Formulation and Evaluation of Paliperidone Floating Tablets
Received: 20-Sep-2022 / Manuscript No. ijrdpl-22-76178 / Editor assigned: 22-Sep-2022 / PreQC No. ijrdpl-22-76178 / Reviewed: 06-Sep-2022 / QC No. ijrdpl-22-76178 / Revised: 10-Sep-2022 / Manuscript No. ijrdpl-22-76178 / Accepted Date: 16-Oct-2022 / Published Date: 17-Oct-2022 QI No. / ijrdpl-22-76178
Abstract
The aim of present work was to develop and evaluate the floating tablets of Paliperidone. The effect of polymer concentration and type of polymer were examined. The floating drug delivery system (tablets) was prepared by direct compression method using Gum Copal, Isapgol husk and Fenugreek extract as polymer and sodium bicarbonates as gas generating agent. All formulations were evaluated for the pre compression and post compression, In vitro buoyancy and In vitro dissolution studies. Pre-compression studies revealed that there was no sign of any interaction between drug and polymers and all formulation showed good flow properties. Results of post compression parameters were found within the limits for all formulations. Among all the formulation F6 showed better buoyancy and drug release profile. The release of drug from the prepared formulations (F6) was found to follow zero order. It was concluded that drug release rate was increased as the concentration of polymer decreased. Isapgol husk showed greater drug release rate as compared to Gum Copal and Fenugreek extract.
Keywords
Paliperidone; Gum Copal; Isapgol husk; Fenugreek extract; Buoyancy; In-vitro evaluation and floating drug delivery system
Introduction
Oral controlled release drug delivery have recently been of increasing interest in pharmaceutical field to achieve improved therapeutic advantages, such as ease of dosing administration, patient compliance and flexibility in formulation. Drugs that are easily absorbed from gastrointestinal tract (GIT) and have short half-lives are eliminated quickly from the systemic circulation. Frequent dosing of these drugs is required to achieve suitable therapeutic activity. After oral administration, such a drug delivery would be retained in the stomach and release the drug in a controlled manner, so that the drug could be supplied continuously to its absorption sites in the gastrointestinal tract (GIT) [1] .Prolonged gastric retention improves bioavailability, increases the duration of drug release, reduces drug waste, and improves the drug solubility that are less soluble in a high pH environment [2] Gastroretentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects. Gastroretentive dosage forms can remain in the gastric region for long periods and hence significantly prolong the gastric retention time (GRT) of drugs. Over the last few decades, several gastroretentive drug delivery approaches being designed and developed, including: high density (sinking) systems that is retained in the bottom of the stomach [3] , low density (floating) systems that causes buoyancy in gastric fluid [4-6], mucoadhesive systems that causes bioadhesion to stomach mucosa [7], unfoldable, extendible, or swellable systems which limits emptying of the dosage forms through the pyloric sphincter of stomach [8,9] ,superporous hydrogel systems [10] magnetic systems [11] etc. The current review deals with floating type gastroretentine drug delivery system.
Basic gastrointestinal tract physiology
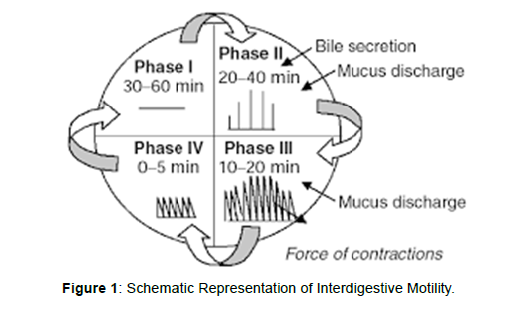
The stomach is divided into 3 regions anatomically: fundus, body, and antrum pylorus. The proximal part is the fundus and the body acts as a reservoir for undigested material, where as the antrum is the main site for mixing motions and acts as a pump for gastric emptying by propelling actions. Gastric emptying occurs during fasting as well as fed states but the pattern of motility is distinct in the 2 states. During the fasting state an interdigestive series of electrical events take place, which cycle through both stomach and intestine every 27 to 3 hours. This is called the interdigestive myloelectric cycle or migrating myloelectric cycle (MMC), which is divided into following 4 phases [12]( Figure 1).
Phase I: This period lasts about 30 to 60 minutes with no contractions.
Phase II: This period consists of intermittent contractions that increase gradually in intensity as the phase progresses, and it lasts about 20 to 40 minutes. Gastric discharge of fluid and very small particles begins later in this phase.
Phase III: This is a short period of intense distal and proximal gastric contractions (4-5 contractions per minute) lasting about 10 to 20 minutes these contractions, also known as ‘‘house-keeper wave,’’ sweep gastric contents down the small Intestine.
Phase IV: This is a short transitory period of about 0 to 5 minutes, and the contractions dissipate between the last part of phase III and quiescence of phase
Need For Gastroretention:
• Drugs that are absorbed from the proximal part of the gastrointestinal tract (GIT).
• Drugs that are less soluble or that degrade at the alkaline pH.
• Drugs that are absorbed due to variable gastric emptying time.
• Local or sustained drug delivery to the stomach and proximal small intestine to treat certain conditions.
• Particularly useful for the treatment of peptic ulcers caused by H.Pylori infections [12].
Factors controlling gastric retention of dosage forms:
There are several factors that can affect gastric emptying of an oral dosage form which include density, size and shape of dosage form, feeding state, biological factors such as age, gender, posture, body mass index, disease state etc.
