Review Article Open Access
From Replacement to Regeneration: Are Bio-Nanomaterials the Emerging Prospect for Therapy of Defective Joints and Bones?
Sheeana Gangadoo, Andrew W Taylor-Robinson* and James ChapmanSchool of Medical & Applied Sciences, Central Queensland University, Rockhampton, Australia
- Corresponding Author:
- Andrew W Taylor-Robinson
School of Medical & Applied Sciences, Central Queensland University
Bruce Highway, Rockhampton, QLD 4702, Australia
Tel: 61749232008
E-mail: a.taylor-robinson@cqu.edu.au
Received date: June 18, 2015; Accepted date: July 10, 2015; Published date: July 17, 2015
Citation: Gangadoo S, Taylor-Robinson AW, Chapman J (2015) From Replacement to Regeneration: Are Bio-Nanomaterials the Emerging Prospect for Therapy of Defective Joints and Bones? J Biotechnol Biomater 5:187. doi:10.4172/2155- 952X.1000187
Copyright: © 2015 Gangadoo S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Healthcare is now regarded as one of the largest costs to any government budget and thus innovative ideas are sought as a means to reduce this spiralling bill. A significant proportion of this expenditure relates to specialised consultative care and post-operative therapy. In order to both mitigate the expense and improve the long-term effectiveness of orthopaedic surgery, including arthroplasty, the conception and creation of new biomaterials for treatment of defective joints and bones in the human body has become an emerging area of translational research over the last decade. In this review, we discuss a series of novel biomaterials and strategies for their therapeutic use that have arisen recently as viable approaches to regenerative medicine.
Keywords
Biomaterial; Arthroplasty; Joint; Ceramic; Polymer; Composite; Translational
Introduction
The notion of incorporating materials in the human body to either enhance health or cure a disease extends back as far as the Neolithic period when civilisations exploited biting ants for use in sutures and stitches [1]. While the concept of implants made of foreign materials did not take off until the 1960s, the last five decades have witnessed increasingly sophisticated approaches. From replacement to regeneration, the nascent field of biomaterials is attracting mounting interest, with current studies focusing mostly on biocompatibility, biotoxicity, biodegradability and lifespan of the implant in the body – issues not considered a thousand years ago. Implant lifetime has proved to be the hardest obstacle to overcome as a consequence of easy loosening of the material in situ due to its slow degradation by chemical and physical reactions such as hydrolysis and surface erosion [2,3]. This prevents substantial healing of the damaged bone before safe biodegradation of the implant. Scientists have therefore begun to focus on the regeneration potential of material rather than replacement; for example, present interest in treating defective joints is aimed solely towards chondrocytes or mesenchymal cells, which are found in healthy articular cartilage. Since the main cause of arthritis is degeneration of cartilage [4], along with scientific findings showing a significant depletion of levels of mesenchymal cells in osteoarthritis [5], attention has been directed towards harnessing these cells to prolong the life of an implant.
The manufacture of implants to be used in replacements has transformed over time, from metals to polymers, with research now turning towards nanotechnology as an emerging strategy. With its recent advance, the prospect of nano-biomaterials, has so far displayed promising results, with nano-sized hydroxyapatite (HA) particles demonstrating greater cell attachment and proliferation than microsized HA particles [6,7]. Mechanical properties were likewise improved with the emphasis on nanohydroxyapatite/collagen/poly-L-lactic acid (PLLA) composite with chitin fibres [8]. Utilising the scanning electron microscopy, Bose et al. confirmed the greater inclination of cells to attach deeply with β-tricalcium phosphate (TCP) than alumina scaffolds [9]. Nano sizes of current biomaterials are able to achieve better integration of biological surfaces due to their considerable surface area to volume ratio and their grain size producing a larger boundary surface equipped with high energy for the adsorption of proteins. Protein adsorption, being an important phase of cell-material interaction, enables the process of cellular adhesion and biological activities to occur on biomaterials, which in turn accomplish cell attachment to the surface [10,11].
Ethics governing the application of biomaterials include the use of animals in research, to which a proportion of the general population is strictly opposed [12]. A number of scientists argue the validity of animal models as the closest way to understand human physiology and would therefore not have moved away from materials first approved in the 1960s. The safe use of these products, as well as the risk/benefit ratio, is scrutinised closely by the US Department of Health and Human Services and Federal Drug Administration, which control biomaterial research. In conclusion, products exhibiting any significant toxicity in preclinical tests are rapidly disproved and do not progress into clinical trials.
This review focuses on recent research performed on joint regeneration, explains the causes of joint defects, types of biomaterials used in arthroplasty, emerging biomaterials, and common controversies in this promising field of biomaterial innovation.
History of Solutions to Joint Defects
Replacement of affected body parts with artificial materials was a solution to shoulder, knees and hip defects as far back as 10,000 BC [13]. As technology has advanced over the years, so has the fabrication of biomaterials and their integration into the human body (Table 1). Arthroplasty is the term given to the principal orthopaedic surgical technique performed on patients with joint defects, which are replaced, reconstructed or realigned [14]. The various types of arthroplasty include interpositional, cup, mould, resurfacing, resectional and silicone replacement.
| Then | Now | |
|---|---|---|
| Hip & Shoulder Joints [17,74] Materials |
Stainless steel fitted to bone with bolts and screws | Low Friction Arthroplasty: Metal femoral stem with coatings Polyethylene acetabular component Acrylic bone cement |
| Benefits | Hard material mimicking bone structure | Ability to adjust leg length Reduces wear due to smaller head Decreased corrosion |
| Limitations | Leaching of metal ions Low wear resistance Infection Corrosion Insufficient strength |
Polyethylene debris contributing to aseptic loosening Irradiation of material through gamma particles |
| Knee Joints [75] Materials |
All stainless steel components | Metal femoral component (Ti allows stainless steel or cobalt chromium with small amount of molybdenum) UHMWPE tibial inserts UHMWPE patella/kneecap |
| Benefits | Rigidity Movement |
Corrosion resistance Frictional movement Optimum articulation Reduced wear due to flatter bearing |
| Limitations | Infection Loosening |
Fixation of the grafts |
Table 1: Past versus present materials commonly used in hip, knee and shoulder arthroplasty.
