Research Article Open Access
From the Mining to the Obtaining of Ferroelectric Ceramics
Rodríguez MDD*Institute of Cybernetics, Mathematics and Physics, 15 Street #551, Vedado, La Habana, CP 10400, Cuba
- *Corresponding Author:
- Rodríguez MDD
Institute of Cybernetics, Mathematics and Physics
15 Street #551, Vedado, La Habana, CP 10400, Cuba
Tel: +1 418-656-2131
E-mail: dolores@icimaf.cu
Received date: February 07, 2017; Accepted date: February 23, 2017; Published date: March 06, 2017
Citation: Rodríguez MDD (2017) From the Mining to the Obtaining of Ferroelectric Ceramics. J Powder Metall Min 6:160. doi:10.4172/2168-9806.1000160
Copyright: © 2017 Rodríguez MDD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Enjoy and learn by looking for the ancient techniques of working the pottery from the clay, led to the obtaining of more convenient materials and better mineral extractive processes. This is what has allowed the development of the great variety of domestic ceramic materials and the emergence of electro technical ceramic. The piezoelectric and ferroelectric ceramics depend on the origin of their raw materials, what has been the process of obtaining them? As well as on the variations of the ceramic process used. Five examples are presented of how the reagents, their purity, granulometry and present phases can modify the properties and characteristics of ferroelectric ceramics. The work will make a panoramic visualization of the materials and their origins, arriving at the obtaining of ferroelectric ceramics and the influence of the raw material (obtaining process) and the properties of the desired product.
Keywords
Ceramic materials; Ferroelectric phases; Piezoelectricity
Introduction
The first question we would ask for the development of this work would be what is a ferroelectric?
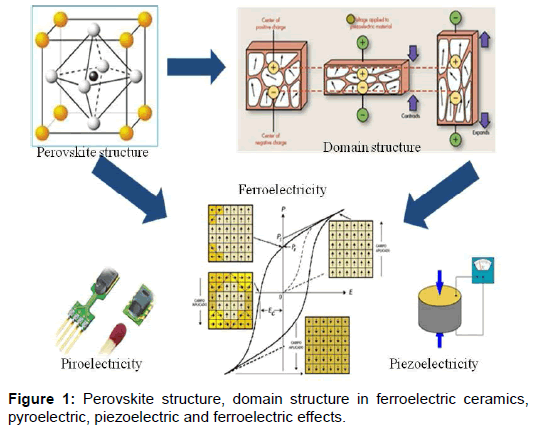
A ferroelectric is a dielectric material that has the characteristic of being piezoelectricity, which is also pyro electric and which is characterized by a hysteresis loop (Figure 1).
These characteristics do not appear in any material, they are manifested only in materials that physically possess one or more ferroelectric phases [1], this being a property that has a select group. Of the 32 point groups of symmetry in which all the crystalline materials can be classified, only 10 of them fulfill the following characteristics [2]:
• Do not have center of symmetry (necessary condition for piezoelectricity)
• Present spontaneous polarization (necessary condition for the piroelectricity)
• Reverse its polarization to an external electric field.
At present many types of ferroelectrics have been studied, in particular we will focus on those with perovskite structure ABO3. The second question that we would ask ourselves would be what is its importance?
These materials are widely used for the manufacture of ultrasonic equipment, for their properties as resonators and filters (clocks, tuning forks, filters for radio and television, telecommunications), delay lines (color television sets), pushbuttons and keyboards (telephones, printers, game machines, calculators, computers) among others and because the ceramic process used is relatively cheap.
The applications of these ceramics range from physicians in diagnosis and physiotherapies to industrial. Modifications are now being made to the obtaining procedures, which allow for cheaper ceramics yet with higher performance. The piezoelectricity is the most interesting characteristic of these materials; however, with the miniaturization of the devices, the Ferro electricity has received a substantial increase for the development of the same in applications as storage of information.
The third question to be asked is: What role does mining and extractive processes play in the properties of ferroelectric ceramics?
This will be answered in the development of the work being the main objective of the same.
Development
Under the general name of ceramics, objects are grouped that differ substantially from one to another in their bases composition, terracotta, stoneware, porcelain, on the exterior in which their shape can be covered by glaze or enamel, and in the how the cooking: temperature, oxidation atmosphere or reduction was carried out. It is a very fine type of earth that is composed of hydrated aluminum silicates that are produced by the decomposition of minerals.
Ceramic is the oldest industry of humankind [3], is a genius idea of man and fruitful since it has developed extensively throughout history not only in quantity but also in the variety of products, some of them, of transcendental importance for Technologies [4].
The manufacture of bricks appears in areas where the stone is scarce and the clay abounds. Ceramic material is the product of various raw materials (mainly clays) made in a powdery or pasty state (to easily communicate the form) and acquiring the stone consistency by physicochemical processes when cooking these clay.
Mining and extractive techniques
Mining is the selective extraction of minerals and other materials from the earth’s crust, from which an economic benefit can be obtained, as well as the primary economic activity related to it.
Depending on the type of material to be extracted, the mining is divided into metallic, non-metallic, ornamental stones and construction.
The mining process involves different stages, which are carried out to develop a mining project:
• The search and estimation of resources
• Extraction of ore
• Pre-treatment of ore
• Reduction to free metals
• Purification
Mining extractive can be classified in opencast mining or subterraneous mining:
Opencast mining
Also denominated of surface is realized by the elimination of the vegetation and of the superior layers of rock, to be able to arrive at the buried deposits. It can be divided into:
• Open pit mining
• Quarries
• Strip mining
• Mountaintop removal mining or mountaintop mining
Subterraneous mining
Sometimes called underground mining is done by building tunnels or galleries, with the goal of penetrating the rock to reach the sites. It is classified according to the access form:
• drift mining
• slope mining
• shaft mining
Or according to the extraction technique: [5,6]
• sinking, within these are found: block caving, sublevel caving y panel caving,
• for “caserones”, within these are found: sublevel stoping, vertical crater retreat (VCR), cut & fill and shrinkage, open stopes and room & pilar.
In order to analyze the extractive techniques used, we must know or present the materials that are developed ferroelectric ceramics. Historically, materials of the lead titanate-zirconate system (PZT) have been developed with different dopant, and more recently leadfree materials due to environmental campaigns to avoid contamination with lead, in particular sodium-potassium niobium, also with different dopant elements. Therefore, we will analyze the extractive processes of the majority elements: Na, K, Ti, Zr, Nb, Pb and of the minority ones: Li, Nb, La, Ta.
