Formulation of the Tazarotene Cutaneous Gel and Its Physicochemical Characteristics
Received: 17-Mar-2020 / Accepted Date: 20-May-2020 / Published Date: 27-May-2020 DOI: 10.4172/2167-065X.1000194
Abstract
Treatment of psoriasis is divided to three main types: moderate treatment (moisturizers, phototherapy and etc.), low treatment (changing the life style) and high treatment include systemic medications.
Tazaroten is one of the most effective and the most applicable dermal medicine in treatment of two mentioned diseases that available in form of gel, cream and foam. This agent is one component of vitamin A derivatives and it is a selective receptor of acetylene retinoid that binds to beta and gamma retinoid acid receptors.
The purpose of this study is formulation of cutaneous gel of Tazarotene 0.1% that is not available in the internal market of medication in Iran.
So, first the basics of medicine prepared after changing in several stages of amounts and proportions of primary materials and the effective agent added to better formulation after related stability tests. Then the stability of the formula evaluated in terms of clarity, pH, lack of two phases in temperature of 4°C, 25°C and 40°C as well as viscosity, and the best formulation was selected in terms of stability. Then the tests of determination of the effective material amount in wavelength 351 nm was performed by method of spectrophotometer and HPLC, release of medicine in plasmatic membrane and dermal absorption in the medium of acetate ammonium buffer with pH=6.5 and 10% acetonitrile on the final formulation.
The final formulation contained 85% effective material that was in the optimal range in terms of releasing and dermal absorption, while adhere to Higouchi kinetics model.
Keywords: Tazaroten; Gel; Releasing; Formulation
Introduction
Tazarotene is a third-generation retinoid which is effective for topical treatment of psoriasis and acne vulgaris and has cosmetic benefits for photoaging [1].
However, there are adverse effects accompanied with its use where local cutaneous irritation including burning, itching, erythema, peeling or dryness occurs in approximately one-quarter of patients using tazarotene [2].
Tazarotene (TZR), 6-[2-(4,4-dimethylthiochroman-6-yl) ethynyl] nicotinic acid ethyl ester is a member of a new generation of receptor selective, synthetic retinoids for the topical treatment of mild to moderate plaque psoriasis, acne vulgaris and photoaging.
In comparative trials 0.1% tazarotene gel demonstrated the highest irritation score followed by tazarotene cream compared to different concentration of tretinoin cream (Thielitz). In a recent study, tazarotene foam 0.1% was found to be an alternative to tazarotene gel with less systemic exposure (Jaratt). Therefore, the development of new effective topical drug delivery system intended to modulate tazarotene release rate, enhance its localization in the skin and reduce its percutaneous absorption might minimize its adverse effects and could be of particular usefulness.
Dermal safety studies also indicated that TZR did not show phototoxic or photoallergic potential [3]. However, mild to moderate local cutaneous irritation, with burning, itching, erythema, peeling, and/or dryness, was observed in approximately 25% of treated patients. So, it is necessary to improve the topical delivery and reduce the adverse effects of TZR using a carrier with the ability of skin targeting. A literature survey revealed a high-performance liquid chromatography (HPLC) method for determination of TZR and its major active metabolite with mass spectroscopy [4].
Isocratic RP-HPLC method with UV detection has been described for quantitative and related substance determination of TZR.12 However, HPLC-based separation methods may not be suitable for the determination of the drug from lipid-based delivery systems such as ME formulations.
The formulations contain various lipophilic excipients that are not soluble in commonly used organic solvents used in RP-HPLC methods. Further, extraction of the drug from such lipophilic excipients may not be achieved easily, and such excipients may get adsorbed on stationary phase. Hence, analysis of TZR, particularly from lipid-based delivery systems, would be difficult with respect to identification of suitable solvents and stationary phases [5].
Tazarotene is a retinoid prodrug which is converted to its active form, the cognate carboxylic acid of tazarotene (AGN 190299), by rapid deesterification in animals and man. AGN 190299 (“tazarotenic acid”) binds to all three members of the retinoic acid receptor (RAR) family:
RARα, RARβ, and RARγ but shows relative selectivity for RARβ, and RARγ and may modify gene expression. The clinical significance of these findings is unknown.