Hip and shoulder replacement procedures are essentially more advanced than knee surgeries as the joints are characteristically different; hip and shoulder joints act as ‘ball and socket’ joints and knee joints operate as hinges, providing the bone with the ability to bend and straighten [15]. In a total hip and shoulder arthroplasty, the femoral head, at the top end of the thigh bone, is removed and replaced with a metal prosthesis attached to a metal stem implanted in the hollow centre of the bone. The acetabulum is replaced with a metal socket held in place with screws or cement. A plastic liner is then fixed in the middle of the femoral head and acetabulum to provide a smoother gliding surface [16].
In 1821, White was the first to perform hip arthroplasty – pain was reduced and mobility of the material was achieved. However, the component could not be deemed all that successful as stability of the material failed. In addition, other solutions around that time treating degenerated joints involved the removal of calcium deposits and injured cartilage. Barton undertook the first osteotomy in 1826 on an ankylosed hip, indicative of the joint suffering abnormal adhesion and rigidity. Although the patient was walking successfully from three months after the operation, six years later they were immobile [17]. It would not be until 1891 that the first hip implant fixation would be achieved. Developed by Glück, the implantation was made of an ivory ball and socket joint which was fixed to the bone utilising nickel-plated screws to replace the degenerated femoral heads of the affected hips [18].
Interpositional Hip Arthroplasty
Interpositional hip arthroplasty appeared around the 1900s, developed by Helferich, whereby tissue-like materials interposed separating inflammatory surfaces of bones. The first interpositional knee arthroplasty was achieved by Verneuil in 1863. French surgeon Foedre applied pig bladder tissue as a material for interpositional hip surgeries, which Baer continued to use in various surgeries in hospitals [17]. Pig bladders are known for their ability to maintain stress caused by load bearing and intra-articular pressure [17]. This tissue proved surprisingly effective but prompted ethical concerns from patients; Sir Robert Jones therefore developed a more conventional material – a strip of gold foil – which was used to cover reconstructed femoral heads. This technique showed great promise, with one patient retaining effective motion for 21 years [17]. In 1923, following his unsuccessful trials with glass mould placed between the femoral head and acetabulum, Smith- Peterson developed a corrosion-resistant scaffold of ‘vitallium’, an alloy of 65% cobalt, 30% chromium and 5% molybdenum introduced to dentistry around that period. His method supported fifty years of solid clinical data, providing the first predictable results of interpositional hip arthroplasty [19]. Although this has proven to be a successful longterm intervention, it does not enable the bone to grow and regenerate and may induce limited motion overtime – serving to stress the point that biocompatibility is a necessary feature of contemporary implants.
Cup Arthroplasty
In cup arthroplasty, the cup replacing the acetabulum underwent various modifications in the past. In 1956 McKee and Watson-Farrar accomplished the first ‘total’ hip replacement utilising an acetabular metal cup cemented into place, which lasted over 20 years [13]. Using acetabular metal cup prostheses Huston and Insall pioneered total knee replacements from 1968 to 1972. Sir John Charnley, in 1962, had earlier first developed a cup made from ultra-high molecular weight polyethylene (UHMWPE); the material was favoured over metal or ceramic surfaces, the latter developing poor fixation. As an option for long-term management, however, it proved a poor choice since the material has been noted to detach easily due to undesirable reactivity in vivo of debris or wear material [20]. This caused further loosening of the component, thereby contributing to osteolysis. In order to improve long-term management and durability, Charnley replaced the allpolyethylene acetabular prosthetic with a metal-backed design [21,22]. The metal component itself was gradually superseded by ceramic surfaces and implants consisted of a ‘ceramic femoral head articulating against an UHMWPE acetabular cup’, as described by Ingham and Fisher [23]. Implant life expectancy is typically less than 25 years with regular check-ups required, thus making this method cost ineffective and unreliable. Other solutions to polyethylene wear included sterilisation by ethylene oxide. Loosening of the component occurred not only due to material wear but to other factors such as poor design specificity and mechanical causes [18].
The potential of articular joint regeneration was not recognised till the 1990s. Having been slow to gather initial interest, the prospect of regeneration has appealed increasingly to scientists across the world, with natural restoration of joints and bones desired as an advancement over replacement with mechanical implants. The ability to restore joint function by inducing cartilage formation could resolve limitations associated with artificial implants – such as wear and tear, loosening and biocompatibility. The use of native cells reduces the likelihood of the body rejecting the implantation because of immunological recognition of the material as foreign and therefore antigenic. Due to limited reliable comparisons and standardisation of screening procedures, this new aspect of joint arthroplasty remains under development [24].
Causes of Joint Defects
Joint defects occur mainly as a result of rupture to cartilage and/or the inability to regenerate the damaged tissue. Cartilage consists principally of an extracellular matrix composed of proteoglycans, an important component of connective tissue responsible for cell proliferation and differentiation, and collagen fibres, which are produced and maintained by chondrocyte cells within the matrix. Chondrocytes are provided by the differentiation of mesenchyme tissues during embryogenesis and so fewer chondrocytes and mesenchymal stem cells are also the cause of joint defects, which can occur from either mechanical means, such as injuries, tumours, lesions and inflammation, or from genetic mutations [25,26].
Genetic mutation of Gdf5 and Gdf6 genes, as observed in rats, have either led to the non- production of the enzyme bone morphogenetic protein (BMP) or to a non-functional enzyme [27]. BMP enzymes act as growth factors required for heart, neural and cartilage development as well as for postnatal bone formation [28].
A commonly observed joint defect is arthrogryposis, a congenital condition in which permanent shortening of two or more joints occurs in the body. Amyoplasia is the most prevalent form of arthrogryposis, whereby skeletal muscle is replaced by dense fibrous tissue and fat [29]. Osteoarthritis, another prime example of joint disease, occurs from asymptomatic lesions in articular cartilage, which degenerates slowly causing pain. Recent research on joint prostheses has focused on the restoration of extracellular matrix chondrocytes and hyaline cartilage, an important component of the articular scaffold. Studies are currently centred on three significant parameters; prime candidates for cell differentiation and growth, tri-dimensional scaffolds and elite surrounding environmental factors [24].
Timeline of Biomaterials
Biomaterials may be considered as any structures or substances able to interact with the biological system of the body, whether they are manufactured naturally or synthetically. Although the concept of integrating materials in the body has existed for a very long time – the ‘biting ants’ example – it was not until the development of the hip arthroplasty procedure in the 1960s that it was named by Charnley [30]. Figure 1 depicts a short history of biomaterials since 10,000 BC.