Extractive mining of the interest elements
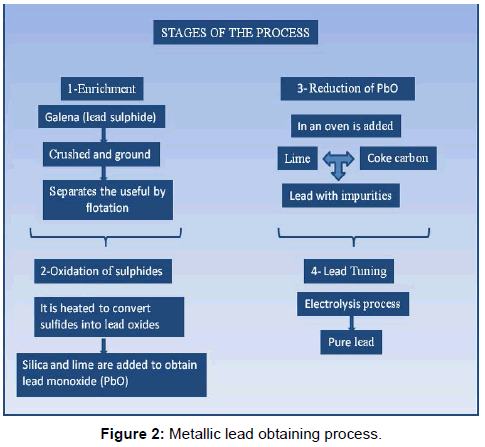
Lead (Pb): is widely distributed throughout the planet in the form of galena, cerusite and anglesite, which is lead sulfide. It occupies the 36th place in abundance among the elements of the earth’s crust.
The extraction of lead from the galena [7,8] is carried out by calcination the ore, converting it into oxide and reducing the oxide with coke in high furnaces. The process of obtaining the metallic lead is summarized in Figure 2, however to obtain the ferroelectric ceramics the lead is in the form of oxide (PbO) or carbonate (PbCO3), which would be what is obtained from the second and third steps of the process.
The structural forms in which lead minerals can be found are four: galena, cerusite, anglesite and litharge, distributed geographically throughout most of the planet but with greater abundance in America, Europe and Africa and Australia (Table 1).
| Pb Mineral | Chemical formula | Crystal system | Density (g/cm³) | Location |
|---|---|---|---|---|
| Galena | PbS | cúbico | 7.6 | Chile, Bolivia, Peru, Mexico, USA and Spain |
| Cerusita | PbCO3 | Ortorrómbico | 6.58 | Nassau, Saxony, England, USA and Spain |
| Anglesita | PbSO4 | Ortorrómbico | 6.3 | Namibia, Germany, USA, Spain, Australia and Mexico |
| Litargirio | PbO | Tetragonal | 9.35 | Appears in conjunction with Galena or Hydro-Cerucite |
Table 1: Crystallographic forms in which the lead mineral appears in the earth, also density and global location.
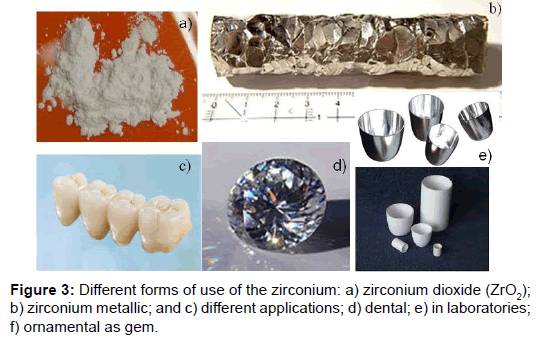
Zirconium (Zr): Zirconium [9] is a hard metal with characteristics similar to steel. It is one of the elements of the periodic table with more presence and greater distribution in the terrestrial crust. In nature, it is only found combined with other elements giving rise to minerals such as zircon, whose chemical formula is ZrSiO4 or as a zircon oxide (ZrO2). It is a transition metal, shiny, greyish white, hard, corrosion resistant and steel-like appearance. The main commercial source of zirconium is zirconium (zirconium silicate, ZrSiO4), which is found mainly in deposits in Australia, Brazil, India, Russia and the United States, as well as in smaller deposits around the world. 80% of zircon mining is produced in Australia and South Africa. As a curious fact, the first practical application of zirconium was using it as a flash in photography (Figures 3-6).
There are two main methods for obtaining the zirconium: the Kroll and the Van Arhel process.
The so-called Kroll process is the most commonly used for obtaining zirconium. It is a process of reductive chlorination in which the preparation of the chloride is made to go to a second phase of reduction using magnesium.
The Van Arhel process is based on the dissociation of zirconium iodide. Through this process is obtained zirconium of greater purity than with the Kroll process. When dissociating the zirconium iodide a metallic zirconium sponge is obtained, Which is melted to obtain the zirconium ingot (Figures 7-9).
Applications
• 90% of the consumption is used, in the form of alloy (zircaloy) in nuclear reactors, due to its resistance to corrosion and to have very low neutron capture section.
• As an additive in steels obtaining very resistant materials. Also in alloys with nickel for the chemical industry for its resistance against corrosive substances, especially for valves, pumps, pipes and heat exchangers.
• Manufacture laboratory crucibles, furnace lining and refractory material in ceramic and glass industries.
• Yttrium stabilized zirconium oxide is widely used in dentistry for the manufacture of fixed prostheses, removable prostheses and implant abutments. In addition, it is used for the replacement of joints since it is a mateiner bioinerte like the titanium.
• Heat exchanger, vacuum tubes and light bulb filaments.
• For military purposes it is used as an incendiary agent, such as the Dragon Breath Cartridge.
• In alloys to obtain materials with superconductivity and magnetism at low temperatures.
• In jewelry; Is an artificial gem called zirconite that imitates the diamond.
• In an industry in its infancy: the manufacture of cutting blades, are extremely durable and resistant to steel.
• Used as an additive to make synthetic sand.
Titanium (Ti): Titanium [10,11] receives its name from the ancient Greek, white earth (its rust is of the purest whites). It was discovered in 1790 by the English chemist William Gregor and in 1795 the German chemist Martin Klaproth gave it the name titanium.
It is the fourth most common metal in nature, is present in igneous rocks, materials formed by decomposition of igneous rocks, many minerals, especially those with iron and all plant and animal organisms.
Titanium is extracted first from rutile (titanium oxide), abundant in coastal sands. For this, the titanium must undergo a refining process before, to prevent its reaction with substances such as nitrogen, oxygen and hydrogen.
To obtain pure titanium, from the minerals that contain it, is mostly used the so-called Kroll Method, which consists of the reduction of titanium tetrachloride with magnesium, in an atmosphere of argon or helium that prevents its oxidation. The process is the following:
• Obtaining of titanium tetrachloride by chlorination at 800° C, in the presence of carbon, according to the reaction:
2 FeTiO3 + 7 Cl2 + 6 C → 2 TiCl4 + 2 FeCl3 + 6 CO
• The TiCl4 is reduced with magnesium or ground sodium in an inert atmosphere according to:
TiCl4 + 4 Na → 4NaCl + Ti If Sodium is used (Na)
TiCl4 + 2 Mg → Ti + 2 MgCl22 If Magnesium is used (Mg)
The Kroll method, traditionally used since 1937, has been tried to modify to obtain titanium powder used in the coating of reinforcements and aerospace components, transport and chemistry to increase the strength of surfaces.
Titanium dioxide is a compound whose formula is (TiO2). It is in a black or brownish form known as rutile. The natural forms found less in nature are the anatasite and brooquita. Both the pure rutile and the anatasite are white. The basic black oxide, (FeTiO3), is in natural form as the mineral called ilmenite. Titanium dioxide is the main commercial source of titanium. Approximately 95% of the titanium consumed is in form of titanium dioxide, due has many industrial applications.
Sodium (Na): Sodium is a chemical element of symbol Na (from the Latin, natrium) with atomic number 11, was isolated by Sir Humphry Davy in 1807. It is a soft alkaline metal, silverish, very abundant in nature, being in the sea salt and halite ore. It is very reactive, burns with yellow flame, oxidizes in the presence of oxygen and reacts violently with water. Sodium is present in large quantities in the ocean in ionic form. It is also a component of many minerals and an essential element for life [12].