Psoriasis: The mechanism of tazarotene action in psoriasis is not defined. Topical tazarotene blocks induction of mouse epidermal ornithine decarboxylase (ODC) activity, which is associated with cell proliferation and hyperplasia. In cell culture and in vitro models of skin, tazarotene suppresses expression of MRP8, a marker of inflammation present in the epidermis of psoriasis patients at high levels. In human keratinocyte cultures, it inhibits cornified envelope formation, whose build-up is an element of the psoriatic scale. Tazarotene also induces the expression of a gene which may be a growth suppressor in human keratinocytes and which may inhibit epidermal hyper proliferation in treated plaques. However, the clinical significance of these findings is unknown [6].
Acne: The mechanism of tazarotene action in acne vulgaris is not defined. However, the basis of tazarotene's therapeutic effect in acne may be due to its anti-hyper proliferative, normalizing-ofdifferentiation and anti-inflammatory effects. Tazarotene inhibited corneocyte accumulation in rhino mouse skin and cross-linked envelope formation in cultured human keratinocytes. The clinical significance of these findings is unknown.
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Little parent compound could be detected in the plasma. Tazarotenic acid was highly bound to plasma proteins (>99%). Tazarotene and tazarotenic acid were metabolized to sulfoxides, sulfones and other polar metabolites which were eliminated through urinary and fecal pathways. The halflife of tazarotenic acid was approximately 18 hours, following topical application of tazarotene to normal, acne or psoriatic skin.
The aim of this study was to provide a gel formulation of tazarotene %1.
Materials and Methods
Materials used in this research are: Carbomer 934, poloxamer 407, PEG 400, Phenytoin 80 (polysorbate 80), BHT, NaEDTA, Triethanolamine, Acetonitrile, Ammonium acetate and Tazarotene. The different levels of the gel were made by using Carbomer 934. Then, each of the basic physical properties, consistency, uniformity and stability was evaluated and the best base for the gel formulation was selected. In the next step and during the constructing the amount of tazarotene % is added to the base.
In order to manufacture of base, in addition to the Jelifan (Which is essential for gels manufacturing), the two materials were water and for cleaning and poloxamer 407.
Before entering the active ingredient in the gel base, before entering the active ingredient in the gel base, testing related to gels evaluation was conducted, as below:
Control tests on the base were
• Morphological stability tests
• Measurement of pH
• The test on the cutaneous spreading
• Heat and cold cycle test3
Stability gel centrifugation method
• Adding active ingredient
• Expansion of the ternary diagram in the project area in order to achieve better formulations.
At this stage, the gel re-tested and finally we achieved 14 appropriate formulations. After finding better formulations, it is time to Adding other oxipants. Additional tests on final formulations were:
• Spectrophotometric method to determine the amount Tazarotene.
• Evaluation of Tazarotene releasing from basic gel by using plasma membrane in formulations 65 and 74.
• Study and statistical analysis of the test which results obtained from the process of active ingredients releasing relating to Tazarotene 1/0% gel.
• Study of Rats’ abdominal percutaneous absorption on formulations 65.
• Determination of the viscosity and rheological behavior.
• The test to determine the amount of active ingredient of the Tazarotene gel by using HPLC in formulations 65.
Results
Firstly, in order to ensure the active ingredient, it was proceeded to take FTIR absorption spectrum. After building the bases of all three regions of the diagram was created:
• Two-phase region.
• Non-transparent area.
• Transparent area.
Formulations 2 to 6, 10 to 13, 17 to 20, form non-transparent area on the diagram. This region contains 80-40% of water, 20-60% of poloxamer 407 and 10%- 40% of Carbomer 934. Other formulations form transparent area on the diagram. Formulations 37 to 39, 46 to 48, 54 to 56 and 61 on the diagram of the two-phase form. Gel was formed in this region. Amongst formulations 57, 58, 62 to 64, 67 to 69, 72, 76 to 78, 79, 80 and 81 constitute the opaque region and seen particles in the formulations in the 76 to 81 that show that Carbomer 934 does not disperse well and creates a non-uniform distribution in the formulation.
The overall result is that when low levels of Carbomer 934 higher levels of the used water, the gel is not formed. On the other hand, the percentage of Carbomer 934, reduction in poloxamer 407 and also in the amount of water creates products with high turbidity.
After studying the physical properties of the gels, reached the 14 best formulation. Due to the similarity of approximate amount of the Carbomer 934 we attempted to make 3 formulations 49, 65 and 74.