In biomaterial research, scaffold is the term used to describe an implant as a ‘biomaterial construct for tissue engineering’, as defined seminally by Shi [31]. A model scaffold must demonstrate both complete integration of implants with the host and total restoration of biological function and preservation of smooth communication between the host and the damaged tissue [31]. Scaffolds made from calcium phosphates contain bioactive features, often combined with regenerative properties, which facilitates the construction of a base that can later be modified for maximum efficiency. Scientists have been able to mimic most tissues in the human body by mixing these elements with biodegradable polymers [32]. Titanium is currently the most widely used metal in fracture fixation. Fracture materials include ceramics, organic polymers, metal-based polymers and composites. While nano-structured scaffolding has emerged recently as holding potential for prostheses and implants, it should be noted that neither toxicity nor biodegradability has been fully investigated.
Bioceramics
The term ‘bioceramic’ is typically used to describe a ceramic material applied to the processes of mending and restoration of diseased or defective skeletal or muscular parts of the body [33]. Bioceramics have been utilised since the 1930s and commonly started from a need for dental implants. Bioceramics may be divided into three distinct classes:
• Bioinert - Characteristics typically include strength, hardness, high resistance and chemical inertness, making such ceramics favourable for bone and dental implants. Examples include alumina (Al2O3), which is polycrystalline, zirconia (ZrO2) and carbon (C);
• Bioactive - Capable of forming direct chemical bonds with bone and soft tissues; glass ceramics and bioglass are classified as bioactive ceramics;
• Bioresorbable - Active contributions are made to the metabolic processes in the living organism. A good example of this material is tricalcium phosphate (Ca3O8P2).
Ceramics that are often considered in the reconstruction of damaged and fracture joints are high-density alumina and metal bioglass coatings, which enable heavy load-bearing [34,35]. Benefits of such biomaterials include biocompatibility and non-cytotoxicity. These are offset by limitations such as innate brittleness and a susceptibility to fracture at the femoral head region (Table 2). In one case, a 25-year old woman reported pain and hip dislocation 20 months after surgery, whereupon X-ray images showed disintegration of the ceramic femoral head [36]. Modifications to fabricated ceramic material, involving combinations of both alumina and zirconia, produce significant reliability with low susceptibility to hydrothermal instability when compared to monolithic alumina and zirconia [37]. Improvements can also be achieved through proof-testing, hot isocratic pressing and laser marking (Table 3).
| Material | Limitations (‘Before’) |
|---|---|
| Alumina ceramics Pure alumina [76] |
Brittle Slow crack growth leading to in vivo failure |
| Pure zirconia[77] | Fabrication of ceramic forms requires stabilisers Hydrothermal stability |
Table 2: Limitations of Alumina and Zirconia as pure monolithic ceramics.
| Material [78] | Improvements (‘After’) |
|---|---|
| Phase-stabilised zirconia | Higher fracture toughness Larger static and fatigue strengths |
| Yttria-stabilised ceramics can be destabilised during process of steam sterilisation | Decreased frictional torque Reduced level of polyethylene debris production Increased surface roughness |
| Alumina matrix reinforced with zirconia particles, zirconia-toughened alumina (ZTA) | Higher toughness values Alumina matrix constrains zirconia particles, toughening ceramic host material Greater hardness of composites with alumina matrix |
Table 3: Improvements achieved through composite fabrications and stabilisation.
Due to recurrent maintenance of natural ceramic biomaterial for use in joint structure replacement, the focus of research was instead directed towards a means of regeneration [38]. Lusquiñosa et al. successfully constructed a bioactive ceramic glass implant, of threedimensional geometry, for bone restoration of low load properties [39]. Rapid manufacturing time of the material was achieved with the facility to alter composition and to enable tailoring to an individual patient’s needs. In order to construct a ceramic implant, particles of bioactive glass are injected into a carrier gas-powder stream that is subjected to a thermal cycle system. The particles are thereby melted onto a substrate using heat emitted from a laser beam. As the laser beam retracts, the material solidifies and layers are built sequentially. For application of a rapid prototype based on laser cladding, the regenerative biomaterial will exhibit other valuable properties including an ability to boost growth and to enable recovery of damaged bone. Following processing, the finished material will preserve a homogeneous composition with success achieved in regenerating joint structures with maximum strength recovery [39].
The process of bone substitution requires the biomaterial to have structural support and to be capable of osteogenicity and resorption. Demirel and Aksakal modified the structure and mechanism of action of HA bone grafts by introducing sol gel additives [40]. Following further development, the mechanical properties exhibited good osteoconductivity and bone regeneration. Results of studies in animal models showed high-energy adsorption proportional to increased toughness and fracture resistance without detection of an immune response. However, two distinct limitations were observed while during HA bone graft manufacture, namely properties of low solubility and reduced in vivo resorption [40]; these are considered significant factors contributing to the biodegradation rate of a bioceramic [41].
The most commonly used bioceramics include inert alumina, zirconia and hydroxyapatite (Ca10(PO4)6(OH)2), a type of calcium phosphate with great compression and bond strength. Other materials include pyrolytic carbon, diamond-like carbon (DLC), porcelain, bioglass and glass ionomers.
Organic polymers
Polymeric materials can be classified as 1) ‘addition’ – self-addition of one or more different monomers; and 2) ‘condensation’ – monomeric units connected by intermolecular elimination of small molecules [42]. Both categories contain polymers which possess a broad range of molecular weights. Natural polymers are essentially starch, cellulose and proteins. Synthetic polymers comprise plastics, elastomers and fibres [42]. Polymers may be identified further as inorganic, such as ceramics, metallics and composites, and organic, which consists of nucleic acids, polysaccharides and proteins. Backbone length and structure, pendant or side groups populating the backbone, and crosslinking chains all contribute properties which collectively determine the physical and chemical properties of a polymer [43]. Low protein adsorption reduces immune recognition by host macrophages and implies weaker cell adhesion, a property which can be amended further with the attachment of specifically modified proteins onto the surface polymer to elicit a specific response [38].
Huang et al. assembled a biphasic structure by introducing a chitosan thermogel system evenly to a demineralised bone matrix (DBM). The scaffold platform thus formed has a close physical resemblance to the structure of native cartilage [44]. When combined with an affinity peptide, bone-derived mesenchymal stem cells (BMSC) are targeted specifically. The scaffold may be equipped with an E7 peptide through chemical conjugation, a protein used to arrest cell death and support cell cycle progression in the Papillomaviridae family of viruses [45]. The addition of the peptide achieves increased cell adhesion, enabling high cell numbers to be retained, and consistent proliferation on the sol-gel matrix, further enabling the structure to maintain solid strength. In studies performed over 24 weeks after implantation, controls showed little or no filling of defects, osteophyte formation and, ultimately, cartilage degeneration. In contrast, the biofunctional material E7-CSDBM – E7 affinity peptide, chitosan hydrogel and demineralised bone matrix composition – exhibited moulding indicative of unchanged smooth cartilage development in defect areas without any signs of detachment or disintegration [45]. This biofunctional combination may prompt further research on major implant limitations encountered in long-term management such as implant loosening and wear particulates.
