Research Article Open Access
Formulation and Evaluation of Fast Dissolving Tablets of Diclofenac Sodium by Novel Hole Technology
| Renati Damodar1*, Babji Movva1, Mallikarjun PN1, Chaitanya Pasumarthy2, Nishanth Kona3 and Varsha PV4 | ||
| 1Department of Pharmaceutics, Vignan Institute of Pharmaceutical Technology, Visakhapatnam, Andhra Pradesh, India | ||
| 2Mother Teresa College of Pharmacy, Ghatkesar, Hyderabad, India | ||
| 3Department of Pharmaceutics, Sree Siddaganga College Pharmacy, Tumkur, Karnataka, India | ||
| 4Department of Biotechnology, GITAM University, Visakhapatnam, Andhra Pradesh, India | ||
| Corresponding Author : | Renati Damodar Department of Pharmaceutics Vignan Institute of Pharmaceutical Technology Visakhapatnam, Andhra Pradesh, India Tel: +918500932050 E-mail: renatidamodar@gmail.com |
|
| Received May 27, 2014; Accepted July 28, 2014; Published July 31, 2014 | ||
| Citation: Damodar R, Movva B, Mallikarjun PN, Pasumarthy C, Kona N, et al. (2014) Formulation and Evaluation of Fast Dissolving Tablets of Diclofenac Sodium by Novel Hole Technology. J Mol Pharm Org Process Res 2:116. doi: 10.4172/2329-9053.1000116 | ||
| Copyright: © 2014 Damodar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Molecular Pharmaceutics & Organic Process Research
Abstract
Research has done to prepare Diclofenac Sodium quick dissolving tablets by Hole technology. Once these quick dissolving tablets contact with gastro enteric fluids, the fluid can enter the hole within the tablet and immediate breaking of the tablet goes to take place. This quick disintegration of tablets is additionally influenced by the formation of latest absolute space. The ready FDTs were subjected to numerous pre and post formulation studies. Its disintegration and dissolution rates were compared with the management formulation (without hole). In-vitro drug release of FDTs (DH6) showed virtually 100.92% of the drug was discharged at sixth minute, whereas the management formulation D12 shows the 99% drug release at 20th minute. Overall, this method is novel and most helpful for formulation into quick dissolving tablets.
| Keywords | |
| Hole technology; Diclofenac sodium; Novel fast dissolving tablets; Hole formation | |
| Introduction | |
| The oral drug delivery has been celebrated for many years because the most generally used route of administration among all the routes that have been explored for the general delivery of drugs via varied pharmaceutical product of different indefinite quantity forms [1]. The explanations that the oral route achieved such popularity could also be partly attributed to its simple administration still because the ancient belief that by oral administration the drug is still absorbed because the foodstuffs that are ingested daily [2]. Pharmaceutical product designed for oral delivery and presently available on the prescription and overthe counter markets are largely the immediate-release sort, that are designed for immediate unreleased of drug for speedy absorption. These fast-dissolving tablets ensure complete solubilization of tablet through surface erosion, leading to elimination of lag time for disintegration thereby offering quicker absorption and speedy onset of action [1-3]. | |
| Despite increasing interest in controlled- unreleased drug delivery systems, the most common tablets are those supposed to be enclosed whole and to disintegrate and unreleased their medicaments quickly within the gastro enteral tract. In additional recent years, increasing attention has been paid to formulating not solely fast dissolving and / or disintegrating tablets that are enclosed, but conjointly orally disintegrating tablets that are indented to dissolve and/or disintegrate quickly within the mouth [4-6]. | |