Halites are the ores associated with the production of sodium chloride, although it is usually associated with magnesium chloride, calcium and potassium. A mining activity is carried out in two possible ways:
• Ore is extracted and pulverized until the desired appearance
• Water is pumped and dissolved with the minerals extracting a kind of mud-brine that is then dried by evaporation.
The methods used depend largely on the geological characteristics of the salt deposits. However the way it is used in ceramics is in Sodium Carbonate (Na2CO3), is a white and translucent salt, used among other things in the manufacture of soap, glass and dyes. It can be found in nature or obtained artificially, thanks to a process devised and patented in 1791 by French Nicolas Leblanc, which consists of:
1ero- Common salt with sulfuric acid
2 NaCl + H2SO4 → Na2SO4+ 2 HCl
2do- Na2SO4 with limestone and coal:
Na2SO4 + CaCO3 + 2 C → Na2CO3 + CaS + 2 CO2
Later this method was replaced by the one devised by the Belgian chemist Ernest Solvay, his method was able to lower the process and eliminate some of the problems presented by the Leblanc method. This method uses as raw materials sodium chloride (common salt), ammonia and calcium carbonate [13,14].
Potassium (K): constitutes about 2.4% by weight of the earth’s crust being the seventh most abundant. Due to its solubility, it is very difficult to obtain the pure metal from its minerals. However, in ancient seabed and lakes there are large deposits of potassium minerals (carnallite, polyhalite and sylvite) in which the extraction of the metal and its salts is economically viable [15].
Potassium carbonate is a white water-soluble (alcohol-insoluble) salt of the chemical formula K2CO3. Form strong alkaline solutions. It is usually formed as a product of the chemical reaction between potassium hydroxide or caustic potassium (KOH) and carbon dioxide (CO2). Is a hygroscopic substance, which often appears as a waterbased solid. It is commonly used for the manufacture of soap and glass and is the main component of caustic potash as found in nature.
Potassium carbonate was obtained from the leaching of ash from wood or other burned vegetables. The first industrial method to obtain it is the Leblanc process already in disuse. Nowadays, potassium carbonate is commercially produced from the reaction between carbon dioxide and potassium hydroxide obtained by electrolysis of the potassium chloride according to:
KCl + H2O → KOH + HCl
2KOH + CO2 → K2CO3 + H2O
Niobium (Nb): is a chemical element of 41 atomic number, located in 5 group of the elements periodic table. It is a transition metal, ductile, gray, soft and little abundant [16]. It is found in the mineral niobita or columbita. It is mainly used alloy in steels, conferring them a high resistance by adding only 0.1% of Nb. It is also used in welding, industries: nuclear, aerospace, electronics, optics, medical, numismatics and jewelry. In the last three applications, its low level of toxicity makes it highly quoted and with advantages over other materials.
It is estimated that niobium is the 33 most common elements on the Earth’s surface, but is not found free in nature. It is found in combination with other mineral elements, such as tantalum, both of great importance in today’s microelectronics industry. Australia is the world’s largest producer, so are Brazil, Nigeria, China, Thailand and the Scandinavian countries. Recent research estimates that most of the potential global reserves of tantalite are located in Africa, 80% of which are in the territory of the Democratic Republic of Congo.
The metal is obtained in two stages:
1- Separation of tantalum by solvents and transformation into Nb2O5 [17].
2- Nb2O5 is reduced in two stages with corbon
• First one at 800° C, NbC is formed,
• In the second stage the NbC at 2000° C, acts as a reducer of the oxide and the metal is produced.
In addition, the extracted ore is finely milled and separated by flotation and magnetic separation of high intensity.
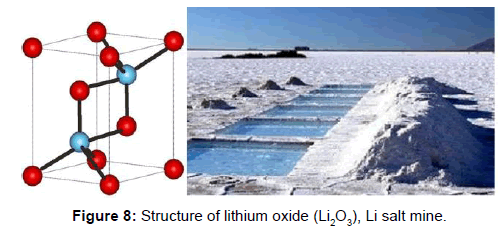
Lithium (Li): is a chemical element of atomic number 3. In the periodic table, it is in-group 1, between the alkaline elements. In its pure form, a soft, silver-white metal rust quickly in air or water. It is the lighter solid element and is used especially in heat conducting alloys, in electric batteries and their salts in the treatment of bipolar disorder [18].
It is the lightest metal, like the other alkaline metals, it is univalent and highly reactive, although less than sodium, so it is not found free in nature.
It was discovered by Johann Arfvedson in 1817 in the spodumena and lepidolite of a petalite mine (LiAl (Si2O5)2) on Utö Island (Sweden) that he was analyzing. In 1818 C.G. Gmelin was the first to note that lithium salts make the flame a bright red color. Both attempted, unsuccessfully, to isolate the element from their salts, which eventually got William Thomas Brande and Sir Humphrey Davy by electrolysis of lithium oxide.
In 1923 the German company Metallgesellschaft AG started producing lithium by electrolysis of molten lithium chloride and fused potassium chloride.
Since 2010, lithium batteries have become the primary method of replacing pollutants with fossil fuels. The “triangle of lithium” composed of the salar Uyuni in Bolivia, salar Atacama in Chile and salar Hombre Muerto in Argentina account for between 50 and 85% of the world’s reserves of that mineral [19]. There are other smaller salar such as Puna in Argentina and Manaure in Colombia, as well as in Afghanistan, recently discovered, whose magnitude is yet to be precisely determined. The accelerated growth in the use of lithium-ion has caused the accelerated increase of its price in 7 times its value in six years.
Applications
• Heat transfer applications due to their specific high heat.
• Anode for electric batteries, due to their high electrochemical potential.
• As a drying agent, due to its high hygroscopicity in form of chloride and bromide of lithium.
• In medical treatments of psychopathologies such as manias and bipolar depression, lithium salts (Li2CO3) are used.
• Stearate is a general purpose lubricant in high temperature applications.
• In the synthesis of organic compounds, for the coordination of ligands through the lithiated intermediate.
• As an air purifier extracting carbon dioxide, used in spacecraft and submarines.
• In alloys used in aeronautical construction as well as in the manufacture of ceramics and lenses.
• In nuclear applications.
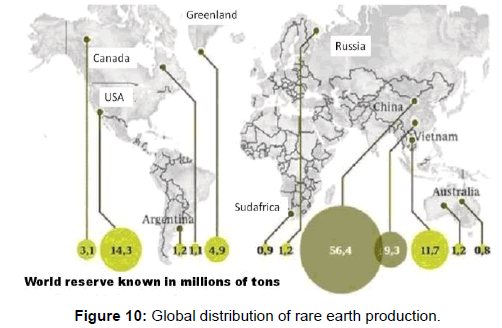
Lithium is industrially obtained from the electrolysis of molten lithium chloride (LiCl). The main minerals from which it is extracted are lepidolite, petalite, espodumena and amblygonite (Figure 10).
