Foregut Structure and Physiology of Brown Planthopper, Nilaparvata lugens (Stål), Unveiled via Synchrotron Radiation X-ray Tomography
Received: 02-Aug-2024 / Manuscript No. DPO-24-144502 / Editor assigned: 05-Aug-2024 / PreQC No. DPO-24-144502 (PQ) / Reviewed: 19-Aug-2024 / QC No. DPO-24- 144502 (QC) / Revised: 26-Aug-2024 / Manuscript No. DPO-24-144502 (R) / Published Date: 02-Sep-1899
Abstract
Synchrotron radiation X-ray tomographic microscopy is a non-destructive technology used in physiology or taxonomy for imaging and analysis. It has become an emerging and progressive technology in insect science. The structural details of these insects can be used as critical information on their interaction with virus pathogens. For example, the Brown Planthopper (BPH) (Nilaparvata lugens, Stål) is a devastating pest of rice plants, causing significant yield loss in East Asia. BPH plays an significant role in transmitting Rice-Ragged Stunt Virus (RRSV), a pathogen from the oryzavirus genus. In this study, we utilized synchrotron radiation X-ray tomographic microscopy to investigate BPH's internal anatomy. This method could identify and characterize the internal structures in the mouthparts and foregut structures, such as the precibarium and food meatus, cibarium chamber and diaphragm. Our findings highlighted the significance of the precibarium and cibarium in BPH feeding, shedding light on vector-pathogen interactions. Moreover, this study demonstrated the broader applications of advanced imaging and behavioral analysis techniques in entomology. This research contributed valuable knowledge to insect-vector interactions and has implications for crop protection and agriculture.
Keywords: Insect pest; Mouthpart structure; Nilaparvata lugens (Stål); Physiology; Synchrotron radiation X-ray tomography
Introduction
The Brown Planthopper (BPH) Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), is a destructive insect pest that poses a significant threat to rice production in East Asia. Infestations of BPH can cause severe damage to rice plants by direct feeding and oviposition, leading to the devastating phenomenon known as “hopper burn,” where rice plants wither and wilt under dense BPH populations. Additionally, BPH plays an important role in the transmission of RRSV, a pathogen belonging to the oryzavirus genus in the Reoviridae family [1,2]. RRSV was first identified in Indonesia and the Philippines in 1976-1977 and has since spread to various rice-growing regions in Southeast Asia and southern China, becoming one of the most significant rice pathogens in these areas [3]. Leaves of infected plants typically exhibit chlorotic yellowish-white stripes and young leaves may turn completely yellow or white. Moreover, RRSV causes stunting, darker leaf color, leaf distortion, galls on leaf veins and suppression of flowering [4]. The BPH horizontally transmits RRSV in a persistent propagative manner, but vertical transmission through eggs or rice seeds does not occur [5]. N. lugens acquire the RRSV virus when it feeds on infected rice plants. It initially enters the epithelial cells of the midgut and then progresses to the visceral muscles surrounding the midgut. Subsequently, it disperses throughout the visceral muscles of both the midgut and hindgut, ultimately reaching the salivary glands [6]. During subsequent sap feeding, the virus present in the saliva is transmitted to the host rice plant [7]. Despite our current knowledge of the invasion route within the insect body, the precise mechanisms facilitating the transmission of this virus from its insect vector to the rice plant remain largely unexplored. Understanding the distribution of RRSV within the BPH’s foregut is important for elucidating vector-pathogen interactions. However, studying these foregut structures poses numerous challenges, primarily due to their scale, intricate internal complexity and delicate nature, as they are deeply located within the insect’s head. Traditionally, imaging of the foregut and mouthparts in insects has been accomplished using Scanning Electron Microscopy (SEM) to observe surface structures and bacterial localization in the precibarium. Transmission Electron Microscopy (TEM) has also been utilized to investigate biofilm interactions with chitinous surfaces within insects [8]. SEM and TEM require laborious sample preparation and provide only twodimensional images that must be reconstructed into composite images. The histological sectioning with subsequent staining and light microscopic investigation is probably better suited for studying the distribution of bacteria within a host organism than TEM because, with light microscopy, it is simpler to study larger areas/volumes. Moreover, SEM and TEM are inherently destructive, limiting visualization to a single opportunity and requiring timeconsuming, technically challenging and costly sample preparation.