| Fast dissolving tablets (FDTs) are solid single-unit dosage forms that are place in the mouth, allowed to disperse/dissolve in the saliva and then swallowed without the need for water. FDTs are appreciated by a significant segment of the population, particularly children and elderly who have difficulty in swallowing conventional tablets or capsules. The most desirable formulation for use by the elderly is one that is easy to swallow and easy to handle. Since such a tablet can disintegrate in only a small amount of water in the oral cavity, it is easy to take for any age patient, regardless of time or place. For example, it can be taken anywhere at any time by anyone who do not have easy access to water. It is also easy to dose the aged, bed-ridden patients, or infants who have problems swallowing tablets and capsules. Recently, many companies have researched and developed various different types of fast-dissolving tablets by adopting different techniques. However, some of these technologies have disadvantages. New equipment, such as freeze-driers and specially molded tableting machines, were required for their production. Furthermore, these formulations were difficult for the aged to handle because of inadequate strength [7-12]. | |
| The Diclofenac sodium is a non steroidal anti inflammatory drug. It comprise a large family of weak acidic drugs whose pharmacological effects result primarily from the inhibition of cyclooxygenase (COX) an enzyme that catalyses the first step in the synthesis of prostaglandins from arachidonic acid and other precursor fatty acids. Since its solubility is very high in upper G.I. and need of fast releasing action in case of acute pain it is formulated as fast dissolving tablet [13-18]. | |
| Therefore the objectives of present research investigation were to formulate fast-dissolving tablets with sufficient hardness for handling and be manufactured by commonly used production methods and equipment. | |
| Materials and Methods | |
| Materials | |
| Diclofenac Sodium was obtained as a gift sample from Hetero drugs limited Laboratories, Hyderabad, India. Sodium starch glycolate, cross carmellose sodium, mannitol, sodium saccharine were procured from Yarrow Chem. Products, Mumbai, camphor was procured from Saptagir camphor limited, Anantapur. Potassium dihydrogen phosphate, methanol Reagent was procured from Merck limited, Mumbai. Lactose, magnesium stearate and talc I.P were procured from Molychem, Mumbai, India. All other chemicals were of analytical grade. | |
| Preparation of FDTs by novel hole technology | |
| All the ingredients in Table 1 were accurately, 100mg camphor tablets were prepared by taking plain camphor granules and compressed into tablets. Diclofenac sodium, super disintegrants and exponents were mixed in a container. Talc and Magnesium stearate after passing through sieve # 60 mixed and blended with initial mixer in the container. This mixture is then placed in the die cavity and at the centre of the die cavity, previously compressed camphor tablets were kept then compressed into tablets. These tablets containing tablet in tablet. i.e. Camphor tablet is present in Diclofenac tablet. After compression, these tablets were dried at 60°C by keeping the tablets in a hot air oven until complete removal of camphor to make tablets with hole at the ce | |
| Evaluation of Tablets | |
| Pre compression parameters | |
| Characteristics like tapped density, bulk density, carr’s index, hausner,s ratio were studied for powder blend of formulations which are ready to compress in to tablets [19-35]. | |
| Post compression parameters | |