Lanthanides or “rare earths”: The series of lanthanides is the group of chemical elements that follow the lanthanum in-group IIIB of the periodic table. Their atomic distinction is that they occupy in electronic sublevel 4f. At first, they were only the elements with atomic numbers from 57 to 71, because the minerals in which they were accumulated were unknown and were isolated as oxides (or “lands”) hence their name [20]. Compared with many other elements, rare earths are not “rare”, their absolute abundance in the lithosphere is relatively high, but the study of these elements has been hazardous because of the similarity of their chemical characteristics. At this moment, this group is composed of: Lanthanum (La 57), Cerium (Ce 58), Praseodymium (Pr 59), Neodymium (Nd 60), Prometio (Pm 61), Samario (Sm 62), Europio (I 63), Gadolinium (Gd 64), Terbium (Tb 65), Dysprosium (Dy 66), Holmium (Ho 67), Erbium (Er 68), Tulio (Tm 69), Ytterbium (Yb 70), Lutetium (Lu 71), Yttrium (Y 39) and Scandium (Sc 21), for having similar characteristics [21-23].
Lanthanides are found in many minerals, mainly in the monazite (which is essentially a lanthanide orthophosphate); their distribution in minerals is La, Ce, Pr and Nd constitute approximately 90%, with Cerium being the most abundant element and Tulio the scarce. Pure lanthanides are silver metals with high boiling points. They react slowly with air, except Samarium, Europium, and Ytterbium, which are much more reactive with oxygen. Metals are preparations of fluorides or oxides by treatment with a strong metal reducer, such as calcium, or salts of chlorides and flowers by electrolysis at high temperatures. Lanthanides are typically insolubilized in a group by precipitating their insoluble hydroxides, oxalates or phosphates. Then they are separated by an ion exchange.
Until recently the only commercial use of rare earths was as a mixture of metal, mainly cerium, lanthanum, and neodymium, for lighter stones. However commercial production of these rare earths today is growing because of its multiple applications in metallurgy (to remove sulfur and oxygen), obtaining permanent magnets with a demand growth of 15% per year in the last 10 years. In production of television screens and energy efficient lamps; such as catalysts, ultraviolet light filters in glass, batteries for hybrid vehicles, dissimilar electronic components of mobile telephony and laptops (Figure 11).
In particular, the lanthanum is used in the production of piezoelectric ceramics increasing the dielectric permittivity and the gadolinium can decrease the temperature of the phase transitions of the PZT.
Ceramic Method for Piezoelectric Ceramics
Under the name of the general term of pottery, objects are grouped that differ substantially from one another in the composition of their base, terracotta, stoneware, porcelain, on the exterior in which their shape can be covered by glaze or enamel, and in the How the cooking, temperature, oxidation atmosphere or reduction was carried out.
Pottery is the oldest industry of humankind, is a genius idea of man and fruitful since it has developed extensively throughout history not only in quantity but also in the variety of products, some of them, of transcendental importance for Technologies.
The manufacture of bricks arises in areas where the stone is scarce and the clay abounds. Ceramic material is the product of various raw materials (mainly clays) made in a powdery or pasty state (to easily communicate the form) and acquiring the stone consistency by physicochemical processes when cooking these clay soil.
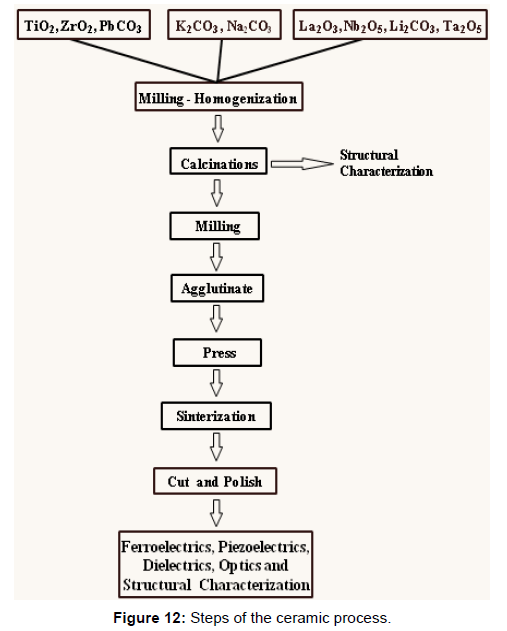
Ceramics are the synthesis of one or more chemical compounds, which for the traditional ceramic process are usually oxides and/ or carbonates. There is a strong influence on the desired final characteristics of the starting materials used. For the ceramist, it is important to consider the process (Figure 12) as a whole in formulating the powder composition [24] (Figure 12).
The interaction of the materials during the ceramic process is so complex that the total quantitative description of all the events and their interactions is not possible. To understand the role of powders it is necessary to join a significant number of factors, such as the characteristic of the powders and agglomerates of the starting materials. If they are large or if the tendency is to form dense agglomerates, intimate and homogeneous mixing will be difficult or impossible. Also if the particles are hard (abrasive) can mean a source of contamination during the grinding process. These interacting factors may have a dominant effect on the formation kinetics of the compound during subsequent heat treatments [25].
Grinding and Calcination
The operation with mills is used to homogenize powders, reduce particle sizes and break agglomerates. It is of special attention in the process of technical ceramics the type of mill to be used and the time of grinding. It has been observed that the particle size decreases and the amount of contaminant increases with the grinding time.
Calcinations is one of the most studied parts of the ceramic process [26,27], since it has a strong influence on the characteristic of the powders. Four stages [28,29] have been found for the case of ceramics type PZT.
Stage 1: Begins the decomposition of the starting materials (carbonates, oxalates, nitrates).
Stage 2: Lead oxide reacts with titanium dioxide.
Stage 3: The composition of the desired compound is initiated; the zirconium dioxide is reacted with the mixture obtained in the previous step.
Stage 4: Homogenization of the reaction.
The selection of the reactants, grinding time and calcinations regimes is sustained on the experience obtained by previous studies [30,31]. To confirm that occurred the formation reaction of the PZT system completely without the presence of other phases, is carried out X-ray diffraction on the calcined material.
Sintering
This stage of the ceramic process has as its object the union between particles, reducing the porosity and obtaining the ceramic body. During the same, the porosity of the samples decreases from 35-50% at the initial time to 5-10% at the end of the process.
The force that governs sintering is the reduction of the surface energy of the particle. This reduction is achieved by the transport of matter from the center of the particles to the “neck” or contact between particles, these causes a reduction of the surface area that leads to a reduction of the free energy in the system, thus ruling the sintering [32]. In the process, the following steps are well defined:
• Removing the binder.
• Reaction of components
• Densification.
• Grain growth.
