Fright of Covid-19 and its Future: A Review
Received: 30-May-2020 / Accepted Date: 20-Jul-2020 / Published Date: 27-Jul-2020 DOI: 10.4172/2165-7386.1000369
Abstract
From Dec 2019 the disease Covid-19 stated being epidemic in China and Outbreak throughout the world. WHO announced it pandemic on 11th Feb 2020.The most common symptoms of COVID-19 patients are fever, dry cough and tiredness.
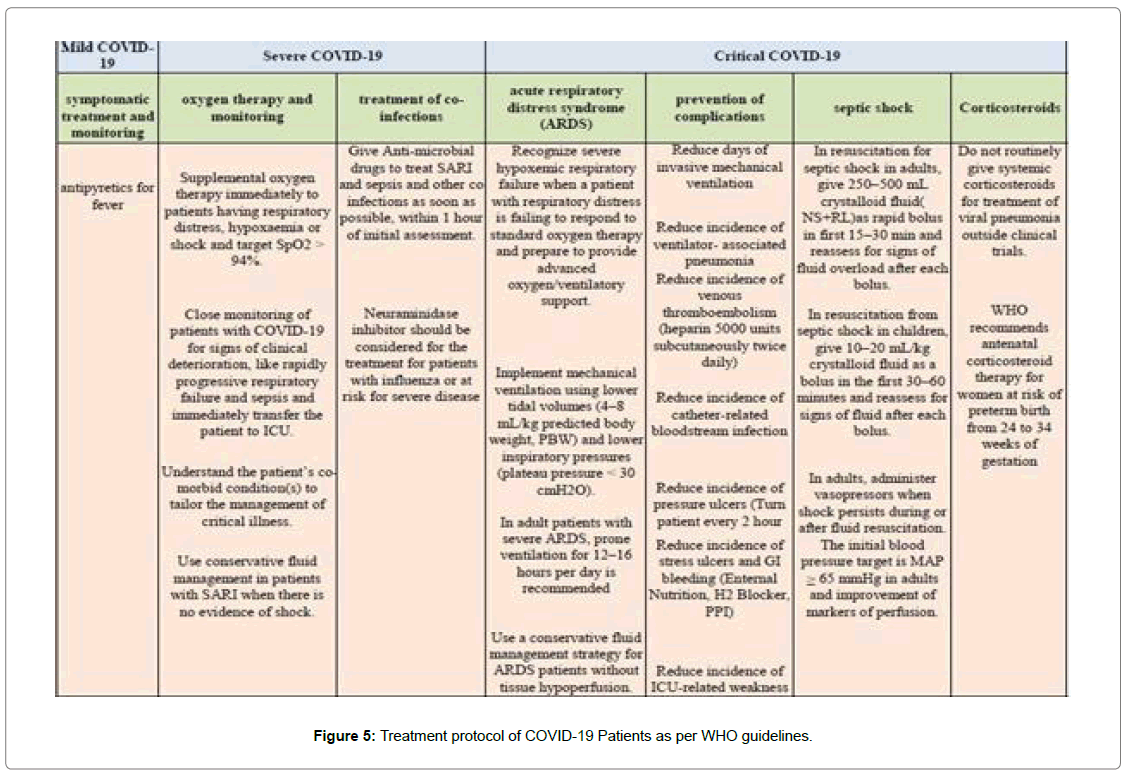
There are mainly two broad categories of test for the diagnosis of Covid-19s, e.g. the RT-PCR test and Antibodies detection test. There is no specific treatment but the symptomatic treatment must be categorized like symptomatic treatment, oxygen therapy, treatment of co-infections, prevention of complications and septic shock.