For treatment of osteoarthritis and the possibility of bone regeneration, Kang et al. investigated the use of nano- and microparticles [46]. These were prepared by ionic gelation of kartogeninconjugated chitosan using tripolyphosphate anion, with the aim of increasing solubility and biocompatibility within the body. A combination of selective differentiation of mesenchymal cells from kartogenin and high level drug delivery from chitosan at nano size allowed for a greater drug effect over an increased retention time. Sustained release for 7 weeks in vitro was achieved, where particles induced chondrogenic differentiation of human BMSC, subsequently increasing bone cell repair and reducing degeneration [46]. The main focus of regeneration typically is stem cell growth. Heo et al. applied a similar technique using as an alternative material gold nanoparticles enveloped in a biodegradable hydrogel solution. Both in vitro and in vivo this proved successful in achieving osteogenic cell proliferation and differentiation from human adipose-derived stem cells (ADSC) [47]. Nanoparticles allow an increased drug dosage to be delivered relative to size due to a higher surface area to volume ratio. Their relatively small size also enables effortless penetration into tissues.
Naturally-occurring polymers attain biocompatibility and reabsorption by host materials more easily than do synthetic polymers. Unlike the latter, natural biomaterials are not required to undergo physical or chemical modification; their similarity to macromolecule elements, proteins, enables instant recognition by cells and facilitates association with biological functions. The main drawback is their limited reproducibility compared to that of synthetic polymers. This is the reason why synthetic polymers such as ultrahigh molecular weight polyethylene (UHMWPE) and polymethylmethacrylate (PMMA) are used more commonly than are the natural polymers cellulose and rubber.
Metal-based polymers
Metal-based polymers have been used in joint replacements since the mid-1800s in hip and knee prostheses. However, due to particular boundaries, long-time use of metallic implants showed rapid deterioration of the material. These boundaries include the release of wear particles that causes activation of host immune mechanisms, leading to local inflammation [48]. They are also susceptible to bacterial adhesion and lack of assimilation with surrounding tissues over an extended time [49], thus making metals especially unfavourable as implants.
Dapunt et al. showed the effects of metallosis on use of metalon- metal implants included an uncontrolled inflammatory response, damaging the bone and thereby contributing to loosening of the implant [48]. This resulted in patients experiencing pain and decreased motion range. Protective outer layers for metal implants are currently under investigation for their characteristics that render the material to be unreactive, providing two main benefits: reduced degradation caused by harsh environments; and reduced inflammation attributable to chemical stability [50]. Titanium implants, for example, form a passive oxide coating at high solubility. This layer protects the implant from challenging conditions, such as high pH, as a result of the unreactivity of the metal. Since the underlying metal degrades slower, release of particles is also averted and hence an inflammatory reaction is not induced. An additional benefit of this technique is that it permits longer term implant fixation [51,52].
This passive layer, however, can dissolve as pH levels drop to an acidic environment. In a recent study, Dorn et al. applied simulation to evaluate the corrosion behaviour of tantalum-coated material compared to that of a titanium alloy [53]. Utilising both wet and dry assemblies, tantalum-coated cobalt-chromium modular necks suffered no corrosion or chemical attack. Corrosion of titanium usually occurs at pH levels < 4; hence, in the wet assembly bathed in calf serum the titanium coating dissolved much faster than when dry [53]. A study conducted by Du et al. showed tantalum polishing rates did not strongly depend on pH, ranging between a high of 27.4 nm per minute at pH 1 and a low of 8 nm per minute at pH 5 [54]. Hence, with a higher load bearing and a lower degradation rate, tantalum appears a more reliable coating than titanium to prolong implant longevity. Other protective layers currently used which promote extended corrosion resistance include cobaltchromium alloys and zirconium oxides. The electrochemical corrosion, Ecorr, of Co-Cr alloy is nearly four times less than for Pd-Ag, 34 mV SCE-1 to 120 mV SCE-1 [55]. The electrochemical corrosion signifies the corrosion between a metal surface and an electrolyte solution.
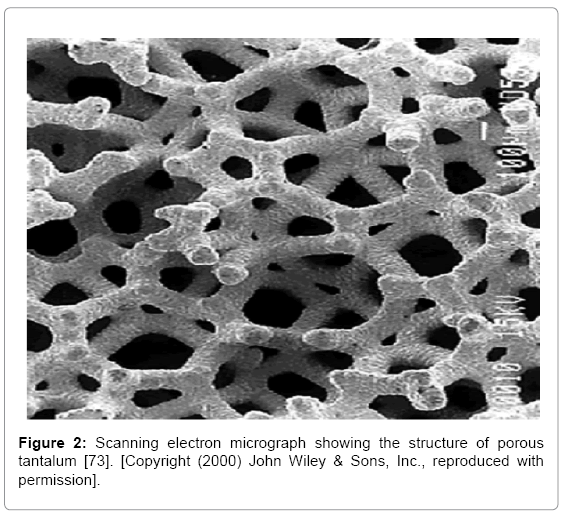
As well as acting as a protective coating, tantalum is also manufactured as a bulk constituent of implants for its ability to mimic traditional bone graft structure. Rapid growth of soft tissue and formation of blood vessels on the surface and inside the matrix have been observed from x-ray images [56]. With significantly smaller and interconnecting pores, tantalum can induce major bone growth [57,58] (Figure 2). Fixation and shear strength is also significantly stronger in tantalum than other metals used as implants [59]. In a segmental long bone defect of a rabbit radius non-union model, porous cylindrical titanium scaffolds were found to have superior biochemical properties over HA scaffolds with regard to the repair process of the bone. The maximum failure load was significantly greater since strength decreases with larger pores of HA [60]. The relative expense of tantalum has led to development of a more cost-effective product, porous titaniumniobium alloy, which exhibits essentially the same corrosion resistance and strength properties [61].