The sintering process is divided into two regions. The first region (and the most critical during heating) is between 60° C and 350° C [33]. In this region, the moisture and the binder are volatilized and extracted. Before starting densification, it is necessary to remove the binder, which has fulfilled its function in the compaction process, as well as the residual moisture contained in the compacted ceramics; this operation must be carried out very slowly, to avoid cracks and pores that decrease the density of the ceramic body. For simple shapes of shaped parts a heating of approximately 10° C/min can be performed, being tolerated without structural difficulties.
In the second region from 350° C to the sintering temperature, the reaction of the raw material residues that have not yet done so in the calcination step occurs, and then the densification process begins.
It must be taken into account if the material is volatile or not, in the PZT the lead is a highly volatile element as in the KNN the sodium and the potassium are it, reason why the process must be realized in closed crucible and with atmosphere control. After sintering, chemical analyzes are performed to confirm the desired stoichiometric or to determine the obtained [34,35].
The percentage of binder used to facilitate the molding of the ceramic by dry pressing is about 5% by weight, depending on the binder used; the most common are starch and polyvinyl alcohol.
Results and Discussion
In this section, we will present examples of how they influence the origin of the reagents, their purity, granulometry, crystalline phase and the process of obtaining in the characteristics and properties of the piezoelectric and ferroelectric ceramics. There is an extensive literature that generalizes these aspects [36], but here we will present specific examples of each behavior (Tables 2-4).
| Country | Thousands of tons | % of total |
|---|---|---|
| Australia | 1291 | 30,6 |
| South Africa | 850 | 20,1 |
| Canada | 767 | 18,2 |
| Norway | 382 | 9,1 |
| Ukraine | 357 | 8,5 |
| Total of 5 countries | 3647 | 86,4 |
| Total world | 4221 | 100 |
Table 2: Main titanium oxide producers and production in 2003.
| Potassium mineral | Chemical formula | Crystal system | Density (g/cm³) |
|---|---|---|---|
| Carnallite, | KMgCl3·6H2O | Orthorhombic | 1,60 |
| Polyhalite | K2Ca2Mg (SO4)4·2H2O | Triclinic, pinacoidal | 2,77 |
| Sylvite | KCl | Cubic | 2,00 |
Table 3: Forms in which the potassium mineral is presented, chemical formula of the same, crystalline system and density.
| Sample | Reagent | ||
|---|---|---|---|
| PbCO3 (%) | ZrO2 (%) | TiO2 (%) | |
| a) | Merck (99) | BDH (99) | Sigma (99.5) |
| b) | Reachim (99.5) | Riedel (99) | Riedel (99) |
Table 4: Reagents used.
Reagents of equal purity and different granulometry
The formation reaction (calcination) of PZT ceramics is analyzed to obtain the composition Pb(Zr0.52Ti0.48)O3. Combinations of raw materials that react completely in the shortest possible time are analyzed, were used the reagents show in Table 4.
The starting reagents are analyzed to corroborate the purity and crystallographic structure provided by the manufacturers. Differential thermal analysis (ATD) was performed according to reference 27. In this example, the granulometry of lead carbonate was a determining factor in the PZT formation reaction process. For PbCO3 Merck the particle size was <1 μm and for PbCO3 Reachin (blue band) it was 0.01-5mm. The reagents for the TiO2 and the ZrO2 presented similar granulometry (Tables 2-4).
This implies that in the process of obtaining the PZT, a longer grinding time is required for the Reachin PbCO3 than for the Merck. If identical processes are carried out, what occurs in Figure 13, for the mixture a) the peaks present between 200 and 460°C correspond to the stages of dissociation of the carbonate and formation of lead oxide in its structural modifications tetragonal (litargirio) and Rhombic (massicot) [37], these transformations produce weight loss, as evidenced in the TG curve (Figure 13). The formation of lead titanate (PT) is considered to occur between 450-650°C [38,39], in this mixture appears an exothermic peak at 588°C associated with this process, as observed this process does not imply either gain or loss weight. In the range of 700- 900°C appears lead zirconate (PZ) and lead titanate-zirconate (PZT) formation.
For the mixture b), the peaks present between 200 and 512°C correspond, in addition to the dissociation of the lead carbonate, already explained above, to structural water and hydroxides present in the composition. Although the peak associated to the PT appears 10°C lower than case a), the rest of the processes between 700-900°C appear shift at higher temperatures. This ratifies the importance of granulometry in the reaction process and formation of the desired product.
Mixing of phases in raw materials
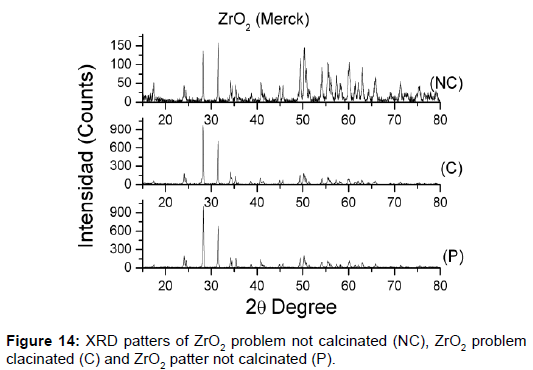
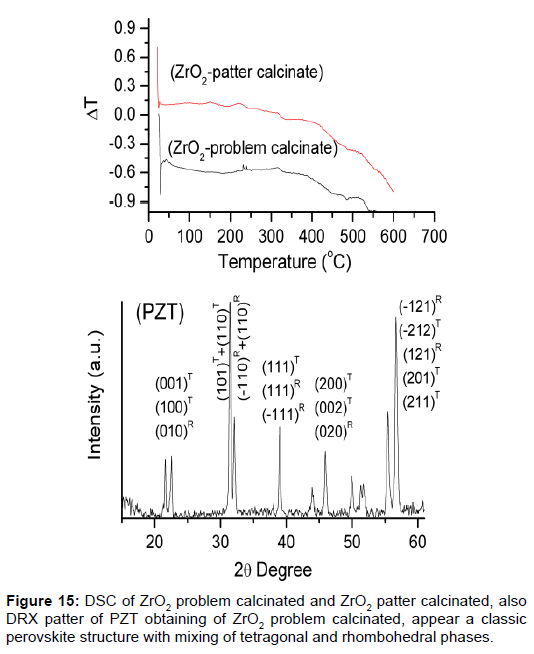
The unknown mixtures of phases in the row materials provoke properties not wanted. It is for that, very important analyze the reagents used to confirm the information of the suppliers [40]. Structural characterizations of all ceramics process are very important for the results, in this case the zirconium powders there were different activity, and were necessary study what happened. Was made the XRD and DSC (Figure 14), both show the difference between the zirconium powders.
The solution was calcinated, made against XRD, and observed what happened. Ceramics obtained with the zirconium powders calcinated have a good structural resolution. The XRD show the patter of perovskita with tetragonal and rhombohedra phase (Figure 15). The properties for sensor construction are the best.