| All the prepared tablets were subjected to various physical characteristics like Crushing strength, Friability, Thickness, Diameter, Hole depth, Disintegration time, Wetting time, Weight variation, Drug content (Table 3). | |
| Weight variation test | |
| Weight variation test was done by weighing 20 tablets individually, by using electronic balance. Calculating the average weight and comparing the individual tablet weight to the average weight [21-23]. | |
| Tablet thickness | |
| The thickness was measured by placing tablet between two arms of the Vernier calipers. 5 tablets were taken and their thickness was measured. | |
| Tablet hardness | |
| The tablet hardness, which is the force required to break a tablet in a diametric compression force. The hardness tester used in the study was Monsanto hardness tester, which applies force to the tablet diametrically with the help of an inbuilt spring. | |
| Tablet friability | |
| The friability of the tablets was measured in a Roche friabilator (M/s. Elite Scientific & Equipments.). Tablets of a known weight (Wo) or a sample of 20 tablets are dedusted in a drum for a fixed time (100 revolutions) and weighed (W) again. Percentage friability was calculated from the loss in weight as given in equation as below. The weight loss should not be more than 1%. Determination was made in triplicate. | |
| % Friability = 100(Wo-W) / Wo | |
| characterization of prepared tablets | |
| In vitro disintegration time | |
| In the disintegration time study, the tablets were taken and introduced in each tube of disintegration apparatus, and the tablet rack of the disintegration apparatus was positioned into a 1-litre beaker containing 900 ml of distilled water and time of disintegration was recorded at 37 ± 2°C [23-28]. | |
| In the wetting time study | |
| In wetting time study a piece of tissue paper folded twice was placed in a petridish (with internal diameter 6.5 cm) containing 5 ml of distilled water. A tablet was placed on the paper and the time for complete wetting of the tablet was measured in seconds [36-42]. | |
| Drug content analysis | |
| Total 10 tablets were weighed and powdered .The powder equivalent to Diclofenac Sodium was taken and dissolved in 0.1N HCl. After that an aliquot of the filtrate was diluted and analyzed spectrophotometrically (Elite UV- 150 double beam spectrophotometer.) at 314 nm [43,44]. | |
| In vitro release studies | |
| The in vitro dissolution study was carried out in the USP dissolution test apparatus (M/s Lab India (Model – DS 8000) type 2 (paddle). 900 ml of the dissolution medium (Phosphate buffer pH 6.8) was taken in vessel and the temperature was maintained at 37 ± 0.5°C. The speed of the paddle was set at 50 rpm. 5 ml of the dissolution medium was withdrawn and the same amount of fresh medium was replenished to the dissolution medium. The sample withdrawn was filtered and diluted with Phosphate buffer pH 6.8 prior to analysis in the UV Spectrophotometer (Elite UV- 150 double beam spectrophotometer ) at 285 nm [45-47]. | |
| Results and Discussion | |
| Compatibility studies | |
| Infrared spectroscopic study: Fourier transformed (FTIR) spectrum of Diclofenac, Drug with different excipients were obtained on a FTIR (Perkin-Elimer) using the KBr disk method. The prominent IR absorption peaks of Diclofenac showed at 3381.57 cm-1 due to NH stretching of secondary amine. Peak at 1572.66 cm-1 due to –c=o stretching of carboxyl ion. Peak at 745.35 cm-1 because of c-cl stretching. All these principal IR peaks of Diclofenac Sodium were present in all formulations. This clearly indicates that there is no interaction between drug and carrier. | |
| Pre compression parameters | |