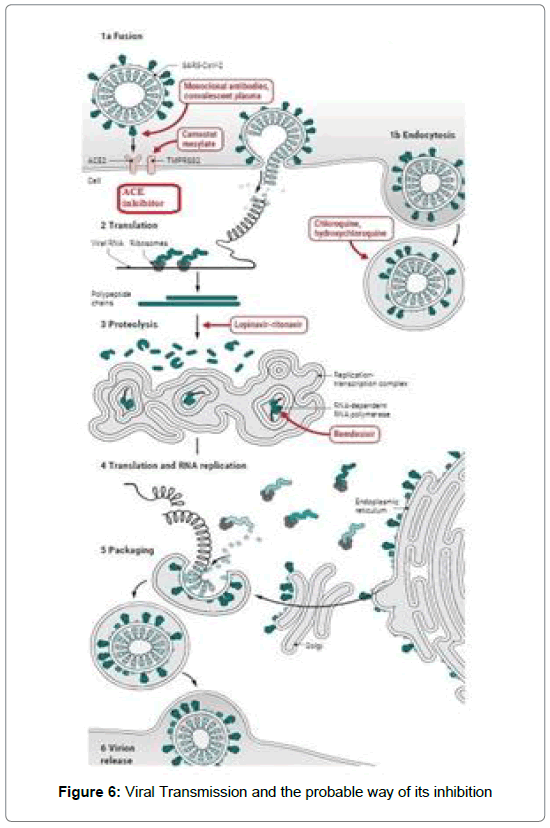
The viral replication takes place after binding with the ACE2receptor, injecting the genome into the host cell, Translation and replication multiply the RNA based nuclear material. After that packaging is takes places and ultimately viral multiplication successfully completed in the presence of various enzymes. The replication can inhibit by blocking the receptor binding or by enzyme inhibition.
Several Clinical Trials and Researches are going on though out the world. A Chinese research team has found a compound called “11a” is less toxic and more effective. Another research said that Interferon-beta-1-b may be a key component for the treatment of COVID-19. Remdesivir, an Ebola drug shows 31% faster recovery than a placebo. Chinese based vaccine’s first human clinical trial was successful and Research on vaccine of Oxford University’s human clinical trial is going “very Well”.
In such a short time, we have advanced our knowledge and research about the virus and the disease a lot so in future we will must overcome and get a peaceful life.
Keywords: Covid-19; SARS-CoV-2; Covid-19 pandemic; RT-PCR; Covid-19; Outbreak; Antibodies Detection Test; Oxygen Therapy
Introduction
Corona, the word comes from ‘Crown” and when examined closely under microscope, the round virus has a ‘crown’ of proteins called peplomers jutting out from its centre in every direction [1]. These proteins actually help the virus to bind with the host. Corona viruses are a previously known large family of viruses that causes illness of common cold to more severe like pneumonic condition such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The novel corona virus is a new stain of corona virus that has not previously identified in human. Around December 2019, the novel corona virus was appeared in human from the city of Wuhan located in Huei Province, China. The actual cause of appearance is still unknown, Japan’s Nobel Laureate Tasuku Honjo has claimed that Novel Corona virus (SARS-CoV-2)” is manmade [2] as well as other scientist assumed that its natural occurring and 1st spread in human from a fish market of Wuhan, China [3]. Novel corona virus is now named “Severe acute respiratory syndrome corona virus 2 (SARS-CoV-2)” as per ICTV announcement on 11th feb 2020 [4]. The disease caused by SARS-CoV-2 is called “Coronavirus Disease” 2019 (COVID-19). From Dec 2019 the disease stated being epidemic in China and Outbreak throughout the world. WHO announced it pandemic on 11th feb 2020 but till that a lot of COVID-19 confirmed cases has been found throughout the world and thus it made a worst situation not only the health condition but also to the economy.