X-ray Computed Tomography (CT) imaging has been widely used for medical imaging for decades and has recently been developed as a non-destructive diagnostic imaging tool for a wide range of biological samples. Based on the same principles as those of medical X-ray computed tomography imaging systems, micro-CT is useful for soft and mineralized tissues, with minimal sample preparation required to produce Three-Dimensional (3D) images with excellent contrast [9]. CT has recently been applied in entomology as a means of non-destructively analyzing the internal anatomy of insects. This has been performed in studies on the head structures of Priacma serrata Leconte, the external and internal morphology of bees and ants (Hymenoptera), the wingbeats of blowflies in a synchrotron-based study performing micrometer-resolution, time resolved microtomography, the structural characteristics of the earliest lineages of insects and the use of X-ray microtomography images for identification of Pheidole knowlesi species group [10- 16]. In recent years, Synchrotron Radiation X-Ray Tomographic Microscopy (SRXTM) has emerged as a non-destructive imaging tool for a wide range of biological samples. Like medical X-ray computed tomography, SRXTM can produce high-resolution Three-Dimensional (3D) images with excellent contrast for soft and mineralized tissues, requiring minimal sample preparation [9]. As a result, SRXTM has been successfully applied in entomology to non-destructively analyze the internal anatomy of various insect species, facilitating breakthroughs in the visualization of preserved specimens and real-time internal processes of living organisms [17- 19]. In this method, the X-rays generated from the synchrotron light source exhibit significantly higher intensity across multiple orders of magnitude compared to those from X-ray tubes. Additionally, the X-rays emitted from the synchrotron light source maintain a parallel beam geometry. As a result, the detector captures an X-ray projection of the sample at the same size, due to the synchrotron light, whereas the X-ray tube, operating as a fan beam, results in a larger and less defined X-ray projection of the sample.
Herein, we employed SRXTM imaging to focus on the foregut’s cibarium and precibarium in the BPH, aiming to better characterize internal structures and provide insights into vector-pathogen interactions. We identified four key sites in the foregut: The precibarium and food meatus, the cibarium chamber’s interior and the diaphragm. Leveraging the power of SRXTM. SRXTM imaging to specifically target the sites of pathogen and biofilm formation in the foregut of the BPH to better characterize the internal structures and develop a framework for studying these vector-pathogen interactions. We virtually dissected the foregut to determine the BPH’s feeding behavior, using this novel, detailed 3D structure of the entire insect, we proposed a plant phloem sap-sucking model based on the actual movement of feeding apparatuses during the feeding process. This research could open new avenues for understanding vector-pathogen interactions and contribute to more effective strategies for managing this significant agricultural pest and mitigating the impacts of RRSV on rice production in East Asia.
Materials Methods
Insect collection and maintenance
A Benign Prostatic Hyperplasia (BPH) N. lugens colony from the Ubon Ratchathani Rice Research Center Thailand in 2019 and raised in multiple tent-shaped, 30 cm × 30 cm × 30 cm bugdorms (MegaView Science Co., Ltd., Taichung City, Taiwan) were set up, with 20-30 male and female adults in each bugdorm. Khao Dawk Mali (KDML)105 rice plants (7 days-14 days old) in pots were placed in each bugdorm to provide ample feeding. Plants were replaced every 2-3 weeks. The planthoppers and rice plants were kept under a 12-hour light:12-hour dark cycle at 28°C ± 2°C and 60% ± 5% relative humidity in the Entomology Laboratory of Suranaree University of Technology. Only adult planthoppers were used throughout this study.