| The Bulk density of all the formulations were within the range of 0.51 ± 0.005 to 0.56 ± 0.005 g/ml and Tapped density was found to be in the range of 0.61 ± 0.03 to 0.66 ± 0.03 g/ml (good flow property). The Angle of repose of powder blends of all formulation was found to be in the range of 19.21 ± 0.12 to 29.62º ± 0.2 (good flow property). The calculated Carrs index of all formulations was found to within the range of 13.84 ± 0.15 to 17.74% ± 0.16 (good flow property). The calculated Hausners ratio of all the formulations was found to be in the range of 1.162 ± 0.13 to 1.21 ± 0.03 (good flow property). The values of pre-compressional parameters evaluated were within the prescribed limits and indicated good free flowing properties. | |
| Post compression parameters | |
| The post compression parameters of all batches were studied and shown in Table 2. The crushing strength of tablets prepared by hole technology were within the range of 3.5 ± 0.25 to 3.5 ± 0.5 kg/cm2. The loss of percentage of weight in friability was found to be 0.39 ± 0.08 to 0.53 ± 0.06 which is less than 1% which indicates tablets has good mechanical resistance. The thickness and diameter of prepared tablets was found to in the range of 3.812 ± 0.02 to 4.0 ± 0.03 mm and 12.80 ± 0.05 to 12.90 ± 0.04 mm respectively. The hole depth of all formulations prepared by hole technology was found to be in the range of 1.66 ± 0.22 to 1.75 ± 0.04 mm. The wetting time of all formulations prepared by hole technology was found to be in the range of 21 ± 0.49 to 44 ± 0.61 sec. The disintegration time of all formulations prepared by hole technology was found to be in the range of 14 ± 0.53 to 33 ± 0.65 sec and was shown in Table 4. The weight variation of all formulations prepared by hole technology was found to be in the range of 497.5 ± 0.19 to 498.5 ± 0.18 mg. The drug content of all formulations prepared by hole technology was found to be in the range of 98.9 ± 1 to 101.5 ± 1.25%. | |
| In vitro dissolution studies | |
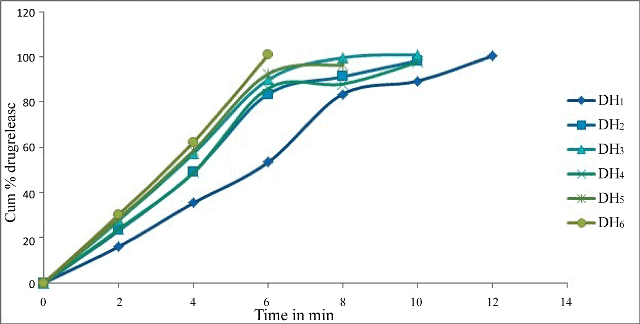
| The in vitro dissolution study was carried out in the USP dissolution test apparatus (M/s Lab India (Model – DS 8000) type 2 (paddle). 900 ml of the dissolution medium (Phosphate buffer pH 6.8) was taken in vessel and the temperature was maintained at 37 ± 0.5°C. The results were shown in Table 4. The cumulative% of drug release of formulations prepared by hole technology was found to be, DH1 showed 100% drug released at 12 min. DH2 showed 98.35% drug released at 10 min, DH3 showed 100.92% drug released at 10 min, DH4 showed 97.71% drug released at 10 min, DH5 showed 96.42% drug released at 8 min, DH6 showed 100.92% drug released at 6 min. The cumulative% of drug release of formulations prepared by direct compression was found to be, D7 showed 100.28% drug released at 50 min, D8 showed 96.42% drug released at 40 min, D9 showed 100.8% drug released at 25 min, D10 showed 99.64% drug released at 45 min, D11 showed 100.9% drug released at 45 min, D12 showed 98.35% drug released at 20 min. | |
| The T50 and T90 were calculated for all formulations and were shown in Time for 50% of dissolution was found to in the range of 3 min 30 sec to 5 min 36 sec for formulations with hole technology. Time for 50% of dissolution was found to in the range of 3 min 30 sec to 6 min for formulations with direct compression. Time for 90% of dissolution was found to in the range of 5 min 36 sec to 10 min for formulations with hole technology. Time for 90% of dissolution was found to in the range of 17 min to 40 min for formulations with direct compression. From the results DH6 was selected as best formulation since it showed total drug release in 6 minutes. | |
| Stability studies of optimized formulation | |