Different phases of one reagents and similar results
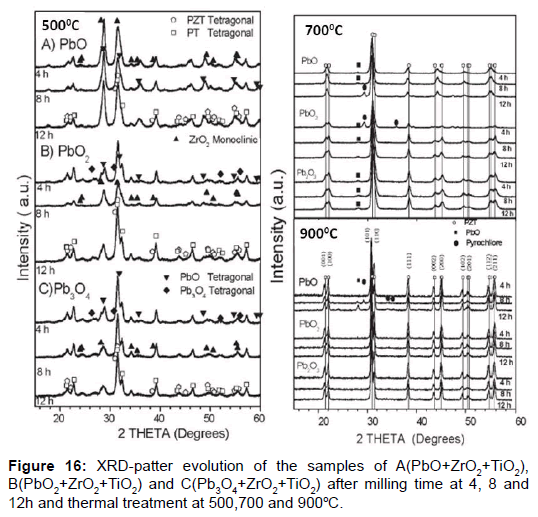
In this example were used different lead oxide PbO, PbO2 and Pb3O4 as Pb sources, were mixed with TiO2 and ZrO2 following stoichiometric ratios to obtain PZT samples with a composition of Zr/Ti=53/47, near to the morphotropic phase boundary (MPB) [41]. In this case was studied the milling time and calcinations temperature to obtain perovskite structure. The previous study of row materials contributes that: in PbO were identified orthorhombic and tetragonal phases, whereas in PbO2 and Pb3O4 platnerite and minium were identified, (both having tetragonal phase), the samples were identified as A(PbO), B(PbO2) and C(Pb3O4).
The results of milling and thermal process shows that A samples need more temperature for obtain the wanted result, only obtained at 900°C but too appear pyrochlore phase undesirable, for B and C samples this result was obtained at 700°C with pyrochlore phase, that disappear completely at 900°C (Figure 16). The most important result is present in Table 5.
| Sources of Pb | Tsint. | Emax | Pr | Ec | Pr/Pmax | ρ (g/cm3) | TC (°C) |
|---|---|---|---|---|---|---|---|
| Pb3O4 | 1250 | 53.62 | 34.02 | 11.3 | 0.99 | 7.47 | 396 |
| PbO | 1250 | 55.34 | 31.35 | 18.1 | 0.94 | 7.54 | 394 |
Table 5: Some results of ferroelectrics ceramic obtained of different lead sources.
In this example the author obtain by mechanical activation process reduce the particle size, accelerating the process of obtaining of the PZT (350°C), compared to the synthesis of traditional methods, the ceramics powders are homogeneous and with sub-micrometric size. Although for the PbO and the Pb3O4 similar results are obtained, they are better those obtained for the Pb3O4, because it has smaller coercive fields (Ec) and bigger remaining polarization (Pr) (Table 5).
Differences caused by variations in the ceramic process
Milling process produce different influence as grain size of powder and specific initial surface that causes difference in final density of material. BaTiO3 ceramics obtained from powders of BaCO3 and TiO2 stoichiometric, exhibit properties that reveal the presence of a memory effect related to the value of specific surface of the starting powders. This magnitude change alternatively with maxima and minima depending on the milling time, a lattice distortions of BaCO3 takes place, no similar effects were detected for TiO2 (Figure 17).
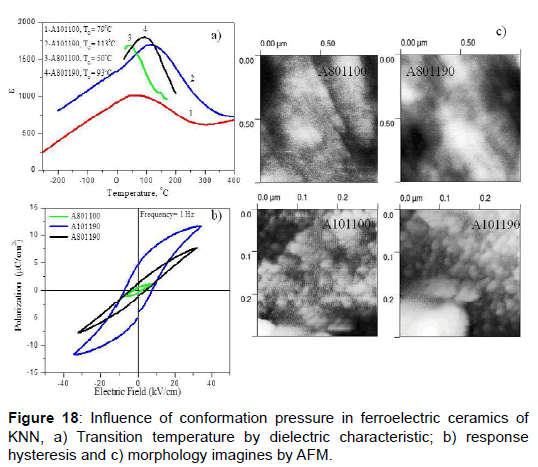
In the second examples in this topic, we present the influence of conformation pressure and sintering temperature in ceramics of niobate of sodium-potassium (KNN) [42,43]. The increase of the conformation pressure from 10 MPa to 80 MPa causes a great effect in the transition temperature (Figure 18a), decreasing it near room temperature; additionally there is an increase of the grain size (Figure 18c) and smaller polarization state (Figure 18b). The information obtained from the distribution of ferroelectric domains indicates that a greater extension of the domain size and the presence of greater area fraction of out-of-plane domains appear to be associated with good ferroelectric behavior, as in the case of samples A101190 and A801190. The microstructure was studied with atomic force microscopy, AFM. The grain size for samples obtained at bigger pressure (A801100 and A801190) was 1.0 μm, while for samples at smaller pressure (A101100 and A101190) was 28 ± 8 nm.
Other examples in KNN ceramics was obtained by López-Matus [44], modifying the process presented previously had milled with mechanic-synthesis. This allowed diminishing the time of having milled from 12 h to 1.5 h. The results are comparable to those of a PZT.
Introduction of dopants to modify properties
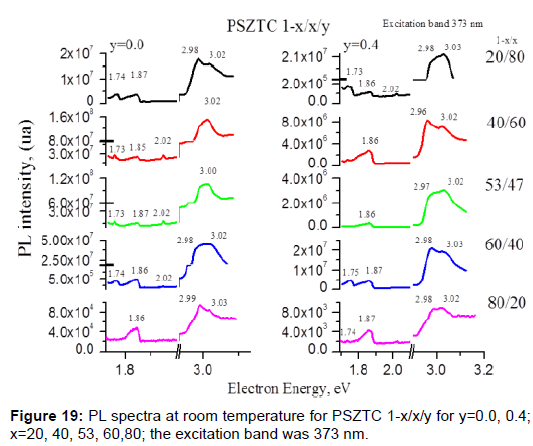
We will present three examples that show how small concentrations of an element can change significantly the properties of the material. Both examples are in PZT ferroelectric ceramics. In this example, the visible photoluminescence effect is analyzed at room temperature in a sintered Pb0.95Sr0.05(Zr1-xTix)1-yCryO3 perovskite-type structure system, doped with Sr and Cr. The excitation bands used were 267, 325, 373, 457, 635 and 680 nm, but the best result was obtained at 373 nm [45]. The intensity and energy of such emission in this system have been studied by changing the molar Cr concentration (0<y<0.005) and the Ti content (x), with x=0.20, 0.40, 0.53, 0.60 and 0.80, on both sides of the morphotropic phase boundary (MPB) zone (Figure 19 and Table 6). The principal emission bands are at the energies 1.73, 1.87 and 3.03 eV. The changes that were caused by Zr or Ti ions in the symmetry presented in the rhombohedral or tetragonal side of the MPB are more important.
| Samples | Emission energy (eV ) for different l excitation (nm) | |||||
|---|---|---|---|---|---|---|
| 267 nm | 325 nm | 373 nm | 457 nm | 635 nm | 680 nm | |
| PSZTC 80/20/0.0 | 3.99 | 3.36 | 1.87 | 2.84 | 1.79 | 1.74 |
| 4.09 | 3.46 | 3 | 2.52 | 1.85 | 1.77 | |
| 3.04 | 2.57 | |||||
| 2.61 | ||||||
| PSZTC 80/20/0.4 | 3.98 | 2.19 | 1.87 | 2.47 | 1.79 | 1.71 |
| 4.24 | 2.65 | 2.03 | 2.49 | 1.84 | 1.73 | |
| 3.36 | 2.66 | 2.56 | 1.86 | 1.75 | ||
| 3.49 | 2.99 | 1.89 | ||||
| 3.04 | ||||||
| 3.08 | ||||||
Table 6: Room temperature PL spectra fixing the excitation band at 267, 325, 373, 457, 635 and 680 nm for PSZTC 80/20/0 and PSZTC 80/20/0.4. Similar results were obtained for the rest of the samples in study.