Effect Of Dosage Form Size & Shape
Small size tablets are emptied from the stomach during the digestive phase while large size units are expelled during the house keeping waves found that floating unit with a diameter equal or less than 7.5 mm had larger gastric residence time (GRT) compared to nonfloating units but the GRT was similar for floating and non-floating units having a large diameter of 9.9 mm. They found that GRT of nonfloating units were much more variable and highly dependent on their size which are in the order of small < medium < large units. Moreover, in supine subjects, size influences GRT of floating and non- floating form. Tetrahedron and ring shaped devices have a better GRT as compared with other shapes [13,14].
Density:
The density of a dosage form also affects the gastric emptying rate and determines the location of the system in the stomach. Dosage forms having a density lower than the gastric contents can float to the surface, while high density systems sink to bottom of the stomach [16]. Both positions may isolate the dosage system from the pylorus. A density of < 1.0 gm/ cm3 is required to exhibit floating property [15].
Gender, Posture & Age:
Generally females have slower gastric emptying rates than male. The effect of posture does not have any significant difference in the mean gastric retention time (GRT) for individuals in upright, ambulatory and supine state. In case of elderly persons, gastric emptying is slowed down [16].
Effect of Food & Specific Gravity:
To float FDDS in the stomach, the density of dosage form should be less than gastric content i.e.1.0 g/cm3. Since, the bulk density of a dosage form is not a sole measure to describe its buoyant capabilities because the magnitude of floating strength may vary as a function of time and gradually decrease after immersing dosage form into fluid as a result of development of its hydrodynamic equilibrium. Various studies have shown the intake of food as main determinant of gastric emptying rather than food. Presence of food is the most important factor effecting GRT than buoyancy. GRT is significantly increased under fed condition since onset of MMC is delayed. Studies show that GRT for both floating and non-floating single unit are shorter in fasted subjects (less than 2 hour), but significantly prolonged after meal (around 4 hour) [12].
Natures of meal & frequency of food
Feeding of indigestible polymers or fatty acid salts can change the motility pattern of the stomach to fed state, to increase gastric emptying rate and prolonging the drug release. Diet rich in protein and fat can increase GRT by 4-10 hours [12].
Type of formulation
Multiple unit formulation show a more predictable release profile and insignificant impairing of performance due to failure of units, allow co-administration of units with different release profile or containing incompatible substances and permit a large margin of safety against dosage form failure compared with single unit dosage form [13].
Future potential
Floating dosage form offers various future potential as evident from several recent publications. The reduced fluctuations in the plasma level of drug results from delayed gastric emptying. Drugs that have poor bioavailibility because of their limited absorption to the upper gastrointestinal tract can be delivered efficiently thereby maximizing their absorption and improving their absolute bioavalability.
Buoyant delivery system considered as a beneficial strategy for the treatment of gastric and duodenal cancers.
• The floating concept can also be utilized in the development of various anti-reflux formulations.
• Developing a controlled release system for the drugs, which are potential to treat the Parkinson’s disease.
• To explore the eradication of Halico-bector pylori by using the narrow spectrum antibodies [14].
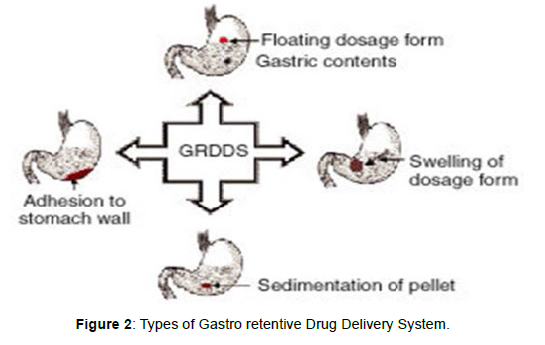
Classification Of Gastro retentive Drug Delivery System
The main approaches that have been examined for gastroretentive dosage forms (GRDFs) are: low density of GRDF that cause buoyancy above gastric fluid (Floating system), high density which retain the dosage form in the body of stomach, concomitant administration of drugs or excipients which slow the motility of the GIT, bioadhesion to gastric mucosa, swelling to a large size which prevents emptying of dosage form through the pyloric sphincter [17, 18] (Figure 2).
Floating drug delivery systems
A floating dosage form is useful for drugs acting locally in the proximal gastrointestinal tract. These systems are also useful for drugs that are poorly soluble (or) unstable in intestinal fluids. The floating properties of these systems help to retain in the stomach for a long time. Various attempts have been made to develop floating systems, which float on the gastric contents and release drug molecules for the desired time period. After the release of a drug, the remnants of the system are emptied from the stomach. Based on the mechanism of buoyancy, two different technologies have been used in development of floating drug delivery systems.
These include:
A) Effervescent system.
B) Non- Effervescent system.
A) Effervescent Systems:
Effervescent systems10 include use of gas generating agents, carbonates (e.g. Sodium bicarbonate) and other organic acid (e.g. citric acid and tartaric acid) present in the formulation to produce carbon dioxide (CO2) gas, thus reducing the density of the system and making it float on the gastric fluid. An alternative is the incorporation of matrix containing portion of liquid, which produce gas that evaporate at body temperature. These effervescent systems further classified into two types:
• Gas generating systems.
• Volatile liquid or vacuum containing systems. Gas Generating Systems:
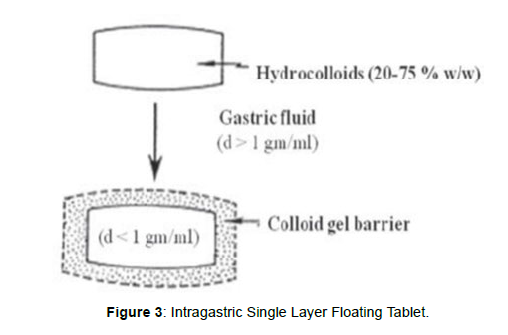
Tablets Intragastric Single layer Floating Tablets or Hydrodynamically Balanced System (HBS)
These formulations have bulk density lower than gastric fluids and thus float in the stomach that increases the gastric emptying rate for a prolonged period, [19] (Figure3). These are formulated by intimately mixing the gas (CO2) generating agents and the drug within the matrix tablet. The drug is released slowly at a desired rate from the floating system and the residual system is emptied from the stomach after the complete release of the drug. This leads to an increase in the gastric residence time (GRT) and a better control over fluctuations in plasma drug concentration Figure 3.