| Optimized formulation was exposed for accelerated conditions as per ICH guidelines. (40ºC / 75% RH for a period of 3 months. Tablets were evaluated for physicochemical properties, drug release. The Stability studies on optimized formulation of Diclofenac Sodium fast dissolving tablets were conducted according to the ICH guidelines. The various parameters tested during studies of Diclofenac Sodium fast dissolving tablets the formulation were withdrawn at suitable intervals (initial and 1 month) and analyzed visually for physical appearance and evaluated for different tests. The tablets showed no visual differences and compiled with description. The percentage of drug release from the formulation DH6 at different intervals of time is given in Table 5 and was found to be matching with specification. The results were shown in Table 5. From the results, it can be concluded that the formulation DH6 of Diclofenac fast dissolving tablets was stable at accelerated conditions of temperature and humidity. | |
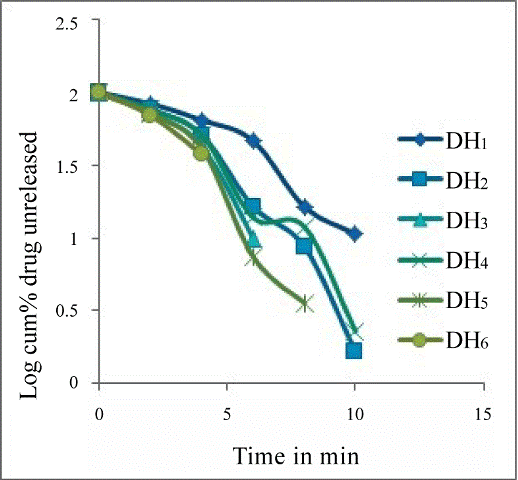
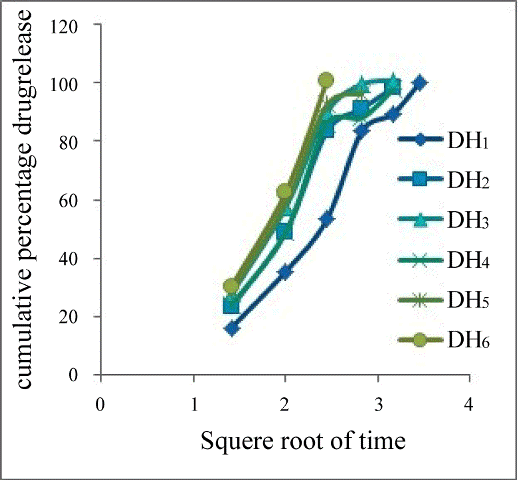
| Drug release kinetics | |
| Ter kinetics. Especially batch DH6 showed R2 value of 0.9948 indicating the drug release followed zero order kinetics. The R2 values of all formulations were showed in Table 6. | |
| Conclusion | |
| The tablets prepared with hole technology showed all the parameters like hardness, friability, weight variation within the limits. All the formulations with increased concentrations of super disintegrants showed better drug release compared to the formulations with less concentration of super disintegrants. The formulation DH6 with 30 mg of SSG showed disintegration in 25 sec and a wetting time of 33 sec and showed total drug release in 6 min. The formulation is effectively useful in treatment of acute pains. | |
| Acknowledgements | |
| The authors are thankful to Dr. L Rathaiah, and Chairman of Lavu Educational Society for providing facility to carry out the above research work. | |
References
- Prakash V, Maan S, Deepika, Yadav SK, Hemalatha et al. (2011) Fast disintegrating tablets: Opportunity in drug delivery system. J Adv Pharm Technol Res 2: 223-235.
- Ishikawa T, Watanabe Y, Utoguchi N, Matsumoto M (1999) Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter-taste-masked granules by the compression method. Chem Pharm Bull 47: 1451-4.
- Sammour OA, Mohammed A. Hammad, Nagia A Megrab, Ahmed S Zidan (2006) Formulation and optimization of mouth dissolve tablets containing rofecoxib solid dispersion. AAPS Pharm Sci Tech 7: E167-E175.
- Bhowmik D, Chiranjib B, Krishnakanth, Pankaj, Margret Chandira R et al. (2009) Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research 1: 163-177.
- NehalSiddiqui, Garima Garg, Pramod Kumar Sharma (2010) Fast Dissolving Tablets: Preparation, Characterization and Evaluation: An Overview. International Journal of Pharmaceutical Sciences Review and Research 4: 015.
- Schiermeier S, Schmidt PC (2002) Fast dispersible ibuprofen tablets. Eur J Pharm Sci. 15:295-305.
- MohitShrivastava, Deepak Chourasiya, Sanjay Soni, Deepak Patidar, Rajesh Jatav et al. (2012) Formulation and In-Vitro Evaluation of Mouth Dissolving Tablets of Phenytoin Sodium Using Different Disintegrating Agents. Int J Nov Drug Deliv Tech 2: 249-255.
- Sehgal Prateek, Gupta Ramdayal, Singh Umesh Kumar, ChaturvediAshwani, Gulati Ashwini, et al. (2012) Fast Dissolving Tablets: A New Venture in Drug Delivery. Am J Pharm Tech Res 2: 4.
- Deepak Sharma (2013) Formulation Development and Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate for Respiratory Disorders. ISRN Pharmaceutics 2013: 1-8.
- Sharma D, Reetika Chopra, NeenaBedi (2012) Development and Evaluation of Paracetamol Taste Masked Orally Disintegrating Tablets Using Polymer Coating Technique. International Journal of Pharmacy and Pharmaceutical Sciences 4: 3.