Synchrotron radiation X-ray tomographic microscopy imaging
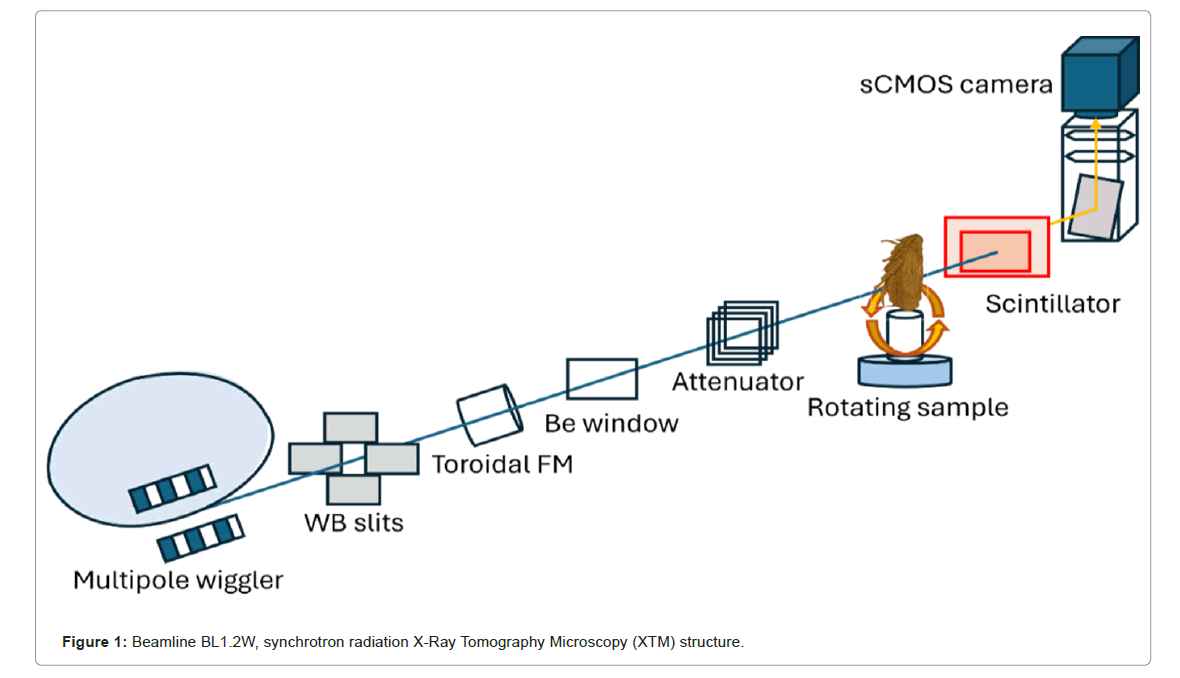
To investigate the mouthpart structures of the BPH N. lugens, we imaged the overall body and dissected the head using SRXTM. Adult truncate-winged 'brachypterous' forms BPH (10 females) were collected and fixed in 6% formaldehyde for 24 h and then washed three times in Phosphate-Buffered Saline (PBS). BPHs were dehydrated in an increasing Ethanol (EtOH) series and preserved using critical point drying. SRXTM was performed in Beamline 1.2W (BL 1.2W) Figure 1, the Synchrotron Light Research Institute (Public Organization; Nakorn Ratchasima, Thailand), using synchrotron radiation X-rays generated from a 2.2-tesla multipole wiggler in the Siam Photon Source (Synchrotron Light Research Institute), operating at 1.2 GV. A filtered polychromatic X-ray beam with a mean energy of 11.5 kV and a distance of 32 m from the source to the sample was used. X-ray projections were collected from 0° to 180° with an angular increment of 0.1°. SRXTM was used to generate 3D images of the samples. The samples were rotated 180° in 0.1° angular increments during imaging. Polychromatic X-rays were attenuated with 350-μm aluminum foil. The energy range was about 5 keV-20 keV and the acquisition time for 1 X-ray projection was about 10 ms. Subsequently, a set of X-ray images was processed to create ‘sinograms’ using Octopus reconstruction software with a filtered back projection algorithm written in lab view (Brusselsesteenweg 708, Gentbrugge, Oost-Vlaanderen 9050, Belgium) [20]. These sinograms were then used to reconstruct CT slices. The assembly of these reconstructed CT slices was processed into a 3D reconstruction of the sample using the Drishti software program (the National Computational Infrastructure's VizLab). Drishti is commonly employed for visualizing and analyzing 3D and tomographic data, as depicted in Figure 2. A scientific complementary metal-oxide semiconductor camera captured the X-ray projections with a pixel size of 1.44 μm. The captured data were preprocessed and reconstructed into 3D images using a filtered back projection algorithm using Octopus reconstruction software written in labview. Drishti software was used to visualize the reconstructed images.
To measure the length of the beak, the adult BPH N. lugens were fast-frozen by liquid nitrogen when they were sucking in phloem sap on a rice plant following [21,22]. Neuropil nomenclature adhered to the guidelines outlined by the Insect Brain Name Working Group, while external sclerites followed the system proposed by Wipfler et al., Labial muscles are sequentially numbered based on their appearance, following the terminology established by Spangenberg et al. [23,24].