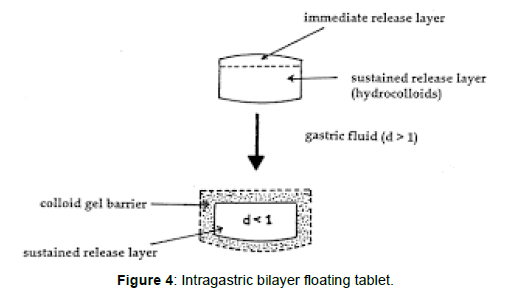
Intragastric Bilayer Floating Tablets These are also compressed tablets [19] containing two layers (Figure 4).
• Immediate release layer and
• Sustained release layer.
Floating capsules
These floating capsules 20 are formulated by filling with a mixture of sodium alginate and sodium bicarbonate. The systems float as a result of the generation of CO2 that was trapped in the hydrating gel network on exposure to an acidic environment.
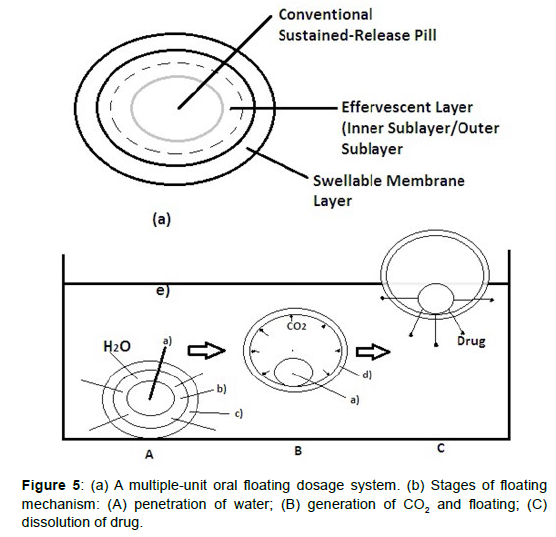
Multiple unit type floating pills
These multiple unit type floating pills20are sustained release pills, known as seeds, which are surrounded by two layers (Figure 5). The outer layer is of swellable membrane layer while the inner layer consists of effervescent agents. This system sinks at once and then it forms swollen pills like balloons which float as they have lower density, when it is immersed in the dissolution medium at body temperature. The lower density is due to generation and entrapment of CO2 within the system. Key: (a) conventional SR pills; (b) effervescent layer; (c) swellable layer; (d) expanded swellable membrane layer; (e) surface of water in the beaker (370C) (Figure 5).
Floating system with ion-exchange resins
Floating system using bicarbonate loaded ion exchange resin was made by mixing the beads with 1M sodium bicarbonate solution, and then the semi-permeable membrane is used to surround the loaded beads to avoid sudden loss of CO2. On contact with gastric contents an exchange of bicarbonate and chloride ions takes place that results in generation of CO2 that carries beads towards the top of gastric contents and producing a floating layer of resin beads [20].
Volatile liquid or Vacuum Containing Systems
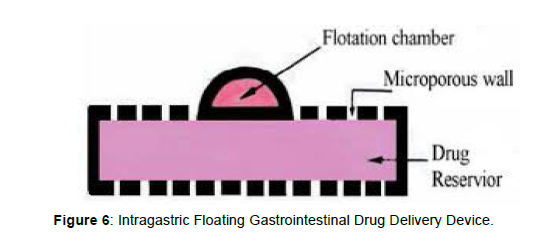
Intragastric Floating Gastrointestinal Drug Delivery System
This system floats in the stomach because of floatation chamber, which is vacuum or filled with a harmless gas or air, while the drug reservoir is encapsulated by a micro porous compartment, [19] (Figure 6).
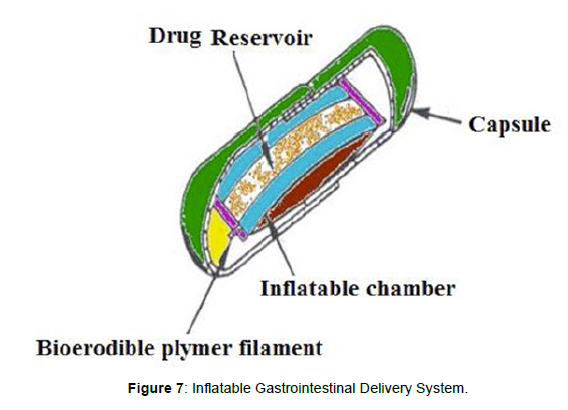
Inflatable gastrointestinal delivery systems
Inflatable chamber are incorporated, which contains liquid ether that gasifies at body temperature to inflate the chamber in the stomach. These systems are fabricated by loading the inflatable chamber with a drug reservoir, which can be a drug, impregnated polymeric matrix, then encapsulated in a gelatin capsule, [19] (Figure 7). After oral administration, the capsule dissolves to release the drug reservoir together with the inflatable chamber. The inflatable chamber automatically inflates and retains the drug reservoir compartment in the stomach. The drug is released continuously from the reservoir into gastric fluid (Figure 7).