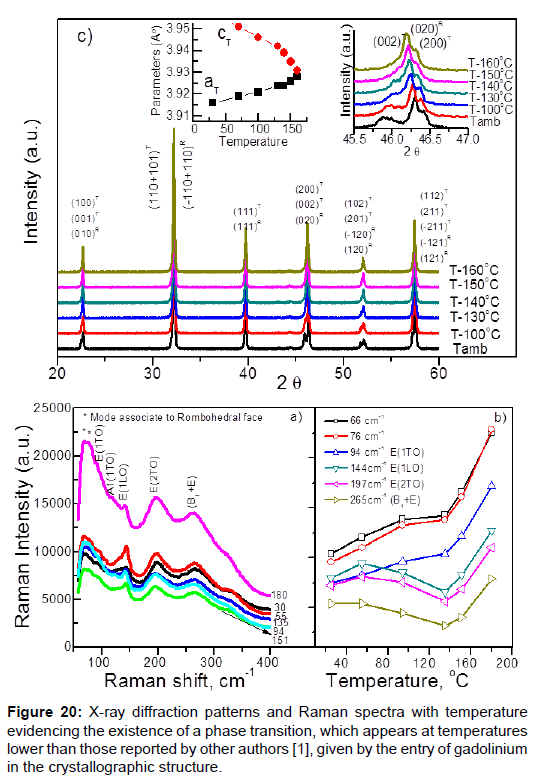
The second example is gadolinium (Gd) doped PZT [46], this causes a decrease in the ferroelectric temperature transition (from rhombohedra ferroelectric phase to tetragonal ferroelectric phase). Determinants by different techniques that said transition was ferroelastic transition (Figure 20).
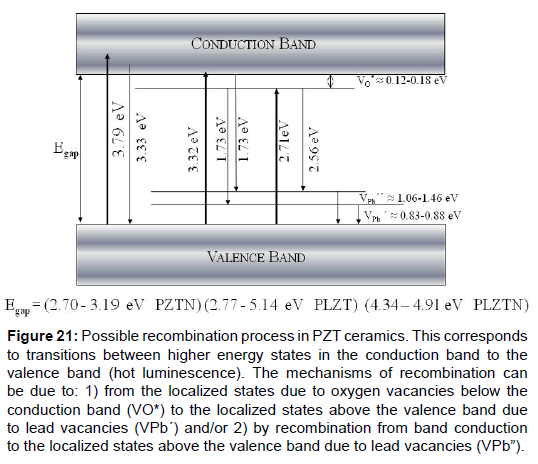
The third examples are La, Nb and La + Nb doped PZT ceramics obtained by traditional ceramic method. In the case it is analyzed how these dopants modify the conduction band (CB), the valence band (VB) and the band forbidden (Eg) of the materials, as well as its dielectric characteristics. From this, it is proposed a model of the possible recombination process of the electrons ejected by photons (Figure 21), measured through the photoluminescence and absorption spectrum of each material [47].
Figure 21: Possible recombination process in PZT ceramics. This corresponds to transitions between higher energy states in the conduction band to the valence band (hot luminescence). The mechanisms of recombination can be due to: 1) from the localized states due to oxygen vacancies below the conduction band (VO*) to the localized states above the valence band due to lead vacancies (VPb´) and/or 2) by recombination from band conduction to the localized states above the valence band due to lead vacancies (VPb”).
Conclusions
The ceramic process has evolved with man’s development, as well as with his needs. As seen in the examples, the influence of each step in the desired product is important. The ceramic process has been used and will continue to be used for its simplicity and versatility, in dissimilar objects from the great industry to the delicacy of an ornamental object.
In the particular case of ferroelectric ceramics with perovskite structure, in order to achieve the desired results, it is very important to pay the highest attention to the purity, crystalline structure and granulometry of the raw materials used. Each of these aspects can cause changes in the process, since the raw materials obtained cannot always be discarded, especially at the laboratory level.
The introduction of dopants and changes to the obtaining process can enrich the material or deteriorate the properties required.
Acknowledgments
The author would like to thank the national programs of CITMA-Cuba and the Sabbatical Programs of CONACYT-Mexico for their financing support of research, as well as Dr. Fathi Habashi of Laval University of Quebec, Canada for suggest me to write this work.
References
- Jaffe B, Cook WR, Jaffe H (1971) Piezoelectric Ceramics. Academic Press, London and New York
- Nye JF (2001) Physical Properties of Cristals: Their representation by tensors and matrices. Claredon Press, Oxford.
- Hartman HL (1992) SME Mining Engineering Handbook. Society of Mining, Metallurgy and Exploration Inc.
- Bednarik RG (1992) Early subterranean chert mining. The Artefact 15: 11-24.
- Hartmann HL, Mutmansky JM (2002) Introductory Mining Engineering. John Wiley & Sons, New Jersey.
- Puhakka T (1997) Underground Drilling and Loading Handbook. Tamrock Corporation.
- Lead. The Essential Chemical Industry. Promoting Science. The University of York.
- Lee AY, Wethington AM, Cole ER (1986) Hydrometallurgical Process for Producing Lead and Elemental Sulfur From Galena Concentrates. Bureau of Mines report of investigations (9055).
- Watt S (2008) The Elements: Zirconium. Marshal Cavendish Corporation, New York.
- Chris Woodford (2003) The Elements: Titanium. Marshal Cavendish Corporation, New York.
- Roza G (2008) Understanding the Element of the Periodic Table: Ti. The Rosen Publishing Group. New York.
- O´Daly A (2002) The Elements: Sodium. Marshal Cavendish Corporation, New York.
- Woodford C (2002) The Elements: Potassium. Marshal Cavendish Corporation, New York.
- (1996) Continuous process of sodium bicarbonate production by Solvay method (based on: “Podr─?cznik do ─?wicze┼?z technologii chemicznej” (Ed. T. Kasprzycka-Guttman), Wydawnictwa UW, Warszawa. Translated by Tomasz Paw┼?owski
- Shakhashiri (2010) Sodium Hydrogen Carbonate and Sodium Carbonate. Chemistry 104-112
- Cooper SK (2007) The Periodic Table: Mapping the Elements. Compass Point Books, New York.