The results of thermal stability
In all three formulations (during 6 months of storage at a temperature of 4, 25 and 40), no change to production phase, change in the color and consistency it was created (Table 1).
| Formulations No. | Carbomer 934 (%) | Poloramer | PEG 400 (%) | Tween 80 (%) | BHT (%) | Na EDTA (%) | Tazarotene | Water (%) |
|---|---|---|---|---|---|---|---|---|
| 49 | 0.45 | 0.25 | 5 | 0. 5 | 0.1 | 0.1 | 0.1 | 93.35 |
| 65 | 0.55 | 0.30 | 5 | 0.5 | 0.1 | 0.1 | 0.1 | 93.35 |
| 74 | 0.65 | 0.20 | 5 | 0.5 | 0.1 | 0.1 | 0.1 | 93.35 |
Table 1: The ratio of materials used in three final formulation.
The results of physical characteristics, ethnic, pH, and percutaneous spreading (Table 2).
| Formulation No. | Clarity | Viscosity | Spreading | PH |
|---|---|---|---|---|
| 49 | +++ | ++ | +++ | 7.94 |
| 65 | +++ | +++ | +++ | 6.76 |
| 74 | +++ | +++ | +++ | 6.72 |
Table 2: Compare the morphological characteristics in formulations 49, 65 and 74.
As seen in the above table, all three formulations are suitable in terms of transparency. However, in terms of consistency, the consistency of the formula 49 is lower than the other two formulations, due to the less of the Carbomer in the base. In terms of pH of formulations 65 and 74, pH has more compatibility with the skin.
The results of stability tests by centrifugation
In centrifugal testing on samples after 15 minutes, no change was observed among phase. The results of the tests which determine the amount of active ingredient in the formulations 65 and 74 by using spectrophotometry.
Diagramming standard
The results of the standard diagramming are as follows (Table 3):
| Concentration (µg/ml) | UV Abs1 | UV Abs2 | UV Abs3 | Mean ± SD |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 1 | 0.118 | 0.122 | 0.12 ± 1.0 | 0.002 ± 12.0 |
| 2 | 0.24 | 0.234 | 0.246 | 24.006 ± 0.0 |
| 3 | 0.398 | 0.4 | 0.402 | 40.002 ± 0.0 |
| 4 | 0.503 | 0.537 | 0.52 | 52.017 ± 0.0 |
| 5 | 0.606 | 0.594 | 0.6 | 6.006 ± 0.0 |
| 6 | 0.745 | 0.774 | 0.76 | 76.014 ± 0.0 |
| 7 | 0.84 | 0.892 | 0.787 | 84.053 ± 0.0 |
| 8 | 0.96 | 0.982 | 0.938 | 96.022 ± 0.0 |
| 9 | 1.12 | 1.099 | 0.141 | 12.21 ± 1.0 |
Table 3: Absorbed Tazarotene in the concentrations of methanol at a wavelength of 351nm, (n=3).
Tazarotene calibration curve based on the values of absorption are as follows:
Y= 0.120x + 0.0047
Correlation coefficient = 0.9996 (Tables 4 and 5)
| Parameters (Units) | Values Tazarotene | |
|---|---|---|
| λmax/nm | 351nm | |
| Linearity Range (µg/ml) | 1.9-0.0 | |
| Molar Absorptivity (1/mol/cm) | 0.00284×103 | |
| Correlation coefficient(r2) | 9996/0 1.9-0.0 | |
| Regression equation (y) | 0.12 x+0.0047 | |
| Intercept, c | 0.0047 | |
| Slope, b | 0.120 | |
| LOD (µg/ml) | 0.106 | |
| LOC (µg/ml) | 0.324-0.0 |
Table 4: Optical parameters.
| Formulation No | Absorbance | Con (µg/ml) |
|---|---|---|
| 65 | 0.974 | 67.31 |
| 74 | 0.968 | 66.89 |
Table 5: Comparing the concentration of active ingredient in the formulation of 65 and 74.
Statistical analysis of the results of release of active ingredient from Tazarotene gel 1/0%
The results of the liberalization process of the gel formulations 65 and 74 were analyzed by Paired- samples T test.
In comparison between the two formulations, there is a significant difference (P<0/05), that the rate of release formulation of 65% 13/1 is equal to the amount of release of the formulation 74.