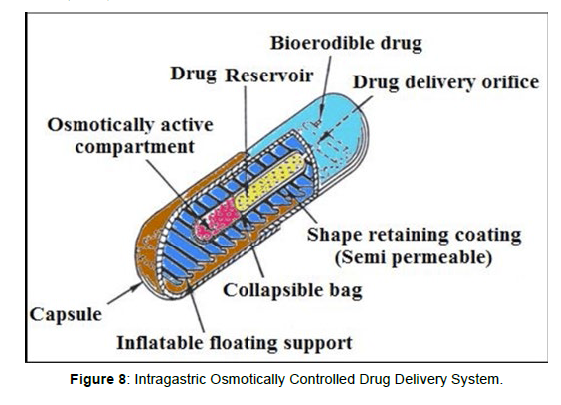
Intragastric osmotically controlled drug delivery system
It is comprised of an osmotic pressure controlled drug delivery device and an inflatable floating support in a biodegradable capsule. In the stomach, the capsule quickly disintegrates to release the intragastirc osmotically controlled drug delivery device. The inflatable support inside forms a deformable hollow polymeric bag that contains a liquid that gasifies at body temperature to inflate the bag. The osmotic pressure controlled drug delivery device consists of two components; drug reservoir compartment and an osmotically active compartment. The floating support is also made to contain a bioerodible plug that erodes after a predetermined time to deflate the support. The deflated drug delivery system is then emptied from the stomach (Figure 8).
Non-effervescent systems
Non-effervescent floating drug delivery systems are normally prepared from gel-forming or highly swellable cellulose type hydrocolloids, polysaccharides or matrix forming polymers like polyacrylate, polycarbonate, polystyrene and polymethacrylate. In one approach, intimate mixing of drug with a gel forming hydrocolloid which results in contact with gastric fluid after oral administration and maintain a relative integrity of shape and a bulk density less than unity within the gastric environment [21]. The air trapped by the swollen polymer confers buoyancy to these dosage forms. Excipients used most commonly in these systems include hydroxypropyl methylcellulose (HPMC) polyacrylates, polyvinyl acetate, carbopol, agar, sodium alginate, calcium chloride, polyethylene oxide and polycarbonates [22]. This system can be further divided into the sub-types:
Single layer floating tablets
These are formulated by intimate mixing of drug with a gel forming hydrocolloid, that swells on contact with gastric fluid and maintain bulk density of less than unity. The air trapped by the swollen polymer confers buoyancy to these dosage forms [19].
Bilayer floating tablets
A bilayer tablet contain two layer one immediate release layer which release initial dose from system while the another sustained release layer absorbs gastric fluid, forming an impermeable colloidal gel barrier on its surface, and maintain a bulk density of less than unity and thereby it remains buoyant in the stomach [19].
Alginate Beads:
Talukdar and Fassihi [32] recently developed a multiple-unit floating system based on cross-linked beads. They were made by using Ca++ and low methoxylated pectin (anionic polysaccharide) or Ca2+ low methoxylated pectin and sodium alginate. In this approach, generally sodium alginate solution is dropped into aqueous solution of calcium chloride and causes the precipitation of calcium alginate. These beads are then separated and dried by air convection and freeze drying, leading to the formulation of a porous system, which can maintain a floating force for over 12 hrs. These beads improve gastric retention time (GRT) more than 5.5 hrs [22- 24].
Hollow microspheres:
Hollow microspheres (microballons), loaded with drug in their outer polymer shells were prepared by a novel emulsion-solvent diffusion method (Figure 9). The ethanol: dichloromethane solution of the drug and an enteric acrylic polymer was poured into an agitated aqueous solution of PVA that was thermally controlled at 400C. The gas phase generated in dispersed polymer droplet by evaporation of dichloromethane formed an internal cavity in microsphere of polymer with drug. The microballons floated continuously over the surface of acidic dissolution media containing surfactant for more than 12 hours in vitro [19] (Figure 9).
Advantages of Floating Drug Delivery System
For any particular medicament or class of medicament hydrodynamically balanced system can be used. It is not restricted to medicaments, which are principally absorbed from the stomach. The HBS are advantageous for drugs absorbed through the stomach e.g. ferrous salts and for drugs meant for local action in the stomach and treatment of peptic ulcer disease e.g. antacids. Medicaments administered barrier on its surface, and maintain a bulk density of less than unity and thereby it remains buoyant in the stomach [19].
Alginate beads
Talukdar and Fassihi [32] recently developed a multiple-unit floating system based on cross-linked beads. They were made by using Ca++ and low methoxylated pectin (anionic polysaccharide) or Ca2+ low methoxylated pectin and sodium alginate. In this approach, generally sodium alginate solution is dropped into aqueous solution of calcium chloride and causes the precipitation of calcium alginate. These beads are then separated and dried by air convection and freeze drying, leading to the formulation of a porous system, which can maintain a floating force for over 12 hrs. These beads improve gastric retention time (GRT) more than 5.5 hrs [22, 23].
Hollow microspheres:
Hollow microspheres (microballons), loaded with drug in their outer polymer shells were prepared by a novel emulsion-solvent diffusion method (Figure 9). The ethanol: dichloromethane solution of the drug and an enteric acrylic polymer was poured into an agitated aqueous solution of PVA that was thermally controlled at 400C. The gas phase generated in dispersed polymer droplet by evaporation of dichloromethane formed an internal cavity in microsphere of polymer with drug. The microballons floated continuously over the surface of acidic dissolution media containing surfactant for more than 12 hours in vitro [19].
Advantages of floating drug delivery system:
For any particular medicament or class of medicament hydrodynamically balanced system can be used. It is not restricted to medicaments, which are principally absorbed from the stomach.The HBS are advantageous for drugs absorbed through the stomach e.g.
ferrous salts and for drugs meant for local action in the stomach and treatment of peptic ulcer disease e.g. antacids [26-28]. Medicaments administered Drugs insoluble in gastric fluid
• Causes G.I irritation
• Inefficient in acidic environment
• Drugs proposed for selective release in the colon.