- Shukla, Dali, Chakraborty, Subhashism, Sanjay S, Mishra B (2009) Mouth Dissolving Tablets I: An Overview of Formulation Technology. ScientiaPharmaceutica 77: 309-326.
- Siddiqui, Md.Nehal, Garima Garg, Pramod Kumar Sharma (2010) Fast Dissolving Tablets: Preparation, Characterization And Evaluation: An Overview. International Journal of Pharmaceutical Sciences Review & Research 4: 87-96.
- Bhardwaj V, Bansal M, Sharma PK (2010) Formulation and evaluation of fast dissolving tablets of amlodipine besylate using different super disintegrants and camphor as sublimating agent. American-Eurasian Journal of Scientific Research 5: 264-269.
- Babji Movva, Laxman Kumar D, Mohan Ravi Kumar K (2013) Formulation and Evaluation of Fast Dissolving Tablets of Ranitidine Hydrochloride by Hole Technology. Asian J Pharm Clin Res 6: 143-147.
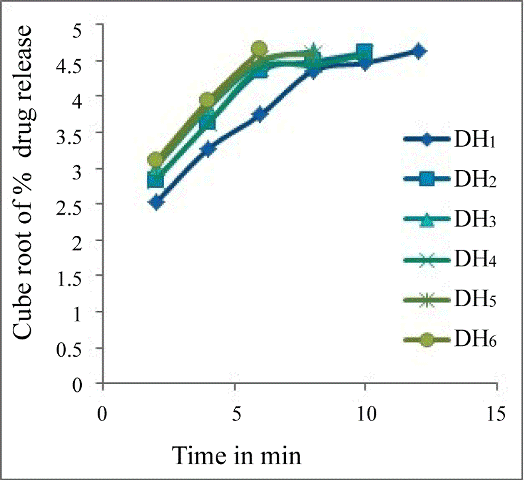
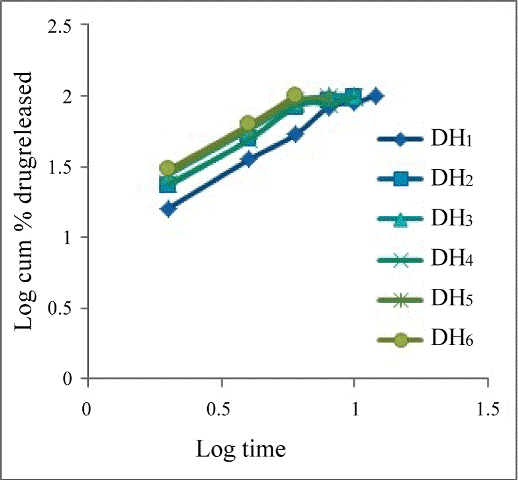
- Kulkarni Upendra, Rao, Raghavendra NG (2011) Design and Development of Aceclofenac Fast Dissolving Tablets by Novel Hole Technology: A Novel Approach to Decrease the Disintegration Time and Increase the patient compliance. Drug Invention Today 3: 91-94. he drug release profiles of the tablets were studied includes zero order (Figure 2), First order (Figure 3), Higuchi square root of time model (Figure 4), Hixon- Crowell model (Figure 5 and Korsmeyerpeppas model (Figure 6). From the results it is implying that the release kinetics from the FDT tablet follows zero ord
- Peddi MG (2013) Novel Drug Delivery System: Liquid Solid Compacts. J Mol Pharm Org Process Res 1:108.
- Madhavi BR, Murthy VSN, Rani AP, Kumar GD (2013) Buccal Film Drug Delivery System-An Innovative and Emerging Technology. J Mol Pharm Org Process Res 1:107.
- Babu B, Meyyanathan SN, Gowramma B, Muralidharan S, Elango K, et al. (2012) Pharmacokinetic Evaluation of Newly Developed Oral Immediate Release and Sustained Release Dosage Forms of Losartan Potassium. J BioequivAvailab 4: 121-127.
- Shakeel F, Mohammed SF, Shafiq S (2009) Comparative Pharmacokinetic Profile of Aceclofenac from Oral and Transdermal Application. J BioequivAvailab 1: 013-017.