Results
Synchrotron radiation X-ray tomographic microscopy imaging
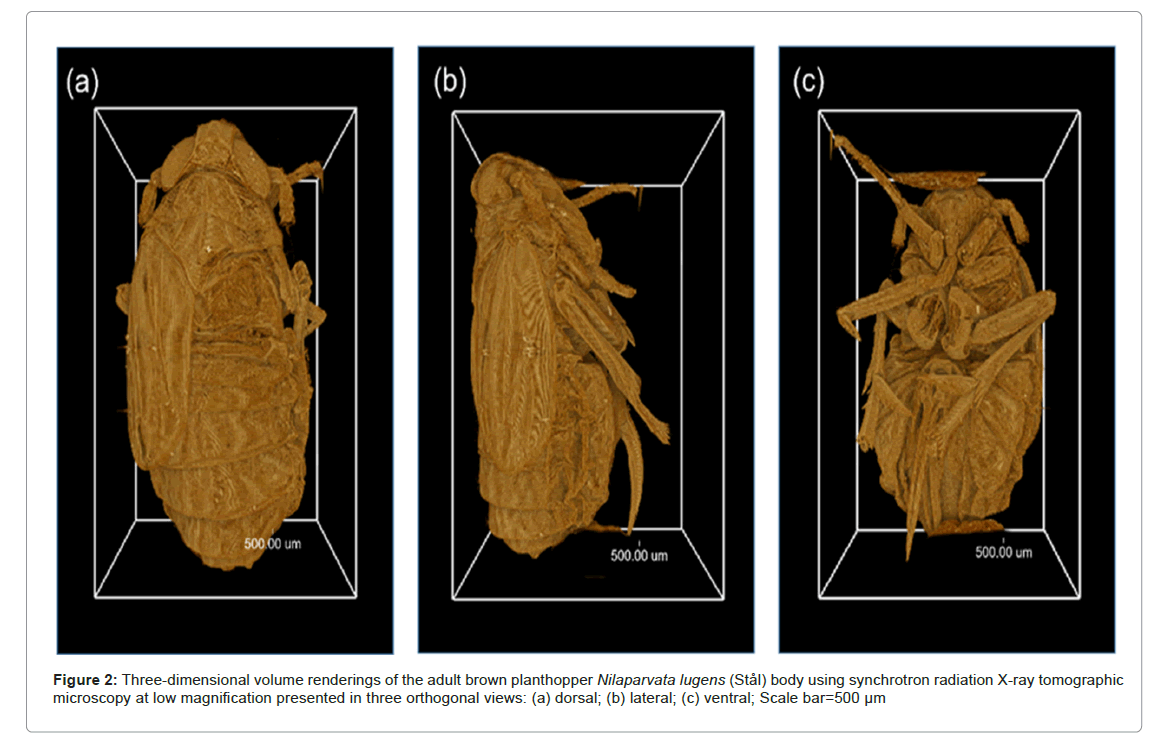
SRXTM imaging provided valuable insights into the internal structures of the BPH body and head, revealing a distinct and intricate anatomy as shown in Figure 2. The method enabled visualization of the mouthparts, including the foregut, cibarium and precibarium, at high resolution. Lower magnification visualizations as showed in Figures 2 and 3 allowed for observing the overall morphology of the entire body and head. The imaging technique facilitated both two-dimensional and 3D investigations of internal structures as well as virtual dissection from any desired plane. The virtually dissected head images (ventral view, Figures 3a and 3c) displayed clear distinctions between the cibarium chamber, precibarium and food meatus. A lateral view of the BPH head also highlighted the various components of the cibarium region.