• Unpredictable adherence owing to state of constant renewal of mucus wall of stomach.
Factors Controlling GRDDS:
Density: Dosage form with lower density can float to [25, 29-31] the surface in the gastric content. Suitable density required for floating property is less than 1.0 gm/ cm3.
Size: Size should be more than 7.5 mm in diameter.
Shape: Either round or spherical shaped dosage form exhibit better property than other shapes.
Single or multiple unit formulation: Due to predetermined release profile multiple units are desirable.
Fed or Unfed State: Gastric retention time is less during fasting condition due to rise in gastric motility.
Nature of Meal: High amount of fatty acid and other indigestible polymers slow down the gastric retention time due to variation in gastric motility.
Frequency of Feed: Low frequency of migrating myoelectric complex (MMC) contributes to GRT up to 400 times which in turn depends on the frequency of food intake.
Caloric Content: A high protein and fat rich diet can increase GRT by 4 to 10h.
Gender: Males have greater GRT than females.
Age: GRT is more in geriatric patients and less in neonates and children. Age above 70 (>70) exhibit longer GRT.
Posture: GRT can vary between supine and upright ambulatory states of the patient.
Disease State: Gastric disease such as diabetes, chron’s disease, hypothyroidism, hyperthyroidism, duodenal ulcers etc fluctuate the GRT.
Concomitant Intake of Drug: Combination of some drugs along with gastric motility enhancers or depressants, affect GRT.
Future Aspects
Gastro-retentive floating dosage form provides various future potential. Delayed gastric emptying time results in the decreased fluctuations in the plasma level of drug. Drugs that have poor bioavailability because of their limited absorption to [32, 33] the upper gastrointestinal tract can be delivered efficiently thereby maximizing their absorption and improving their absolute bioavailability. Buoyant delivery system considered as an advantageous approach for the treatment of gastric and duodenal cancers. The floating concept can also be utilized in the development of various antireflux formulations. Developing a controlled release system for the drugs, which are potential to treat the Parkinson’s disease. This system can also be useful to discover the eradication of Helicobacter pylori by using the narrow spectrum antibodies. Day after day the FDDS shows more promise for a bright future.
Therapeutic efficacy/ Indications
For the treatment of schizophrenia.
Contraindications:
Conditions:
• Breast cancer
• Diabetes
• Increased prolactin in the blood
• Excessive fat in the blood
• Low amount of magnesium in the blood
• Extreme loss of body water
• Low amount of potassium in the blood
• Overweight
Interactions:
Drug interactions:
• The risk or severity of adverse effects can be increased when Paliperidone is combined with Aceprometazine.
• The serum concentration of Acetylsalicylic acid can be increased when it is combined with Paliperidone.
• Amikacin may decrease the excretion rate of Paliperidone which could result in a higher serum level.
• The risk or severity of adverse effects can be increased when Aripiprazole is combined with Paliperidone.
Food interactions
• Avoid alcohol.
Drug Formulation
Methodology
Analytical method development:
a) Determination of absorption maxima
A solution containing the concentration 10 μg/ mL drug was prepared in 0.1N HCL UV spectrum was taken using Double beam UV/VIS spectrophotometer. The solution was scanned in the range of 200 – 400 nm.
b) Preparation calibration curve
10mg Paliperidone pure drug was dissolved in 10ml of methanol (stock solution1) from stock solution 1ml of solution was taken and made (Table 1) up with10ml of 0.1N HCL (100μg/ml). From this 1ml was taken and made up with 10 ml of 0.1N HCL (10μg/ml). The above solution was subsequently diluted with 0.1N HCL to obtain series of dilutions Containing 5, 10, 15, 20, 25μg /ml of per ml of solution. The absorbance of the above dilutions was measured at 284 nm by using UV-Spectrophotometer taking 0.1N HCL as blank. Then a graph was plotted by taking Concentration on X-Axis and Absorbance on Y-Axis which gives a straight line Linearity of standard curve was assessed from the square of correlation coefficient (R2) which determined by least-square linear regression analysis.
| S.No | Drug name | Label Claim | Brand name | Company |
|---|---|---|---|---|
| 1 | Paliperidone | 9mg | Invega | Janssen Cilag International Nv |
Table1 : Drug formulation.
Preformulation parameters
The quality of tablet, once formulated by rule, is generally dictated by the quality of physicochemical properties of blends. There are many formulations and process variables involved in mixing and all these can affect the characteristics of blends produced. The various characteristics of blends tested as per Pharmacopoeia.
Angle of repose
The frictional force in a loose powder can be measured by the angle of repose. It is defined as, the maximum angle possible between the surface of the pile of the powder and the horizontal plane. If more powder is added to the pile, it slides down the sides of the pile until the mutual friction of the particles producing a surface angle, is in equilibrium with the gravitational force. The fixed funnel method was employed to measure the angle of repose. A funnel was secured with its tip at a given height (h), above a graph paper that is placed on a flat horizontal surface. The blend was carefully pored through the funnel until the apex of the conical pile just touches the tip of the funnel. The radius (r) of the base Table 2 of the conical pile was measured. The angle of repose was calculated using the following formula:
Angle of Repose |
Nature of Flow |
|---|---|
| <25 | Excellent |
| 25-30 | Good |
| 30-40 | Passable |
| >40 | Very poor |
Table 2: Angle of Repose values (as per USP).