Prevalence
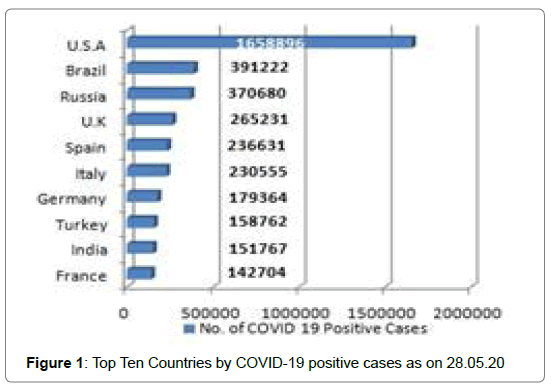
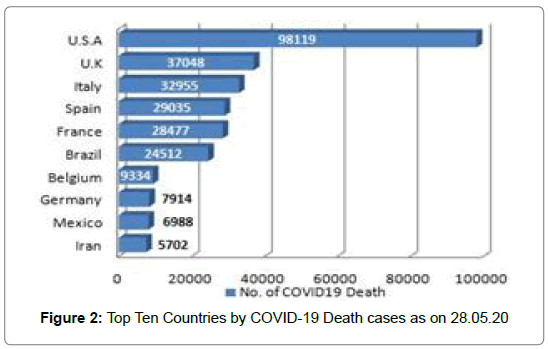
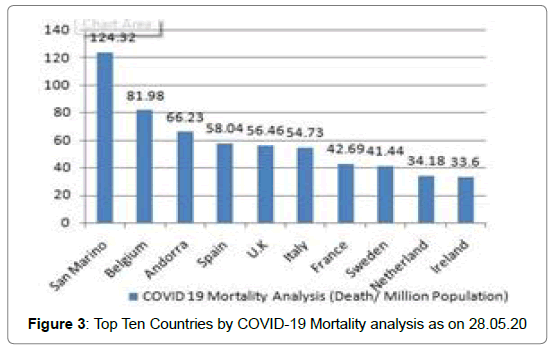
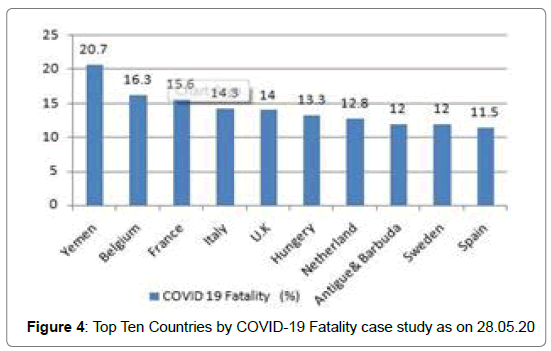
213 Countries and Territories around the world have reported a total of 5556679 confirmed cases of the Covid-19 that originated from Wuhan, China and 351866 deaths according to WHO as on 28.05.2020 [5]. The highest Covid-19 confirmed cases as well as death reported from U.S.A till date [5] (Figure 1 and Figure 2). The mortality rate (death/one million population of a country) is higher in case of European countries [6] (Figure 3 and Figure 4). Male and female have the same prevalence of Covid-19 whereas males are more at risk for worse outcomes and death, independent of age [7]. People with associated health problems like diabetes, Cancer and Cardiovascular disease along with age of over sixty are more severe.
Sign and Symptoms
According to WHO, the most common symptoms of COVID-19 patients are fever, dry cough, and tiredness after a median incubation period of 3 days [8, 9]. Researchers found that other symptoms that are less common but may affect include nasal congestion, breathlessness, headache, conjunctivitis, and sore throat, Diarrhoea, loss of taste or smell or a rash on skin [9].
Test and Diagnosis
The primary screening is to check the body temperature with the help of thermal gun. If the body temperature is more the normal or anyone have cold and cough or flu like symptom, they must go through specific test which can determine if the person is COVID 19 positive or not. If the patient is asymptomatic then the only way is to go for specific test to determine the condition. There are mainly two broad categories of test. Some look for the presence of the virus, e.g. the RT-PCR test. Others are for confirming the presence of the antibodies which arise when a body is attacked by the virus.
For performing the test it is very important to know that how much time has passed since the person was infected. According to Christian Drosten the RT-PCR test performed with throat swabs is only reliable in the first week, after that the virus may disappear from the throat. For infected people tested in the second week, it will be better to take nasal swabs or coughed up material (sputum) can be used. After collecting the swab, the RT-PCR test performed. The test sample is treated with certain chemicals [10, 11] which remove extraneous substances and extract only the RNA from the sample. Reverse transcription polymerase chain reaction (RT-PCR) is a technique that first uses reverse transcription to convert the extracted RNA into DNA and then uses PCR to amplify a piece of the resulting DNA, creating enough to be examined in order to determine if it matches with the genetic code of SARS-CoV-2 [10].