- Rabaa AM, Bautista-Ruíza J, Joyab MR (2016) Synthesis and Structural Properties of Niobium Pentoxide Powders: A Comparative Study of the Growth Process. Materials Research 19: 1381-1387
- Jackson T (2007) The Elements: Lithium. Marshal Cavendish Corporation, New York.
- Rivas CN, El litio de la discordia. Capital.
- Zabre Ramírez H (2007) Tratamiento del mineral Alanita para la obtención de compuestos de elementos de tierras raras ligeras. Temas de Ciencia y Tecnología 11: 13-22.
- Hammond CR (2009) Section 4; The Elements", In: David R Lide (ed.) CRC Handbook of Chemistry and Physics (89thedn) CRC Press/Taylor and Francis, Boca Raton, FL, (electronic version 2009).
- Rare-earth metals. What are rare-earth elements.
- Rare Earth Elements. Critical Resources for High Technology. U.S. Department of the Interior. U.S. Geological Survey. USGS Fact Sheet 087-02
- Wang F (1976) Treatise on Materials Science and Technology, Vol 9, Ceramic Fabrication Processes. Academic Press, Inc., London.
- Kingery WD, Bowen HK, Uhlmann DR (1976) Introduction to Ceramics, John Wiley & Sons, Inc.
- Umakantham K, Bhanumathi A, Rao GN, Ramanan KN (1994) Calcination and sintering studies on modified Lead Titanate ceramics materials. Indian J Pure Appl Phys 32: 756-763.
- Durruthy MD, Fuentes L, Camacho H, Hernández M, Domínguez H (1997) Detalles de la síntesis y control de cerámicas PZT. Revista CENIC, Cíencias Químicas 28: 125-129.
- Martirena HT, Burfoot JC (1974) Grain Size Effects on Properties of Some Ferroelectric. J Phys C: Solid State Phys 7: 3182-3192.
- Matsuo Y, Sasaki H (1965) Formation of Lead Zirconate Titanate Solid Solutions. J Am Ceram Soc 48: 289-291.
- Prieto JJ, Durruthy-Rodríguez MD, Calderón F, Llópiz JC (1986) Cinética de sinterización de cerámicas piezoeléctricas de BaTiO3 (II). Revista Cubana de Física VI, 3: 77-82.
- Victorero A, Prieto JJ, Durruthy-Rodríguez MD (1989) Influencia de la superficie específica inicial de los polvos de TiO2 y BaCO3 en la consolidación final del BaTiO3. Revista Latinoamericana de Metalurgia y Materiales 91: 18-20.
- Braginsky M, Tikare V, Olevsky E (2005) Numerical Simulation of solid state sintering. International Journal of Solids and Structures 42: 621-636.
- Rokhvarger AE, Chigirinsky LA (2004) Engineering of Superconductive Ceramics. Journal of Electronic Packaging 126: 26-33.
- Hernández-García R, Behar M, Ferraz-Dias J, Villanueva-Tagle ME, Durruthy-Rodríguez MD, Calderón-Piñar F, et al. (2012) Determination of Pb, Zr, Ti, Sr, Cr, Nb and La in Lead Zirconate-Titanate ceramics by Particle Induced X-Ray Emission. X-Ray Spectrom 41: 156-163.
- Villanueva-Tagle ME, Larrea-Marín MT, Martin-Gavilán O, Durruthy-Rodríguez MD, Calderón-Piñar F, et al. (2012) Determination of metal impurities in advanced lead zirconate titanate ceramics by axial view mode inductively coupled plasma optical emission spectrometry. Talanta 94: 50-57.
- Xu Y (1991) Ferroelectric Materials and Their Applications. North-Holland, Amsterdam.
- (1974) Thermal Analysis of Minerals and Mountain Rocks. Ed Ñiedra, Leningrad.
- Garcia D (1989) Cerâmicas de Titanato de Chumbo (PbTiO3) dopadas com La, Sr, Nb, MN. Preparaçâo e Caracterizaçâo, Dissertaçâo apresentada ao Instituto de Física e Química de Sâo Carlos, para a obtençâo do titulo de Mestre em Física Aplicada, Departamento de Física e Ciencia dos Materiales, Sâo Carlos.
- Hiremath BU, Kingon AY, Biggers JV (1983) Reaction sequence in the formation of Lead Zirconate-Lead Titanate Solid Solution: Role of Raw Materials. Journal of the American Ceramics Society 66: 790-793.
- Durruthy-Rodríguez MD, Angulo Y, Herrera V, Griffith J, Moreno E, et al. (2009) Study of raw materials for construction of NDT for amusement parks. Proceedings of the XII Workshop on Nuclear Physics, WONP 2009 and VI International Symposium on Nuclear and Related Techniques, NURT 2009.
- Yañez-Limón JM, Rivera-Ruedas G, Sanchez De Jesus F, Bolarín-Miró AM, Jiménez Riobóo R, et al. (2011) Ferroelectric-Materials Aspects, C-16: Synthesis of PZT by sol-gel method and mixed oxides with mechanical activation using different oxides as source of Pb. InTech, Croatia.
- Fuentes J, Portelles J, Pérez A, Durruthy-Rodríguez MD, Ostos C, et al. (2012) Structural and dielectric properties of La and Ti modified K0.5Na0.5NbO3 ceramics. Applied Physics A: Materials Science & Processing 107: 733-738.
- Durruthy-Rodríguez MD, Gervacio-Arciniega JJ, Portelles J, Fuentes J, Pérez A, et al. (2013) PFM characterization of (K0.5Na0.5)0.95La0.05 (Nb0.9Ti0.05)O2.9 ceramics lead free. Applied Physics A: Materials Science and Processing 113: 515-519.
- López Matus AK (2015) Synthesis by mechanochemical activation and characterization of the KNN system doped with La and Li. Thesis for Chemical Engineer degree, Academic Division of Engineering and Architecture, Juárez Autonomous University of Tabasco, Mexico.
- Hernández-García M, Durruthy-Rodríguez MD, Costa Marrero J, Calderón Pinar F, Guerra JDS, et al. (2014) Photoluminescence in Pb0.95Sr0.05(Zr1-xTix)1-yCryO3 ferroelectric ceramic system. Journal of Applied Physics 116: 043510-1-043510-6.
- Portelles J, Fuentes J, Durruthy-Rodríguez MD, Raymond O, Heiras J, et al. (2013) A new phase transition in Pb(Zr0.53Ti0.47)O3:Gd. Journal of Applied Physics 114: 064108-1-064108-4.
- Durruthy-Rodríguez MD, Yáñez-Limón JM (2011) Ferroelectrics, C-27: Photoluminescence in doped PZT ferroelectric ceramic system. InTech, Croatia.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 8140
- [From(publication date):
April-2017 - Apr 21, 2025] - Breakdown by view type
- HTML page views : 7174
- PDF downloads : 966