Tan θ = h / r
Tan θ = Angle of repose
h = Height of the cone, r = Radius of the cone base
Bulk density
Density is defined as weight per unit volume. Bulk density, is defined as the mass of the powder divided by the bulk volume and is expressed as gm/cm3. The bulk density of a powder primarily depends on particle size distribution, particle shape and the tendency of particles to adhere together. Bulk density is very important in the size of containers needed for handling, shipping, and storage of raw material and blend. It is also important in size blending equipment. 10 gm powder blend was sieved and introduced into a dry 20 ml cylinder, without compacting. The powder was carefully leveled without compacting and the unsettled apparent volume, Vo, was read.
The bulk density was calculated using the formula:
Bulk Density = M / Vo
Where, M = weight of sample, Vo = apparent volume of powder
Tapped density
After carrying out the procedure as given in the measurement of bulk density the cylinder containing the sample was tapped using a suitable mechanical tapped density tester that provides 100 drops per minute and this was repeated until difference between succeeding measurement is less than 2 % and then tapped volume, V measured, to the nearest graduated unit. The tapped density was calculated, in gm per L, using the formula:
Tap = M / V
Where, Tap= Tapped Density
M = Weight of sample
V= Tapped volume of powder
Measures of powder compressibility
The Compressibility Index (Carr’s Index) is a measure of the propensity of a powder to be compressed. It is determined from the bulk and tapped densities. In theory, the less compressible a material the more flowable it is. As such, it is measures of the relative importance of inter particulate interactions. In a free- flowing powder, such interactions are generally less significant, and the bulk and tapped densities will be closer in value. For poorer flowing materials, there are frequently greater inter particle interactions, and a greater difference between the bulk and tapped Table 3 densities will be observed. These differences are reflected in the Compressibility Index which is calculated using the following formulas:
Carr’s index |
Properties |
|---|---|
| 5 – 15 | Excellent |
| 12 – 16 | Good |
| 18 – 21 | Fair to Passable |
| 2 – 35 | Poor |
| 33 – 38 | Very Poor |
| >40 | Very Very Poor |
Table 3:Carr’s index value (as per USP)
Carr’s Index = [(tap - b) / tap] × 100
Where, b = Bulk Density
Tap = Tapped Density
Formulation development of floating Tablets
Procedure for direct compression method
• Drug and all other ingredients were individually passed through sieve no ≠ 60.
• All the ingredients were mixed thoroughly by triturating up to 15 min.
• The powder mixture was lubricated with talc.
• The tablets were prepared by using direct compression method by using 6mm punch.
Formulation of Tablets
All the quantities were in mg (Table 4)
Ingredients |
Formulation Code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (MG) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| Paliperidone | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Gum Copal | 6 | 12 | 18 | - | - | - | - | - | - |
| Isapgol husk | - | - | - | 6 | 12 | 18 | - | - | - |
| Fenugreek extract | - | - | - | - | - | - | 6 | 12 | 18 |
| Citric acid | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Sodium bicarbonate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Micro crystalline cellulose | Q.S | Q.S | Q.S | Q.S | Q.S | Q.S | Q.S | Q.S | Q.S |
| Magnesium Stearate | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Talc | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Total Weight | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 4: Formulation composition for Floating tablets.
Evaluation of post compression parameters for prepared Tablets
The designed compression tablets were studied for their physicochemical properties like weight variation, hardness, thickness, friability and drug content.
Weight variation test
To study the weight variation, twenty tablets were taken and their weight was determined individually and collectively on a digital weighing balance. The average weight of one tablet was determined from the collective weight. The weight variation test would be a satisfactory method of deter mining the drug content uniformity. Not more than two of the individual weights deviate from the average weight by more than the percentage shown in the following table and none deviate by more than twice the percentage. The mean and deviation were determined. The percent deviation was calculated using the following formula.
% Deviation = (Individual weight – Average weight / Average weight) × 100 (Table 5)
Average weight of tablet (mg) (I.P) |
Average weight of tablet (mg) (U.S.P) | Maximum percentage difference allowed |
|---|---|---|
| Less than 80 | Less than 130 | 10 |
| 80-250 | 130-324 | 7.5 |
| More than | More than 324 | 5 |
Table 5: Pharmacopoeial specifications for tablet weight variation.
Hardness
Hardness of tablet is defined as the force applied across the diameter of the tablet in order to break the tablet. The resistance of the tablet to chipping, abrasion or breakage under condition of storage transformation and handling before usage depends on its hardness. For each formulation, the hardness of three tablets was determined using Monsanto hardness tester and the average is calculated and presented with deviation.
Thickness
Tablet thickness is an important characteristic in reproducing appearance. Tablet thickness is an important characteristic in reproducing appearance. Average thickness for core and coated tablets is calculated and presented with deviation.
Friability
It is measured of mechanical strength of tablets. Roche friabilator was used to determine the friability by following procedure. Pre weighed tablets were placed in the friabilator. The tablets were rotated at 25 rpm for 4 minutes (100 rotations). At the end of test, the tablets were re- weighed, and loss in the weight of tablet is the measure of friability and is expressed in percentage as
% Friability = [( W1-W2) / W1] × 100
Where, W1 = Initial weight of tablets
W2 = Weight of the tablets after testing
Determination of drug content
Both compression-coated tablets of were tested for their drug content. Ten tablets were finely powdered quantities of the powder equivalent to one tablet weight of Paliperidone were accurately weighed, transferred to a 100 ml volumetric flask containing 50 ml water and were allowed to stand to ensure complete solubility of the drug. The mixture was made up to volume with water. The solution was suitably diluted and the absorption was determined by UV –Visible spectrophotometer. The drug concentration was calculated from the calibration curve.