Isothermal nucleic acid amplification method is just like PCR, amplify a piece of the virus's genome and detect the amplified virus sequences using fluorescent tags. The process is cheap and faster than PCR because they don't involve repeated cycles of heating and cooling the sample [12, 13].
The antigen tests look for proteins from the surface of the virus. In the case of a SARS-CoV-2 these are usually proteins from the surface spikes, and a nasal swab is used to collect samples. There are several drawbacks in this test. It is a very rapid test though the chances of false negative test result are more common and the biggest problem is to find a protein target unique to SARS-CoV-2. WHO said that many scientists doubt whether an antigen test can be made reliable enough against COVID-19.
Chest CT scans are not recommended for routine screening. It’s a non-specific test but it is important for false negative cases confirmation [14].
Serology tests can identify people who were infected and have already recovered from COVID-19, including those who did not know that they had been infected [14]. Most serology tests are in the research stage of development.
Preventive Measures
According to modern medical science prevention is classified into 4 categories from Primordial prevention to Tertiary prevention. The 1st step of prevention should stop the generation of risk factor and for that WHO suggested ‘Lockdown’ which helps to stop the spreading of disease. The next steps are Washing hands and face, drinking plenty of water, use of alcoholic hand sanitizer and lastly it has been added to wearing mask (as because it’s a droplet infection) specially who have flu like symptoms [15].
Treatments
Scientists, researchers, doctors, health workers and all of us are trying our best to get a way which can protect us from this breakneck disease but unfortunately till date there is no specific medicine or vaccine against this disease. Although the most of the COVID-19 patient have uncomplicated or mild illness (81%), some have severe illness with severe pneumonia requiring oxygen (14%) and approximately 5% of the patients require intensive care unit treatment which includes mechanical ventilation [16] [Figure 5].
Older patients with associated disease such as cardiovascular disease and diabetes mellitus, cancer have increased risk mortality. So these patients have high chances of deterioration and must treat under close monitoring [16].
Transmission
The novel corona viruses were classified into family Coronaviridae based on crown or halo-like appearance. SARS-CoV-2 is a Spherical or pleomorphic enveloped particles containing single-stranded (positive-sense) RNA beta with a 30 kilobase genome that encodes viral proteins up to 14 open reading frames (Orfs). The 16 protein replicase-transcriptase consists of multiple enzymes essential for viral genome replication which induced the viral RNA-dependent RNA polymerase, endo and exo-nucleases vital for nucleic acid metabolism [17].
Structural proteins of SARS-CoV-2 form the viral capsid that encapsulates the genome. These structural proteins are also facilitating entry to host cells through the human angiotensin converting enzyme 2(ACE2) receptor.
After binding with the receptor, injecting the genome into the host cell takes place, Translation and replication are the next steps into the host cell which multiply the RNA based nuclear material. After that packaging is takes places and ultimately viral multiplication takes place. In the process of incorporation of the viral RNA material into the host cell, Viruses have to bind with a host receptor then the multiplication takes place with the help of various enzymes and proteins [Figure 6].
The replication can inhibit by blocking the receptor binding or by enzyme inhibition [17].
These may be a possible way to find out a drug active against COVID-19. Several Clinical Trials and Researches are going on though out the world. A china based research team was working on two compounds (“11a” and “11b”) and they have found that “11a” is less toxic and more effective and so they have focused on the compound [18]. Prof. Nevan J. Krogan and his team of University of California San Francisco is working on an pre-existing anti-cancer drug called “PB28” and they have found that the drug is 20 times more potent that hydroxychloroquine at deactivating COVID-19 but after successful animal study, they have found some limitation of their research [14]. In another research of three drug combination ( Interferon beta- 1b, Lopinavir-ritonavir, Ribavirin), the phase II clinical trial has gone successful and Dr. Jenny Lo from Ruttonjee Hospital says that interferon beta 1-b may be a key component for the treatment of COVID-19 [14]. In the other study it has been found that, Remdesivir, an Ebola drug shows 31 % faster recovery than a placebo and as a result drug has now received emergency use authorization from USFDA. Bangladeshi doctors claim Ivermectin with Doxycycline can treat Covid-19 [19]. Roivant scientist is in advance stage of developing for Gimsilumab, a human monoclonal antibody. The drug targets granulocyte-macrophage colony stimutating factor which is proinflammatory cytokine found in high levels in the serum of covid-19 patient. Altimmune has going to develop a single dose intranasal vaccine named “Ad COVID” collaborated with university of Alabama.IMab Biopharma has developed a neutralizing antibody named “TJM2” for the treatment of cytokine storm in Covid-19 patients. Medicago is developing Virus-Like particles (VLP) to fight against Covid-19.