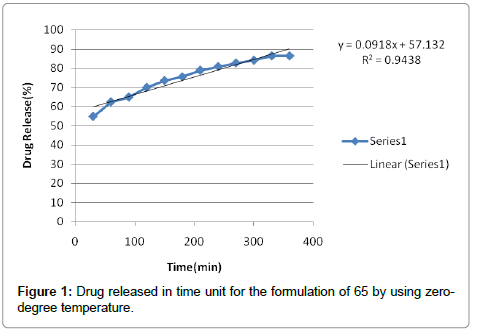
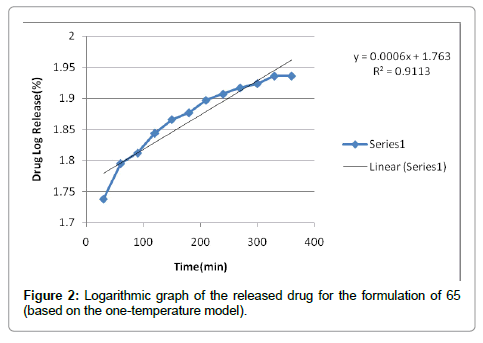
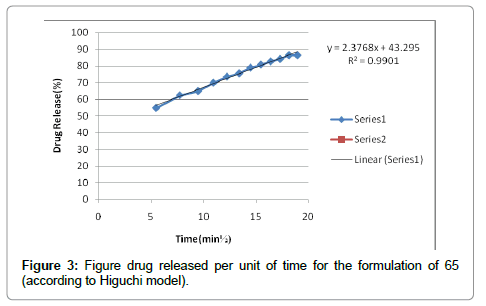
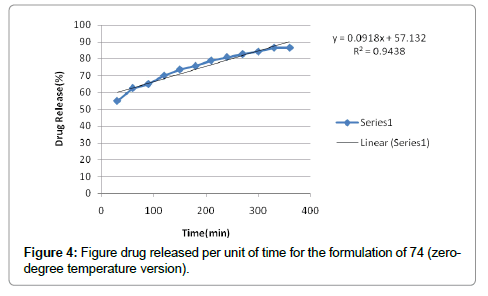
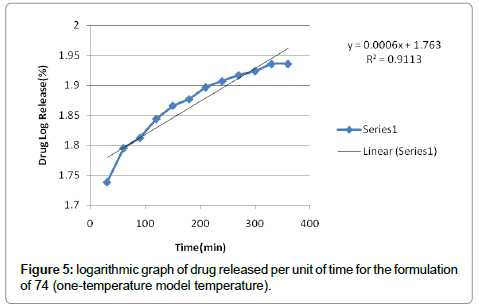
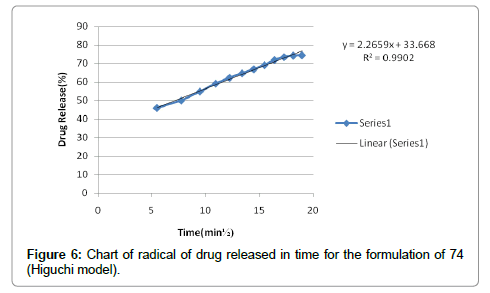
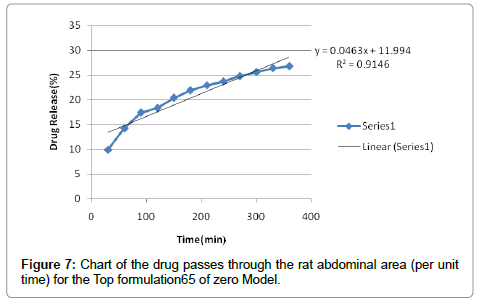
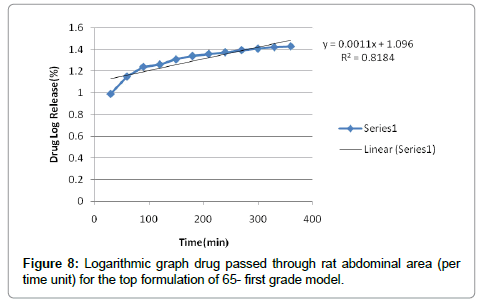
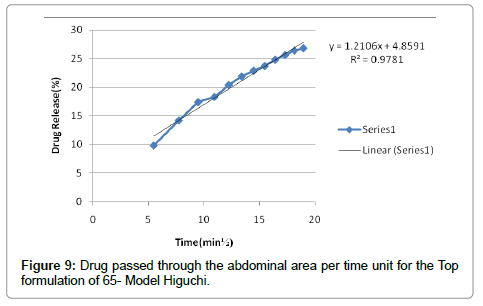
According to the results, cutaneous drug passage after 6 hours is equal to 8/26% and for per unit of skin is at a rate of 93/4% respectively. According to the results of statistical analysis for better comparison, the kinetic model was drawn up by models of: zero, one and Higuchi (Figures 1-6 and Table 6).
| Model of release | R | |
|---|---|---|
| Zero order | 0/9714 | |
| First order | 0/9546 | |
| Higuchi | 0/9950 |
Table 6: The results of the drug release pattern of plasma membrane in Formulation 65.
