiction in China: Current situation and treatments. Addiction 101: 657-665.
- SAMHSA, Centre for Behavioural Health Statistics and Quality (2015)Behavioural health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50).
- OCNNCC (2014) Annual Report on Drug Control in China. Beijing, China: Office of China National Narcotic Control Commission.
- Li M, Tian J, Zhang R, Qiu Y, Wen X, et al. (2014) Abnormal cortical thickness in heroin-dependent individuals. Neuroimage 88: 295-307.
- Liu H, Hao Y, Kaneko Y, Ouyang X, Zhang Y, et al. (2009) Frontal and cingulate gray matter volume reduction in heroin dependence: Optimized voxel-based morphometry. Psychiatry ClinNeurosci 63: 563-568.
- Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, et al. (2006) Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl) 184: 139-144.
- Wang X, Li B, Zhou X, Liao Y, Tang J, et al. (2012) Changes in brain gray matter in abstinent heroin addicts. Drug Alcohol Depend 126: 304-308.
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, et al. (2004) Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry 55: 531-537.
- Yuan K (2010) Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. NeurosciLett: 482: 101-105.
- Meyerhoff DJ,Durazzo TC, Ende G (2011) Chronic alcohol consumption, abstinence and relapse: Brain proton magnetic resonance spectroscopy studies in animals and humans. CurrTop BehavNeurosci 13: 511-540.
- Yücel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, et al. (2007) A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry 12: 611, 691-702.
- Haselhorst R, Dürsteler-MacFarland KM, Scheffler K, Ladewig D, Müller-Spahn F, et al. (2002) Frontocortical N-acetylaspartate reduction associated with long-term i.v. heroin use. Neurology 58: 305-307.
- Verdejo-García A, Lubman DI, Roffel K, Vilar-López R, Bora E, et al. (2013) Cingulate biochemistry in heroin users on substitution pharmacotherapy. Aust N Z J Psychiatry 47: 244-249.
- Greenwald MK (2015) Methadone maintenance dose modulates anterior cingulate glutamate levels in heroin-dependent individuals: A preliminary in vivo (1)H MRS study. Psychiatry Res 233: 218-224.
- Hermann D (2010) MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol 17: 659-667.
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, et al. (2009) The neurocircuitry of impaired insight in drug addiction. Trends CognSci 13: 372-380.
- Volkow ND, Wang GJ, Fowler JS, Tomasi D (2012) Addiction circuitry in the human brain. Annu Rev PharmacolToxicol 52: 321-336.
- Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, et al. (2009) Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn 71: 223-228.
- Qiu YW, Jiang GH, Su HH, Lv XF, Tian JZ, et al. (2013) The impulsivity Behaviour is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals. NeurosciLett 538: 43-48.
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, et al. (2002) Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology 26: 682-691.
- Rogers RD (2004) Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry 55: 594-602.
- Rogers RD (1999) Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19: 9029-9038.
- Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J ClinPsychol 51: 768-774.
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, et al. (2000) Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23: 113-126.
- Brand M, Roth-Bauer M, Driessen M, Markowitsch HJ (2008) Executive functions and risky decision-making in patients with opiate dependence. Drug Alcohol Depend 97: 64-72.
- Darke S (2012)Comparative patterns of cognitive performance amongst opioid maintenance patients, abstinent opioid users and non-opioid users. Drug Alcohol Depend 126: 309-315.
- Pluck G, Lee KH, Rele R, Spence SA, Sarkar S, et al. (2012) Premorbid and current neuropsychological function in opiate abusers receiving treatment. Drug Alcohol Depend 124: 181-184.
- Loeber S, Nakovics H, Kniest A, Kiefer F, Mann K, et al. (2012) Factors affecting cognitive function of opiate-dependent patients. Drug Alcohol Depend 120: 81-87.
- Fishbein DH (2007) Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug Alcohol Depend 90: 25-38.
- Durazzo TC (200) Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: A preliminary investigation. Alcohol ClinExp Res 31: 1114-1127.
- Hasin DS (2007) Prevalence, correlates, disability and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64: 830-842.
- MertensJR (2003) Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: Comparison with matched controls. Arch Intern Med 163: 2511-2517.
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K (2005) Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions and costs. Alcohol ClinExp Res 29: 989-998.
- Parekh RS, Klag MJ (2001) Alcohol: role in the development of hypertension and end-stage renal disease. CurrOpinNephrolHypertens 10: 385-390.
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, et al. (2005) Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 80: 105-116.
- First MB (1998) Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision). New York, NY: Biometrics Research Department.
- Skinner HA, Sheu WJ (1982) Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol 43: 1157-1170.
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, et al. (1988) The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol 49: 225-232.
- Fagerstrom KO, Heatherton TF, Kozlowski LT (1990) Nicotine addiction and its assessment. Ear Nose Throat J 69: 763-765.
- Pennington DL, Durazzo TC, Schmidt TP, Abé C, Mon A, et al. (2015) Alcohol use disorder with and without stimulant use: Brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PLoS One 10: e0122505.