Combination strategies may also be performed in orthopaedic medicine to yield load-bearing benefits while facilitating innovative drug release, such as through construction of an implant utilising both dense and porous titanium. In order to be used in bone regeneration studies, densified titanium scaffolds are coated with a bone growth factor, BMP-2. Porous titanium, on the other hand, contains profound mechanical properties characteristic of hard tissues, bioactivity in bone implants and porosity that promotes controlled drug release [62]. This novel material, used as a drug carrier metal implant, benefits from a larger surface area compared to that of dense titanium due to its porous nature, which enables a higher dose of drug to be loaded onto the material [62]. Porous titanium scaffolds are manufactured by dynamic freeze casting, followed by densification, a process that does not contribute to mechanical corrosion while retaining structural integrity. Characterisation by μ-computed microtomography and scanning electron microscopy shows the material to be tortuous and provides considerable pore connectivity. During fabrication of the porous scaffold, chemical contamination should be avoided in order to prevent embrittlement of the material which would otherwise reduce compression [62].
Composites
Composites comprise two or more different materials with unique properties which are combined to form a new material with increased benefits.
Lu et al. investigated in a rabbit model delivery and bone formation performance of biodegradable hydrogel composite scaffolds for use in osteochondral tissue repair [63]. When compared to delivery of insulin-like growth factor 1 (IGF-1) alone within the chondral layer, early bone formation was observed following delivery of BMP-2 in the subchondral layer with increased bony trabecular islet numbers. Dual delivery of BMP-2 and IGF-1 in separate layers acts synergistically to improve the degree of subchondral bone formation, although not enhancing cartilage repair, ultimately required for good working condition of joints [63].
Silk is a material used frequently in combination with other polymers with the aim of developing regeneration characteristics of the scaffold. In one study, Yodmuang et al. utilised biodegradable and biocompatible silk fibroin from the silk moth Bombyx mori to produce an entirely silkbased fibre-hydrogel composite scaffold for use in cartilage repair [64]. Unlike the previous study [63], cartilage repair would be proactive as the formulation of the biomaterial resembles the architecture of native cartilage; collagen and proteoglycan. Following 42 days in in vitro culture an enhanced chondrocyte response and stronger mechanical compositions from fibre reinforcement were noted, compared to silk hydrogels alone [64]. The fibre is again observed in the reconstruction of anterior cruciate ligament, a difficult portion to heal. Further, a scaffold made from a knitted silk-collagen sponge has been developed for use in knee reconstructions [65]. Increased migration and adhesion of spindleshaped cells into the scaffold, which accelerates tissue reconstruction of the affected joint, occurred at just 2 months post-surgery, with a mature ligament structure observed by 18 months [65].
Nanocomposites
With the integration of nanotechnology in the development of novel biomaterials, it has proved possible to overcome some of the limitations of long-term loosening of implants, with nanomaterials displaying excellent biomimetic activity. A recent example of a titanium-coated nano-patterned implant, manufactured by Brydone et al. and tested in rabbit tibiae, successfully reduced mechanical and biological abrasion, resulting in increased long-term attachment of the implant [66]. Smart implants of defined topographical features are being manufactured for soft lithography surfaces, which improve integration between joint and implant by increasing the surface area of the interface [66]. Utilising cross-linking, Chang et al. designed a nano-scale bacterial cellulose derived from Gluconacebacter xylinus, which demonstrated superiority to plant cellulose due to nano size and large surface area [67]. High crystallinity of the material after drying often leads to poor rehydration as well as reduced mechanical strength. Low rehydration of scaffold may also prevent bacterial cellulose composition from dissolving onto surfaces [67].
The production of a polyetheretherketone/nano-fluorohydroxyapatite (PEEK/nano-FHA) biocomposite demonstrated excellent biocompatibility as well as antibacterial activity, with biofilm formation greatly reduced [68]. Further benefits, as shown in vivo studies, include increased osseointegration, the connection between implant and bone. In addition, 3D microcomputed tomography and 2D histomorphometric analysis have demonstrated that bone volume formation is significantly higher than for PEEK alone [68]. Histomorphometric analysis is simply computer-assisted microscopic analysis of the structure and organisation of a tissue. This quantitative histological assessment of bone modelling enables a detailed insight into bone reconstruction [69]. Chapman et al. manufactured sol-gel coated period four nanoparticles to inhibit biofouling at its initial stage known as micro-fouling, the process of accumulation of bacteria and other microorganisms [11]. The nanoparticles were synthesised using a modified polyol reduction method, characterised using UV-visible spectrophotometry and then doped in prepared sol-gels by centrifugation and spin-coating. Assays for biofouling, glycocalyx and environmental bacterial counts showed that the sol-gel coated nanoparticles reduced biofouling at significant rates compared to sol-gel blanks.
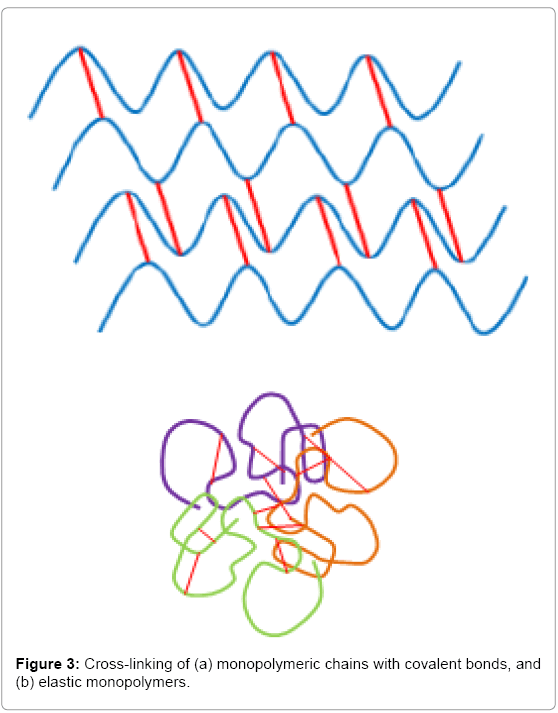
Hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles have been developed successfully by reversible, non-covalent bonds; these exhibit self-healing properties, a huge step towards the goal of regenerative capacity [70] (Figure 3). The composite shows good biocompatibility, bone-to-implant interaction and great adhesion to mineral surfaces of enamel and HA [70]. The next step for this promising material is its application in joint regeneration trials.