- Zheng C, Wang Y (2013) Prediction of Oral Bioavailability: Challenges and Strategies. J BioequivAvailab 6: 10000e47
- Dutta AK, Ikiki E (2013) Novel Drug Delivery Systems to Improve Bioavailability of Curcumin. J BioequivAvailab 6:1000172
- Balderas-Acata JI, Ríos-Rogríguez BuenoEP, del Castillo-García S, Espinosa-Martínez C , Burke-Fraga V et al. (2011) Bioavailability of Two Coated-Tablet Formulations of a Single Dose of Pantoprazole 40 mg: An Open-Label, Randomized, Two-Period Crossover, Comparison in Healthy Mexican Adult Volunteers. J BioequivAvailab 3: 1000063
- Valenzuela F, Davila G, Ibañez Y, Garcia L, Crownover P, et al. (2011) Relative Bioavailability of Chewable and Conventional Film-Coated Tablet Formulations of Sildenafil 100 mg in Healthy Volunteers Under Fasting Conditions. J BioequivAvailab 3:1000087
- Bragatto MS, dos Santos MB, Pugens Pinto AM, Gomes E, Angonese NT, et al. (2011) Comparison between Pharmacokinetic and Pharmacodynamic of Single-Doses of Furosemide 40 mg Tablets. J BioequivAvailab 3: 191-197.
- Feleder EC, Yerino GA, Halabe EK, Carla S, Soledad G, et al. (2011) Single-Dose Bioequivalence of a New Fixed-Dose Combination Tablet Containing Tenofovir Disoproxil Fumarate and Lamivudine. J BioequivAvailab 3: 236-243.
- Pechenkin MA, Balabushevich NG, Zorov IN, Staroseltseva LK, Mikhalchik EV, et al. (2011) Design, In Vitro and In Vivo Characterization of Chitosan-Dextran Sulfate Microparticles for Oral Delivery of Insulin. J BioequivAvailab 3: 244-250.
- Palma-Aguirre JA, Mireya LG, de Jesus CST, Mariel PG, Elisa ZB, et al. (2010) Bioavailability of Two Oral Tablet Formulations of citalopram 20 mg: Single-Dose, Open-Label, Randomized, Two-Period Crossover Comparison in Healthy Mexican Adult Subjects. J BioequivAvailab 2: 018-022
- Bapuji AT, VenkataRavikiran HL, Nagesh M, Syedba S, Ramaraju D, et al. (2010) Bioequivalence Testing - Industry Perspective. J BioequivAvailab 2: 098-101.
- Kanfer I (2010) Strategies for the Bioequivalence Assessment of Topical Dermatological Dosage Forms. J BioequivAvailab 2: 102-110.
- Junior EA, Duarte LF, PirasolVanunci ML, Teixeira ML (2010) Bioequivalence of Two Oral Contraceptive Drugs Containing Ethinylestradiol and Gestodene in Healthy Female Volunteers. J BioequivAvailab 2: 125-130.
- Khattak S, Malik F, Hameed A, Ahmad S, Rizwan M, et al. (2010) Comparative Bioavailability Assessment of Newly Developed Flurbiprofen Matrix Tablets and Froben SR® Tablets in Healthy Pakistani Volunteers. J BioequivAvailab 2: 139-144.
- Shakeel F, Ramadan W, Shafiq S (2009) Solubility and Dissolution Improvement of Aceclofenac using Different Nanocarriers. J BioequivAvailab 1: 0 39-043.
- Roda A, Simoni P, Roda G, Caponi A, Pastorini E, et al. (2009) Pharmacokinetics and Safety of a New 1200 mg Single-Dose Delayed Release MesalazineMicrogranule Formulation. J Bioequiv Availab 1: 044-051.
- Lima R, Vasconcelos T, Cerdeira R, Lefebvre M, Sicard E, et al. (2009) Bioequivalence of Final Tablet Formulation and Research Tablet Formulation of Eslicarbazepine Acetate in Healthy Volunteers. J Bioequiv Availab 1: 093-098.