In vitro Buoyancy studies
The in vitro buoyancy was determined by floating lag time, and total floating time. The tablets were placed in a 100ml beaker containing 0.1N HCL. The time required for the tablet to rise to the surface and float was determined as floating lag time (FLT) and duration of time the tablet constantly floats on the dissolution medium was noted as Total Floating Time respectively (TFT).
In vitro drug release studies
Dissolution parameters
Apparatus -- USP-II, Paddle Method
Dissolution Medium -- 0.1 N HCL
RPM -- 50
Sampling intervals (hrs) -- 0.5,1,2,3,4,5,6,7,8,10,11,12
Temperature -- 37°c + 0.5°c
As the preparation was for floating drug release given through oral route of administration, different receptors fluids are used for evaluation the dissolution profile.
Procedure
900ml 0f 0.1 HCL was placed in vessel and the USP apparatus – II (Paddle Method) was assembled. The medium was allowed to equilibrate to temp of 37°C + 0.5°C. Tablet was placed in the vessel and the vessel was covered the apparatus was operated for 12 hours and then the medium 0.1 N HCL was taken and process was continued from 0 to 12 hrs at 50 rpm. At definite time intervals of 5 ml of the receptors fluid was withdrawn, filtered and again 5ml receptor fluid was replaced. Suitable dilutions were done with media and analyzed by spectrophotometrically at 284 nm using UV-Spectrophotometer.
Application of Release Rate Kinetics to Dissolution Data
Various models were tested for explaining the kinetics of drug release. To analyze the mechanism of the drug release rate kinetics of the dosage form, the obtained data were fitted into zero-order, first order, Higuchi, and Korsmeyer-Peppas release model.
Zero order release rate kinetics:
To study the zero–order release kinetics the release rate data ar e fitted to the following equation.
F = Ko t
Where, ‘F’ is the drug release at time‘t’, and ‘Ko’ is the zero order release rate constant. The plot of % drug release versus time is linear.
First order release rate kinetics
The release rate data are fitted to the following equation
Log (100-F) = kt
A plot of log cumulative percent of drug remaining to be released vs. time is plotted then it gives first order release.
Higuchi release model: To study the Higuchi release kinetics, the release rate data were fitted to the following equation.
F = k t1/2
Where, ‘k’ is the Higuchi constant.
In higuchi model, a plot of % drug release versus square root of time is linear.
Korsmeyer and Peppas release model
The mechanism of drug release was evaluated by plotting the log percentage of drug released versus log time according to Korsmeyer- Peppas equation. The exponent ‘n’ indicates the mechanism of drug release calculated through the slope of the straight Line.
Mt/ M∞ = K tn
Where, Mt/ M∞ is fraction of drug released at time ‘t’, k represents a constant, and ‘n’ is the diffusional exponent, which characterizes the type of release mechanism during the dissolution process. For non- Fickian release, the value of n falls between 0.5 and 1.0; while in case of Fickian diffusion, n = 0.5; for zero-order release (case I I transport), n=1; and for supercase II transport, n > 1. In this model, a plot of log (Mt/ M∞) versus log (time) is linear.
Drug – Excipient compatibility studies
Fourier Transform Infrared (FTIR) spectroscopy
The compatibility between the pure drug and excipients was detected by FTIR spectra obtained on Bruker FTIR Germany(Alpha T).The solid powder sample directly place on yellow crystal which was made up of ZnSe. The spectra were recorded over the wave number of 4000 cm-1 to 550 cm-1.
Results and Discussion
Analytical method
a. Determination of absorption maxima
The standard curve is based on the spectrophotometer. The maximum absorption was observed at 284nm.
b. Calibration curve
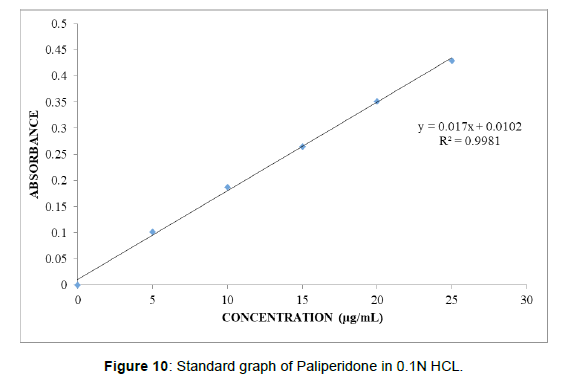
Graphs of Paliperidone were taken in 0.1N HCL (pH 1.2) (Table 6), (Figure 10). Standard graph of Paliperidone was plotted as per the procedure in experimental method and its linearity is shown in Table 8.1 and Fig 8.1. The standard graph of Paliperidone showed good linearity with R2 of 0.999, which indicates that it obeys “Beer- Lamberts” law.
Conc. [µg/mL] |
Abs |
|---|---|
| 0 | 0 |
| 5 | 0.127 |
| 10 | 0.241 |
| 15 | 0.364 |
| 20 | 0.478 |
| 25 | 0.591 |
Table 6: Observations for graph of Paliperidone in 0.1N HCL.
Preformulation parameters of powder blend:
Tablet powder blend was subjected to various pre-formulation parameters. The angle of repose values indicates that the powder blend has good flow Table 7 properties. The bulk density of all the formulations was found to be in the range of 0.42 to 0.57 (gm/cm3) showing that the powder has good flow properties. The tapped density of all the formulations was found to be in the range of 0.52 to 0.63 showing the powder has good flow properties. The compressibility index of all the formulations was found to be below 18.05 which show that the powder has good flow properties. All the formulations has shown the hausner ratio below 1.50 indicating the powder has good flow properties.
Formulation Code |
Angle of Repose |
|---|---|
| F1 | 28.36 |
| F2 | 25.64 |
| F3 | 27.02 |
| F4 | 24.22 |
| F5 | 31.38 |
| F6 | 24.22 |
| F7 | 30.11 |
| F8 | 22.29 |
| F9 | 27.02 |
Table 7: Pre-formulation parameters of blend.