A study performed by Sharma et al. shows promising results for cartilage and joint regeneration; this involved the design of a poly (ethylene glycol) diacrylate (PEGDA) hydrogel [71]. This biomaterial demonstrated deposition and formation of cartilage tissue by mesenchymal stem cells at joint areas. The adhesion of the implant to cartilage and bone tissue was achieved by a chondroitin sulphate adhesive bonded covalently to the hydrogel. A clinical trial, combining the material’s use with microfracture surgery, observed 15 patients; those treated with the implant had significantly reduced knee pain with increased extracellular matrix tissue, thus promoting ligament and cartilage regeneration [71].
Controversies, Ethics and Limitations
The integration of artificial or man-made structures in the human body has, among certain audiences, caused contention and raised questions relating to the loss of human integrity and dignity [12]. Regarding development of implants, how thoroughly tested should these be before their commercial release for widespread clinical application? Since the study of biomaterials is an emerging area and issues of biodegradability and biotoxicity persist, ethical considerations and accountability for these are still to be fully resolved. Concerns over the pace of development notwithstanding, a majority of the general population is broadly supportive of using bio-nanomaterials for reconstructive therapy [72]. It is appreciated that, for example, the quality of life of people with mobility impairment due to defective joints would be enhanced profoundly if they were enabled the use of properly functioning bones and joints. If a biomaterial can itself be regenerated within our own body, would it be processed as artificial or recognised as intrinsic and merely a means of aiding the body to reconstruct itself?
Although dramatic breakthroughs have been achieved in the field of biomaterials, implants still present minor limitations. For example, bioceramics acquire decreased stability and reduced toughness at elevated temperatures, making their manufacture difficult and therefore increased in cost. Ceramic implants have displayed reduced longevity along with continuous loosening and dislocation [35]. In contrast, metals retain their properties at higher temperatures and can also be easily sterilised. However, metal polymers corrode easily in harsh internal environments, including exposure to body acids and enzymes. The possible leaching of metal ions can result in wear and tear of the implant, an undesirable characteristic due to particulate toxicity to tissues. These toxins could easily be absorbed into body systems along with significant nutrients. Thus, biodegradability of implants must be achieved safely over time without toxic contamination of the body. Implants may be broken down and/or excreted without requiring surgical removal [72]. Absorption of toxic biodegradable products into the blood may also be detected with organic polymers. Compared to other biomaterials such as ceramics, metallics and composites, however, production of organic polymers is both rapid and cost-effective. Finally, composites are tough but corrosion-resistant in comparison to metallics. They are also able to support heavy loads while being lightweight and low density. Nonetheless, composites do have limitations, including difficulty in altering shape and topography, which in turn raises the cost of manufacture.
Conclusion
Arthroplasty and other knee joint surgical procedures have been around since 10,000 BC. In recent times, the field of biomaterials has advanced considerably our knowledge of strength requirements, wear minimisation, enhanced biocompatibility and reduced biodegradability. Improvements have been attained through coatings of metallics, replacement of conventional plastics with UHWMPE and the use of technology to manufacture biocompatible scaffolds. The study of bioceramics started mainly with alumina and zirconia, and presently a combination of these two materials has been modified chemically and physically to provide greater toughness and better surface properties. Bioceramics are also being investigated for regenerative properties. Metal implants are given a protective coating in order to reduce degradation, release of wear particles and inflammation. Scientists have also realised that porous metallics provide heightened mechanical properties and more efficient drug release. Development of polymers and composites is focused on utilising natural materials for better integration and biocompatibility; hydrogel is the most commonly used substance in implants. Although replacement has been the conventional practice for knee and hip surgeries since the 1700s, articular regeneration has surfaced as an alternative therapy with the benefit of reconstructing tissue instead of introducing foreign materials into the body, which has well-documented long-term drawbacks. In order to achieve regeneration, it is imperative to understand the basis of chondrocytic and mesenchymal cell proliferation and differentiation, biological and physicochemical properties of scaffolds for protein absorption, and biodegradability of materials. The possibility of regeneration of bone and cartilage tissues raises expectations that limitations historically associated with implants – loosening, wear, tear and bioaccumulation of particles – may be prevented. There would be in effect no need to replace implants continuously, hence reducing costs of knee, hip and shoulder arthroplasty; patients would only require the initial operation. The emergence of nanotechnology has also enabled researchers to produce multi-phasic and biomimetic scaffolds that permit fast protein adsorption and proliferation of cells onto the material’s surface, accelerating regeneration of joints. With antibacterial properties of nanoparticles demonstrated by successfully inhibiting biofouling, they are being used to develop biodegradable and regenerative implants. Due to their large surface area to volume ratio, drug release and absorption is more effective than for conventional implants. Future advances in implant regeneration provide an exciting prospect of enabling orthopaedic patients to regain joint mobility with reduced pain and at less cost due to negation of the requirement for follow-up replacement surgery.
Authors’ Contributions
J Chapman and AW Taylor-Robinson conceived the design of the manuscript. S. Gangadoo undertook an initial literature review supervised by J Chapman. All authors contributed to writing, critically reviewed the paper during preparation and approved the final version.
Acknowledgements
The authors’ research is supported by the CQUniversity Health CRN and the Australian Government's Collaborative Research Networks Program.
References
- Mackenzie D (1973) The history of sutures.Medical History 17:158-168.
- Agrawal CM, Athanasiou KA (1997) Technique to control pH in vicinity of biodegrading PLA-PGA implants. Journal of BiomedicalMaterials Research 38: 105-114.
- Yang LJ, Li J, Jin Y, Li M, Gu Z (2015) In vitro enzymatic degradation of the cross-linkedpolypoly(e-caprolactone) implants. PolymerDegradation and Stability 112: 10-19.
- Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional Course Lectures 47: 487-504.
- Luyten FP (2004) Mesenchymal stem cells in osteoarthritis. Current Opinion in Rheumatology 16: 599-603.
- Heo SJ, Kim SE, Wei J, Kim DH, Hyun YT, et al. (2009) In vitro and animal study of novel nano-hydroxyapatite/poly(epsilon-caprolactone) composite scaffoldsfabricated by layermanufacturingprocess. Tissue Engineering Part A 15: 977-989.
- Shi Z, Huang X, Cai Y, Tang R, Yang D (2009) Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-likecells. Acta Biomaterialia 5: 338-345.
- Li X, Feng Q, Cui F (2006) In vitro degradation of porous nano-hydroxyapatite/collagen/PLLA scaffoldreinforced by chitin fibres. Materials Science and Engineering: C 26: 716-720.