For Cvid-19, there are over 100 vaccines being developed across the world, some are new and some from existing molecules but only a few are going for clinical trials. Still now there is no vaccine ready for use but some of them show good result in clinical trials. Chinese researchers reported in Lancet Medical Journal that the first human trial of experimental Covid-19 vaccine developed by Cansino Biologics Inc was successful and the result represents an important milestone according to Prof. Wei Chen. On the other hand, Researchers of Oxford University described that the clinical trials of covid-19 vaccine on human is going “very Well” [20]. 1st clinical trial has done successfully and they are trying to combine second and third clinical trial to speed up their research. Apart from that a study has been published in New England Journal of Medicine Which helps to explain that Novel corona virus can severely damage patient blood vessels and it causes blood clotting. A process called intussuscepted angiogenesis is 30 times higher in Covid-19 patient lungs. This thrombosis is one of the great mysteries which may open a new way of thinking about Covid-19 and its treatment.
Conclusion
Novel Corona Virus, From Wuhan, China to the whole world completed its journey and honoured as Pandemic by WHO within 3 months. Stated from December 2019 it has affected near about 46 Million people and cause death of near about 4 million people as on 17.05.2020 and continuing its journey. It is very unfortunate but true that still now we are not able to find out any remedy against it. Being Human it is very hard to deal with the situation but we are not going to lose. Several Researches is going on and many groups of various universities, countries are doing clinical trials of vaccines. We should keep in mind that WHO announced that we have to live with the Novel Corona Virus, so we have to change ourselves a little bit. We have to be more cautious, we have to make some guidelines to be safe and stay healthy as well as we has to try more and more. In such a short time we have advanced our knowledge and research about the virus and the disease a lot so in future we will must overcome and get a peaceful life.
Conflict of Interest
There is no conflict of interest.
Financial support and sponsorship
Nil
Acknowledgement
We would like to acknowledge institutional heads for their support.
References
- Sturman LS, Holmes KV, Lauffer MA (1983) The molecular biology of Coronaviruses. Advances in Virus Research 28: 35-112.
- Sheth H (2020) Nobel Laureate Tasuku Honjo refutes claims of novel coronavirus being man-made. Kyoto University Institute of Advanced Study.
- Harapan H, Naoya I, Amanda Y, Winardi W (2020) Coronavirus disease 2019 (COVID-19): A literature review. Journal of Infection and Public Health 13: 667-673.
- WHO ( 2020), Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization.
- Jin JM, Bai P, He W, Wu F and Liuet XF, et al. (2020) Gender Differences in Patients With COVID-19: Focus on Severity and Mortality.†Front Public Health 8: 152.
- Chen N, Zhou M, Dong X, Qu J and Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A Descriptive study. Lancet 395: 507-513.
- Tanu S (2020) A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr 87: 281-286.
- Novel coronavirus (COVID-19): Cases in Nova Scotia: (2020) data visualization.
- Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, et al. (2020) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus [2019-nCoV] infected pneumonia. Mil Med Res. 7: 4.
- World Health Organization (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected..
- Kupferschmidt K, Cohen J. (2020) WHO launches global megatrial of the four most promising coronavirus Treatments.
- Chaubey AK (2020) COVID-19 cure in sight? Bangladeshi doctors claim Ivermectin with Doxycycline can treat coronavirus patients.
- Duddu P (2020) Coronavirus treatment: Vaccines/drugs in the pipeline for COVID-19.
Citation: Chakraborty D, Yolmo NL (2020) Fright of Covid-19 and its Future: A Review. J Palliat Care Med 10: 369. DOI: 10.4172/2165-7386.1000369
Copyright: © 2020 Chakraborty D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2399
- [From(publication date): 0-2020 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1610
- PDF downloads: 789