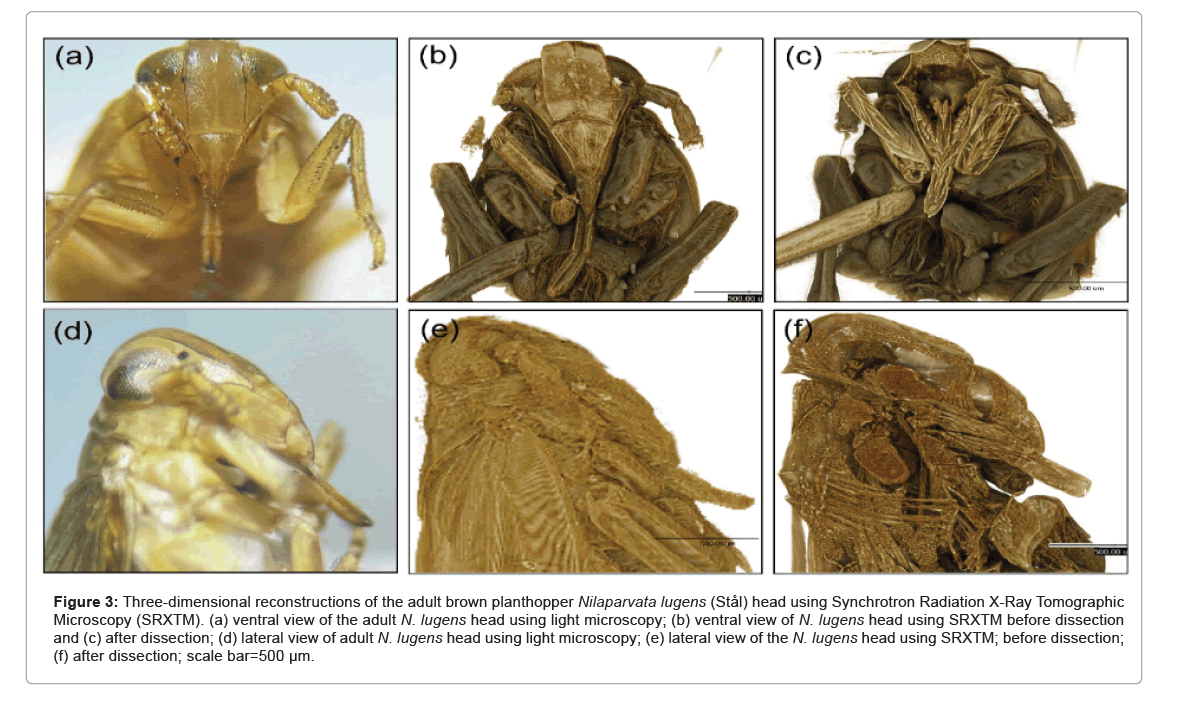
Figure 3: Three-dimensional reconstructions of the adult brown planthopper Nilaparvata lugens (Stål) head using Synchrotron Radiation X-Ray Tomographic Microscopy (SRXTM). (a) ventral view of the adult N. lugens head using light microscopy; (b) ventral view of N. lugens head using SRXTM before dissection and (c) after dissection; (d) lateral view of adult N. lugens head using light microscopy; (e) lateral view of the N. lugens head using SRXTM; before dissection; (f) after dissection; scale bar=500 µm.
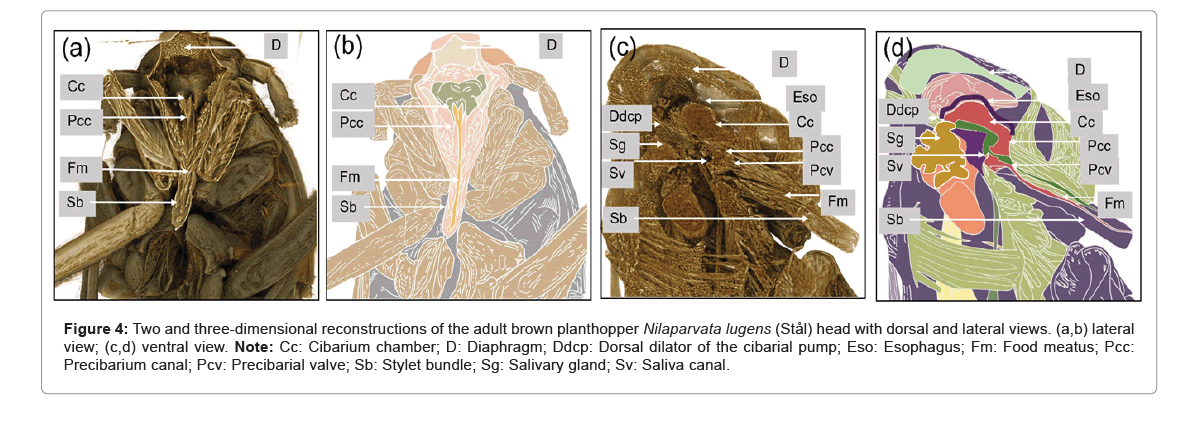
The 2D and 3D reconstructions of SRXTM images of the adult BPH provided a detailed view of the apodeme of the dilator muscle, cibarium chamber and food meatus. The 2D view revealed the precibarium canal, precibarial valve and precibarial valve pit as shown in Figure 4. Additional images of the head (Figures 4b and 4c) showcased the food meatus, cibarium chamber and precibarium, whereas the lateral view depicted specific parts of the cibarium area, including the food meatus, precibarium canal, precibarial valve, precibarial valve muscle and precibarial valve pit.