According to the test results release formulation, formulation 65 was selected as the chosen formulation. Based on regression analysis, charts followed Higuchi model, because its regression to the nearest mode (Figures 7-9).
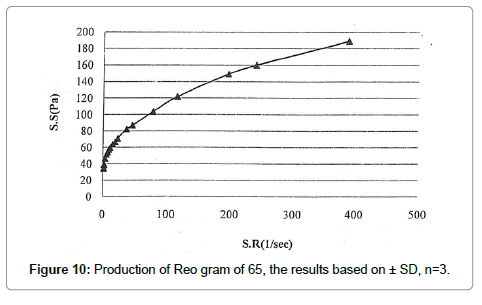
Results of viscosity and rheological behavior The results of the test to determine the amount of active ingredient in the formulation of 65 by HPLC (Table 7 and Figure 10)
| S.R(sec-1) | S.S(Pa)-Up curve |
|---|---|
| 1.84 | 34.1 ± 0.1 |
| 1.95 | 38.3 ± 0.5 |
| 2.33 | 47.24 ± 0.7 |
| 3.87 | 48.1 ± 0.4 |
| 5.79 | 51.33 ± 0.5 |
| 7.71 | 54.08 ± 0.3 |
| 9.63 | 57.27 ± 0.3 |
| 11.56 | 59.39 ± 0.6 |
| 15.43 | 63.71 ± 0.1 |
| 19.23 | 66.28 ± 0.1 |
| 23.46 | 70.76 ± 0.4 |
| 37.12 | 82.32 ± 0.3 |
| 46.15 | 86.72 ± 0.2 |
| 78.51 | 103.4 ± 0.4 |
| 116 | 121.67 ± 0.4 |
| 196 | 149.12 ± 0.2 |
| 240 | 159.90 ± 0.5 |
| 389 | 189.10 ± 0.3 |
Table 7: Results of the application of SR on the formulation 65. Results based on mean ± SD (n=3).
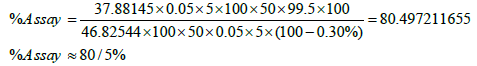
Discussion and Conclusion
Tazarotene a retinoid drug that is already active form of D-esterification quickly becomes tazarotenic acid (which can be connected to all three of the retinoic acid receptors: RARα, RARβ, RARγ). It also selectively responds binds to the β and γ receptors.
References
- Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD (2010) Photoaging: prevention and topical treatments. Am J Clin Dermatol 11:95-102.
- Guenther LC (2002) Topical tazarotene therapy for psoriasis, acne vulgaris, and photoaging. Skin Therapy Letter 7:1-4.
- Bershad S, Singer GK, Parente JE, Tan MH, Sherer DW, et al. (2002) Successful treatment of acne vulgaris using a new method: results of a randomized vehicle-controlled trial of short-contact therapy with 0.1% tazarotene gel. Arch Dermatol 138:481-489.
- Decken A, Mailman A, Mattar SM, Passmore J (2005) Evolution of the pseudo-1,3-dipolar cycloaddition chemistry of SNSMF6 (M=As, Sb) leading to 2,5-dihydroxybenzo-1,3,2-dithiazolylium and 2,7-dicarbonylnaphtha-1,3,2-dithiazolylium salts and their corresponding radicals. Chemical Communications (Cambridge, United Kingdom), pp:2366-2268.
- Jaratt M, Werner CP, Saenz AB (2013) Tazarotene foam versus tazarotene gel: A randomized relative bioavailability study in acne vulgaris. Clin Drug Invest 33:283-289.
- Thielitz A, Gollnick H (2008) Topical retinoids in acne vulgaris: A Systematic Review. Am J Clin Dermtol 9:369-381.
Citation: Taheri T, Mortazavi SA, Siaghi AD (2020) Formulation of the Tazarotene Cutaneous Gel and Its Physicochemical Characteristics. Clin Pharmacol Biopharm 9: 194. DOI: 10.4172/2167-065X.1000194
Copyright: © 2020 Taheri T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3908
- [From(publication date): 0-2020 - Nov 04, 2025]
- Breakdown by view type
- HTML page views: 2999
- PDF downloads: 909