- Beck AT (1978) Depression Inventory. Philadelphia: Centre for Cognitive Therapy.
- Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
- Lejuez CW (2002) Evaluation of a Behavioural measure of risk taking: the Balloon Analogue Risk Task (BART). J ExpPsycholAppl 8: 75-84.
- Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7-15.
- Frahm J, Merboldt KD,Hnicke W (1987) Localized proton spectroscopy using stimulated echoes. Journal of Magnetic Resonance 72: 502-508.
- Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB (2008) A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed 21: 22-32.
- Van Leemput K, Maes F, Vandermeulen D, Suetens P (1999) Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging 18: 897-908.
- Mon A, Durazzo TC, Meyerhoff DJ (2012) Glutamate, GABA and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend 125: 27-36.
- Abé C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, et al. (2013) Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend 130: 30-37.
- Schuff N (2001) Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging.MagnReson Med 45: 899-907.
- Jansen JF, Backes WH, Nicolay K, Kooi ME (2006) 1H MR spectroscopy of the brain: Absolute quantification of metabolites. Radiology 240: 318-332.
- Sankoh AJ, Huque MF, Dubey SD (1997) Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 16: 2529-2542.
- Cohen J (1988) Statistical power analysis for the Behavioural sciences. Hillsdale, NJ: Lawrence Erlbaum Associates.
- Durazzo TC, Meyerhoff DJ, Mon A, Abé C, Gazdzinski S, et al. (2016) Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain N-acetylaspartate and glutamate levels. Biol Psychiatry 79: 481-488.
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, et al. (2004) Frontal lobe GABA levels in cocaine dependence: A two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res 130: 283-293.
- Mashhoon Y (2013) Lower Left Thalamic Myo-Inositol levels associated with greater cognitive impulsivity in marijuana-dependent young men: Preliminary spectroscopic evidence at 4T. J Addict Res Ther.
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, et al. (2005) Methamphetamine users in sustained abstinence:A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 62: 444-452.
- Zhang Y, Tian J, Yuan K, Liu P, Zhuo L, et al. (2011) Distinct resting-state brain activities in heroin-dependent individuals. Brain Res 1402: 46-53.
- Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111: 1444-1451.
- Dom G, Sabbe B, Hulstijn W, van den Brink W (2005) Substance use disorders and the orbitofrontal cortex: Systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry 187: 209-220.
- Durazzo TC (2004) Cigarette smoking exacerbates chronic alcohol-induced brain damage: A preliminary metabolite imaging study. Alcohol ClinExp Res 28: 1849-1860.
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, et al. (2007) Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry 61: 1281-1289.
- Howells FM, Uhlmann A, Temmingh H, Sinclair H, Meintjes E, et al. (2014) (1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res 153: 122-128.
- Meyerhoff DJ (1999) Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: proton magnetic resonance spectroscopic imaging. Addict Biol 4: 405-419.
- Silveri MM (2011) Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res 191: 201-211.
- Cowan RL, Joers JM, Dietrich MS (2009) N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: A 3 T magnetic resonance spectroscopy study. PharmacolBiochemBehav 92: 105-110.
- Mintzer MZ, Copersino ML, Stitzer ML (2005) Opioid abuse and cognitive performance. Drug Alcohol Depend 78: 225-230.
- Pirastu R, Fais R, Messina M, Bini V, Spiga S, et al. (2006) Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies. Drug Alcohol Depend 83: 163-168.
- Rapeli P (2011) Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: Stability and correlates. BMC ClinPharmacol 11: 13.
- Gruber SA, Silveri MM, Yurgelun-Todd DA (2007) Neuropsychological consequences of opiate use. Neuropsychol Rev 17: 299-315.
- Holman BL, Carvalho PA, Mendelson J, Teoh SK, Nardin R, et al. (1991) Brain perfusion is abnormal in cocaine-dependent polydrug users: a study using technetium-99m-HMPAO and ASPECT. J Nucl Med 32: 1206-1210.
- Klingberg T (2010) Training and plasticity of working memory. Trends CognSci 14: 317-324.
- El-Sheikh Sel-G1, Bashir TZ (2004) High-risk relapse situations and self-efficacy: comparison between alcoholics and heroin addicts. Addict Behav 29: 753-758.
- Epperson CN (2002) Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 59: 851-858.
- Wetzel L, Boll TJ (1987) Short Category Test, Booklet Format. 1, Los Angeles: Western Psychological Services.
- Golden CJ (1978)StroopColor and Word Test, Chicago, IL: Stoelting Company.
- Reitan RM, Wolfson D (1985)The halstead-reitan neuropsychological test battery: Theory and interpetation, Tucson, AZ: Neuropsychological Press.
- Kongs S (2000) WCST-64: Wisonsin Card Sorting Test-64 Card Version, Professional Manual. Psychological Assessment Resources, Inc., Lutz, FL.
- Wechsler D (1997) Wechsler Memory Scale - III (WMS - III). San Antonio, TX: The Psychological Corporation.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 12371
- [From(publication date):
August-2016 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 11435
- PDF downloads : 936