- Bose S, Darsell J, Kintner M, Hosick H, Bandyopadhyay A (2003) Pore size and pore volume effects on alumina and TCP ceramicscaffolds. Materials Science and Engineering: C 23: 479-486.
- Misra RDK, Nune C (2014) Biological response to self-assembly of preadsorbedproteins at biointerfaces: significance, cellular activity and perspective. MaterialsTechnology: Advanced Biomaterials 29: B41-B48.
- Chapman J, Weir E, Regan F (2010) Period four metalnanoparticles on the inhibition of biofouling. Colloids and Surfaces: B Biointerfaces 78: 208-216.
- Saha S (2004) Bioethics and biomaterial research. Trends in Biomaterials and ArtificialOrgans 17: 1-3.
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds) (2012) Biomaterials Science: An Introduction to Materials in Medicine. 3rd Edn. AcademicPress.
- Garino JP, Beredjiklian PK (2007) CoreKnowledge in Orthopaedics: Adult Reconstruction and Arthroplasty. Mosby Elsevier.
- Van Putte C, Regan J, Russo A (2014) Seeley's Essentials of Anatomy and Physiology. 10th Edn. McGraw-Hill.
- Buechel FF, Pappas MJ (2015) Principles of Human Joint Replacement: Design and Clinical Application. Springer-Verlag.
- Gomez PF, Morcuende JA (2005) Earlyattempts at hip arthroplasty – 1700s to 1950s. Iowa Orthopaedic Journal 25: 25-29.
- Wang W, Ouyang Y, Poh CK (2011) Orthopaedic implant technology: biomaterials from past to future. Annals of the Academy of Medicine, Singapore 40: 237-244.
- Smith-Petersen MN (1948) Evolution of mouldarthroplasty of the hip joint. Journal of Bone and Joint Surgery, British Volume 30B: 59-75.
- Li S, Burstein AH (1994) Ultra-high molecular weightpolyethylene. The material and its use in total joint implants. Journal of Bone and Joint Surgery, American Volume 76: 1080-1090.
- Bono JV, McCarthy JC, Thornhill TS, Bierbaum BE, Turner RH (eds) (1999) Revision Total Hip Arthroplasty. Springer-Verlag.
- Marya SKS, Bawari RK (2010) Total Hip Replacement Surgery: Principles and Techniques. JaypeeBrothers Medical Publishers.
- Ingham E, Fisher J (2000) Biological reactions to wear debris in total joint replacement. Proceedings of the Institution of MechanicalEngineers, Part H: Journal of Engineering in Medicine 214: 21-37.
- Schwinté P, Keller L, Eap S, Mainard D, Benkirane-Jessel N (2014) Osteoarticularregenerativenanomedicine: advances and drawbacks in articular cartilage regeneration implants. Austin Journal of Nanomedicine&Nanotechnology 2: 1025.
- Baasanjav S, Al-Gazali L, Hashiguchi T, Mizumoto S, Fischer B, et al. (2011) Faulty initiation of proteoglycansynthesis causes cardiac and joint defects. American Journal of Human Genetics 89: 15-27.
- Settle SH Jr, Rountree RB, Sinha A, Thacker A, Higgins K, et al. (2003) Multiple joint and skeletalpatterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. DevelopmentalBiology 254: 116-130.
- Storm EE, Kingsley DM (1996) Joint patterning defects caused by single and double mutations in members of the bonemorphogeneticprotein (BMP) family. Development 122: 3969-3979.
- Chen D, Zhao M, Mundy GR (2004) Bonemorphogeneticproteins. Growth Factors 22: 233-241.
- Bernstein RM (2002) Arthrogryposis and amyoplasia. Journal of the American Academy of Orthopeadic Surgeons 10: 417-424.
- Wroblewski BM (2002) Professor Sir John Charnley (1911-1982). Rheumatology 41: 824-825.
- Shi D (2006) Introduction to Biomaterials. Tsinghua University Press.
- Nadege S, Engel E, Castano O (2014) Hybridorganic-inorganicscaffoldingbiomaterials for regenerativetherapies. Current OrganicChemistry 18: 2299-2314.
- Park J (2008) Bioceramics: Properties, Characterizations, and Applications. Springer-Verlag.
- Hench LL (1991) Bioceramics: from concept to clinic. Journal of the American Ceramic Society 74: 1487-1510.
- Thamaraiselvi TV, Rajeswari S (2004) Biological evaluation of bioceramicmaterials – a review. Trends in Biomaterials and ArtificialOrgans 18: 9-17.
- Tai SM, Parker L, de Roeck NJ, Skinner JA (2014) Recurrentcatastrophicceramicfemoralheadfailure in total hip arthroplasty. Case Reports in Orthopedics 2014: 837954.
- De Aza AH, Chevalier J, Fantozzi G, Schehl M, Torrecillas R (2002) Crack growthresistance of alumina, zirconia and zirconiatoughened alumina ceramics for joint prostheses. Biomaterials 23: 937-945.
- Schoichet MS (2010) Polymerscaffolds for biomaterials applications. Macromolecules 43: 581-591.
- Lusquiños F, delVal J, Arias-González F, Comesaña R, Quintero F, et al. (2014) Bioceramic 3D implants produced by laser assisted additive manufacturing. PhysicsProcedia 56: 309-316.
- Demirel M, Aksakal B (2014) Enhancedboneregeneration in rabbit tibial defects implanted with newlyfabricatedbioceramicbonegrafts. International Journal of AppliedCeramicTechnology 12: 254-263.
- Black J, Hastings G (1998) Handbook of BiomaterialProperties. 1st Edn. Chapman & Hall.
- Brazel CS, Rosen SL (2012) FundamentalPrinciplesof PolymericMaterials. 3rd Edn. John Wiley& Sons.
- Chrisey DB, Piqué A, McGill RA, Horwitz JS, Ringeisen BR, et al. (2003) Laser deposition of polymer and biomaterial films. Chemical Reviews 103: 553-576.
- Huang H, Zhang X, Hu X, Shao Z, Zhu J, et al. (2014) A functional biphasicbiomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials 35: 9608-9619.
- Nishimura A, Nakahara T, Ueno T, Sasaki K, Yoshida S, et al. (2006) Requirement of E7 oncoprotein for viability of HeLacells. Microbes and Infection 8: 984-993.
- Kang ML, Ko JY, Kim JE, Im GI (2014) Intra-articulardelivery of kartogenin-conjugatedchitosan nano/microparticles for cartilage regeneration. Biomaterials 35: 9984-9994.