- Moreira RF, Rigato HM, Borges BC, Sverdloff CE, Oliveira RA,et al. (2009) Effect of Hyperlipemic Food on the ComparativeBioavailability of Two Bupropion Formulations after Administration of a Single Oral Dose of 150 mg in Healthy Human Volunteers. J Bioequiv Availab 1: 103-111.
- Teksin ZS, Agabeyoglu I, Yamac K (2009) Bioavailability of Pentoxifylline-Chitosan Oral Matrix Tablet in Healthy Subjects. J BioequivAvailab 1: 115-120.
- Dewan B, Sahu N (2010) Bioequivalence Study of Troxipide Tablet Formulations. J Bioequiv Availab 2: 050-054.
- Zhang CL, Jiao JJ, Wu YN, Song JQ, Gao WZ, et al. (2011) Study on Pharmacokinetics and Bioequivalence of Cefdinir Dispersible Tablet in Healthy Chinese Volunteers. J Bioequiv Availab 3: 114-117.
- Feng Y, Wang N (2013) The New Generation of Drug Discovery and its Analytical Technologies. J Bioequiv Availab 5: e42.
- Al-Ghananaeem A (2013) Sublingual and Nasal Trans mucosal Drug Delivery for Breakthrough Pain: A Frontier in Cancer Therapy. J Bioequiv Availab 5: e29.
- Harahap Y, Budi Prasaja MM, Lusthom W, Hardiyanti, Azmi F, et al. (2013) Bioequivalence of Trimetazidine Modified Release Tablet Formulations Assessed in Indonesian Subjects. J Bioequiv Availab 5: 117-120.
- Nichols AI, Richards LS, Behrle JA, Posener JA, Fruncillo R, et al. (2013) Effects of Age and Sex on the Pharmacokinetics, Safety, and Tolerability of Oral Desvenlafaxine in Healthy Adults. J Bioequiv Availab 5: 088-094.
- Mascoli V, Kuruganti U, Bapuji AT, Wang R, Damle B (2013) Pharmacokinetics of a Novel Orodispersible Tablet of Amlodipine in Healthy Subjects. J Bioequiv Availab 5: 076-079.
- Nishant T, Sathish Kumar D, Arun Kumar, Phaneendra M (2011) Role of Pharmacokinetic Studies in Drug Discovery. J Bioequiv Availab 3: 263-267.
- Ruiz A, Cuesta F, Parra S, Montoya B, Restrepo M, Archbold R, et al. (2012) Bioequivalence Evaluation of Two Formulations of Lamotrigine Tablets in Healthy Volunteers. J Bioequiv Availab 4: 030-034.
- Parthasarathi D, Gajendra C, Dattatreya A, Sree Venkatesh Y (2011) Analysis of Pharmacokinetic &Pharmacodynamic Models in Oral and Transdermal Dosage Forms. J Bioequiv Availab 3: 268-276.
- Wagh N, Gayakwad NJ, Christina AJM, Bhople A, Thakre A (2013) A Bioequivalence Study of Two Finofibrate Tablet Formulations in Indian Healthy Subjects. J Bioequiv Availab 5: 016-021.
--
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
 |
 |
 |
 |
|||||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Agglutination
- Anovulation
- Bacteriostasis
- Biological Processes
- Biopharmaceuticals
- Catabolism
- Catalysis in Organic Synthesis
- Defoliation
- Deossification
- Digestion
- Drug Discovery Process
- Eburnation
- Ecchymosis
- Effacement
- Erythropoiesis
- Eutrophication
- Green Chemistry in Process Research
- Introversion
- Intussusception
- Molecular and Cellular Biology
- Molecular Pharmacy
- Nanomedicine and Nanoparticle Drug Delivery
- Pharmaceutical Nanotechnology
- Pharmacokinetics and Pharmacodynamics
- Protein Protein interactions
Recommended Journals
Article Tools
Article Usage
- Total views: 23847
- [From(publication date):
August-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 18346
- PDF downloads : 5501