Quality Control Parameters For tablets:
Tablet quality control tests such as weight variation, hardness, and friability, thickness, Drug content and drug release studies were performed for floating tablets Table 8.
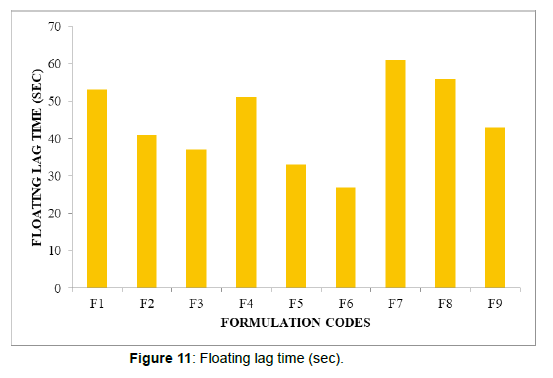
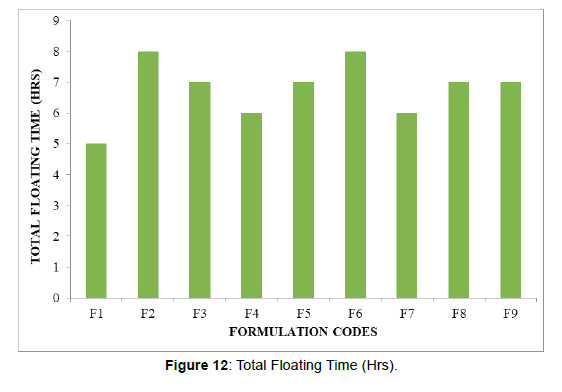
| Formulation codes | Weight | Hardness | Friability (%loss) | Thickness (mm) | Drug content (%) | Floating lag time | Total Floating Time (Hrs) |
|---|---|---|---|---|---|---|---|
| (kg/cm2) | (sec) | ||||||
| F1 | 98.14 | 4.2 | 0.53 | 2.15 | 99.36 | 53 | 5 |
| F2 | 100.02 | 4.9 | 0.62 | 2.69 | 98.14 | 41 | 8 |
| F3 | 99.34 | 4.2 | 0.48 | 2.81 | 97.34 | 37 | 7 |
| F4 | 98.67 | 4.3 | 0.72 | 2.79 | 100.02 | 51 | 6 |
| F5 | 95.34 | 4 | 0.34 | 2.56 | 99.47 | 33 | 7 |
| F6 | 97.33 | 4.7 | 0.53 | 2.11 | 99.33 | 27 | 8 |
| F7 | 99.02 | 4.6 | 0.33 | 2.29 | 97.2 | 61 | 6 |
| F8 | 100.12 | 4.8 | 0.29 | 2.5 | 98.47 | 56 | 7 |
Table 8:In vitro quality control parameters.
All the parameters for SR layer such as weight variation, friability, hardness, thickness, drug content were found to be within limits (Figure 11, 12)
In vitro drug release studies
From the dissolution data it was evident that the formulations prepared with Gum Copal as polymer were retarded the drug release 12 hours. Whereas the formulations prepared with Isapgol husk were retarded the drug release up to 12 hours in the Table 9 Figure 13-15 all ratios. In higher concentrations the polymer was retard the drug release. Whereas the formulations prepared with Fenugreek extract were retarded the drug release in the concentration of 18 mg (F9 Formulation) showed required release pattern i.e., retarded the drug release up to 12 hours and showed maximum of 98.54 % in 12 hours with good retardation.
Time |
Cumulative Percent Drug Dissolved | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (H) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 15.62 | 13.82 | 10.95 | 25.18 | 17.14 | 12.4 | 20.37 | 18.34 | 16.54 |
| 1 | 26.15 | 20.71 | 18.76 | 33.15 | 23.27 | 18.59 | 27.36 | 22.54 | 23.51 |
| 2 | 32.89 | 26.14 | 23.39 | 40.11 | 32.66 | 24.31 | 36.08 | 31.08 | 30.65 |
| 3 | 45.18 | 34.43 | 30.87 | 47.25 | 41.84 | 28.85 | 49.52 | 36.52 | 35.47 |
| 4 | 53.96 | 42.17 | 37.18 | 53.96 | 54.64 | 35.79 | 56.33 | 43.42 | 42.26 |
| 5 | 61.72 | 50.97 | 44.96 | 66.61 | 61.54 | 42.23 | 61.14 | 47.57 | 47.33 |
| 6 | 77.17 | 56.42 | 50.14 | 72.64 | 67.64 | 50.92 | 68.48 | 52.43 | 54.51 |
| 7 | 82.29 | 62.15 | 57.35 | 85.38 | 72.85 | 57.45 | 78.23 | 57.25 | 60.84 |
| 8 | 97.14 | 71.36 | 60.71 | 91.51 | 88.23 | 65.73 | 82.19 | 68.19 | 67.54 |
| 9 | 78.89 | 65.99 | 97.74 | 90.54 | 71.53 | 96.3
References
Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Indexed at, Google Scholar, Crossref Citation: Reshma M, Umar M, Shalini C (2022) Formulation and Evaluation of Paliperidone Floating Tablets. Int J Res Dev Pharm L Sci, 8: 137. Copyright: © 2022 Reshma M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Select your language of interest to view the total content in your interested language Share This ArticleRecommended JournalsOpen Access JournalsArticle Usage
Peer Reviewed JournalsMake the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals Journals by SubjectClinical & Medical JournalsInternational Conferences 2025-26
Conferences by CountryMedical & Clinical Conferences |