- Heo DN, Ko W-K, Bae MS, Lee JB, Lee D-W, et al. (2014) Enhancedboneregeneration with a gold nanoparticle-hydrogel complex. Journal of MaterialsChemistry B 2: 1584-1593.
- Dapunt U, Giese T, Lasitschka F, Reinders J, Lehner B, et al. (2014) On the inflammatoryresponse in metal-on-metal implants. Journal of Translational Medicine 12: 74.
- Ye D, Peramo A (2014) Implementing tissue engineering and regenerativemedicine solutions in medical implants. British Medical Bulletin 109: 3-18.
- Tejero R, Anitua E, Orive G (2014) Toward the biomimetic implant surface: biopolymers on titanium-based implants for boneregeneration. Progress in Polymer Science 39: 1406-1447.
- Linder L, Lundskog J (1975) Incorporation of stainlesssteel, titanium and vitallium in bone. Injury 6: 277-285.
- Suska F, Gretzer C, Esposito M, Emanuelsson L, Wennerberg, A et al. (2005) In vivo cytokine secretion and NF-kappa β activation aroundtitanium and copper implants. Biomaterials 26: 519-527.
- Dorn U, Neumann D, Frank M (2014) Corrosion behavior of tantalum-coated cobalt-chromiummodular necks compared to titaniummodular necks in a simulator test. Journal of Arthroplasty 29: 831-835.
- Du T, Tamboli D, Desai V, Chathapuram VS, Sundaram KB (2004) Chemicalmechanicalpolishing of tantalum: oxidizer and pH effects. Journal of Materials Science: Materials in Electronics 15: 87-90.
- Viennot S, Dalard F, Lissac M, Grosgogeat B (2005) Corrosion resistance of cobalt-chromium and palladium-silveralloysused in fixedprostheticrestorations. European Journal of Oral Sciences 113: 90-95.
- Mohandas G, Oskolkov N, McMahon MT, Walczak P, Janowski M (2014) Poroustantalum and tantalumoxidenanoparticles for regenerativemedicine. Acta NeurobiologiaeExperimentalis 74: 188-196.
- Cohen R (2002) A poroustitaniumtrabecularmetal: basic science. American Journal of Orthopedics 31: 216-217.
- Malkani AL, Price MR, Crawford CH 3rd, Baker DL (2009) Acetabular component revisionusing a poroustantalumbiomaterial: a case series. Journal of Arthroplasty 24: 1068-1073.
- Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ (1999) Characteristics of boneingrowth and interface mechanics of a new poroustantalumbiomaterial. Journal of Bone and Joint Surgery, British Volume 81: 907-914.
- Zhang M, Wang GL, Zhang HF, Hu XD, Shi XY, et al. (2014) Repair of segmental long bonedefect in a rabbit radius nonunion model: comparison of cylindricalporoustitanium and hydroxyapatite scaffolds. ArtificialOrgans 38: 493-502.
- Xu J, Weng XJ, Wang X, Huang JZ, Zhang C, et al. (2013) Potential use of poroustitanium-niobium alloy in orthopedic implants: preparation and experimental study of itsbiocompatibility In vitro. PLoS One 8: e79289.
- Jung HD, Jang TS, Wang L, Kim HE, Koh YH, et al. (2015) Novel strategy for mechanicallytunable and bioactive metal implants. Biomaterials 37: 49-61.
- Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, et al. (2014) Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guidedosteochondral tissue repair. Biomaterials 35: 8829-8839.
- Yodmuang S, McNamara SL, Nover AB, Mandal BB, Agarwal M, et al. (2014) Silkmicrofiber-reinforcedsilk hydrogel composites for functional cartilage tissue repair. Acta Biomaterialia 11: 27-36.
- Shen W, Chen X, Hu Y, Yin Z, Zhu T, et al. (2014) Long-termeffects of knittedsilk-collagenspongescaffold on anteriorcruciate ligament reconstruction and osteoarthritisprevention. Biomaterials 35: 8154-8163.
- Brydone AS, Prodanov L, Lamers E, Gadegaard N, Jansen JA, et al. (2014) Enhancedosseointegration on rabbittibiaeusingtitaniumcoated nano-patterned implants. Bone and Joint Journal 96-B, Suppl 7: 2.
- Chang ST, Chen LC, Lin SB, Chen HH, et al., (2012) Nano-biomaterials application: morphology and physicalproperties of bacterial cellulose/gelatin composites via crosslinking. Food Hydrocolloids 27: 137-144.
- Wang L, He S, Wu X, Liang S, Mu Z, et al. (2014) Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobialactivity and osseointegrationproperties. Biomaterials 35: 6758-6775.
- Kulak CA, Dempster DW (2010) Bonehistomorphometry: a concise review for endocrinologists and clinicians. ArquivosBrasileirosde Endocrinologia e Metabologia 54: 87-98.
- Nejadnik MR, Yang X, Bongio M, Alghamdi HS, van den Beucken JJ, et al. (2014) Self-healinghybridnanocompositesconsisting of bisphosphonatedhyaluronan and calcium phosphate nanoparticles. Biomaterials 35: 6918-6929.
- Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, et al. (2013) Human cartilage repair with a photoreactiveadhesive-hydrogel composite. Science Translational Medicine 5: 167ra6.
- Brey P (2009) Biomedical engineering ethics. In: A Companion to the Philosophy of Technology (Olsen JKB, Pedersen SA, Hendricks VF, eds), Wiley-Blackwell.
- Hacking SA, Bobyn JD, Toh K, Tanzer M, Krygier JJ (2000) Fibrous tissue ingrowth and attachment to poroustantalum. Journal of BiomedicalMaterials Research 52: 631-638.
- Knight SR, Aujla R, Biswas SP (2011) Total hip arthroplasty – over 100 years of operativehistory. Orthopedic Reviews 3: e16.
- Nasab MB, Hassan MR, Sahari BB (2010) Metallicbiomaterials of knee and hip – a review. Trends in Biomaterials and ArtificialOrgans 24: 69-82.
- Hannink RH, Kelly PM, MuddleBC (2000) Transformation toughening in zirconia-containingceramics. Journal of the American Ceramic Society 83: 461-487.
- Piconi C, Maccauro G (1999) Zirconia as a ceramicbiomaterial. Biomaterials 20: 1-25.
- Lawn B (1993) Fracture of BrittleSolids. 2nd Edn. Cambridge University Press, Australia.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15088
- [From(publication date):
August-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10521
- PDF downloads : 4567