Comparatively, the internal morphology of the mouthparts displayed similarities to different Hemiptera species. The food meatus or stylet was connected to the precibarium canal, featuring the precibarial valve as shown in Figure 4. The cibarium chamber, located at the extreme anterior end of the esophagus, presented a sclerotized walled canal with quadrangle cross-sections (Figures 4c and 4d). Positioned vertically in the posterior part of the head, the cibarium chamber’s anterior wall invaginated into the lumen, providing flexibility and ease of expansion. The dorsal dilators of the cibarium chamber originated from the dorsal wall of the head and inserted into its anterior wall. The lateral dilators of the cibarium chamber inserted into the lateral wall, having their origins on the anterior arm of the tentorium.
Figure 4: Two and three-dimensional reconstructions of the adult brown planthopper Nilaparvata lugens (Stål) head with dorsal and lateral views. (a,b) lateral view; (c,d) ventral view. Note: Cc: Cibarium chamber; D: Diaphragm; Ddcp: Dorsal dilator of the cibarial pump; Eso: Esophagus; Fm: Food meatus; Pcc: Precibarium canal; Pcv: Precibarial valve; Sb: Stylet bundle; Sg: Salivary gland; Sv: Saliva canal.
Discussion
Synchrotron X-ray tomographic microscopy imaging
Studying the internal anatomy of small insects, especially those in the order Hemiptera, has historically posed significant challenges due to their minute size and delicate structures. Traditional methods, such as SEM and TEM, require meticulous sample preparation, embedding and sectioning, often leading to difficulties in obtaining satisfactory images of the delicate chitinous material. Additionally, SEM may obscure surface structures due to the sputter-coating of samples. In contrast, SRXTM offers numerous advantages, making it an invaluable tool for entomological research.
SRXTM enables the visualization of external and internal anatomy with exceptional clarity, producing high-definition, dynamic images previously unavailable using other methods. This technique requires minimal sample preparation and the ability to virtually dissect the sample allows for multiple views of the same sample without compromising its integrity. Although soft tissues may not be easily visualized using SRXTM, insects’ chitinous material, including the head structures like the cibarium, precibarium and mouthparts, offers excellent contrast, allowing for detailed examination without the need for staining or complex sample preparation. Notably, our SRXTM images are comparable in quality to static SEM images from previous studies but with the added benefit of the flexibility to repeatedly section samples to expose specific regions of interest [8,25,26].
Using SRXTM, we successfully identified crucial structures such as the precibarial valve and its pit, as previously reported in studies using SEM and X-ray microcomputed tomography [27]. The cibarium valve’s importance in BPH feeding is well-documented, as it controls the flow direction of ingested sap along with the cardiac (esophageal) valve [21]. Additionally, recent research on the fluid dynamics in the functional foregut of BPH first instar nymphs was made possible using SRXTM [21]. Furthermore, SRXTM can be used to study live insects due to its fast-scanning capabilities at synchrotron facilities, presenting exciting opportunities for investigating dynamic processes in live insects. The detailed characterization of the precibarial and cibarial morphology of the BPH corroborated findings from previous publications, where the precibarium was shown to be divided into proximal and distal regions by the flap-like precibarial valve located on the epipharynx. Overall, the SRXTM with the study of structures BPH has resulted in a comprehensive understanding of internal structures, facilitating the development of a phloem sap-sucking model based on the actual movements of the animal’s feeding apparatus during the process.
The BPH N. lugens is a piercing-sucking insect that feeds on the phloem sap of rice plants through stylets, which are modified mouthparts used to penetrate plant tissues. Their feeding behavior makes them an efficient vector of plant viruses. N. lugens acquires the RRSV virus when it feeds on infected rice plants using its stylet (food canal; (Figures 4c and 4d) and two sites have been identified for virus adhesion in the foregut: The precibarium and food meatus, inside the cibarium chamber. The feeding duration is important to acquire a sufficient virus titer, known as the acquisition access period [7]. Then, it enters the midgut’s epithelial cells and progresses to the visceral muscles surrounding the midgut. Subsequently, it disperses throughout the visceral muscles of the midgut and hindgut, ultimately reaching the salivary glands [6]. The time that elapses from initial acquisition to the ability to transmit the virus is known as the latent period. The minimum time required for infective BPH N. lugens from the latent period until inoculation to the host plant is called the inoculation access period. The BPH N. lugens transmits circulative, propagative plant RRSV via salivary glands [28]. The salivary glands are the destination for circulative transmission and viruses reach the salivary glands via the hemolymph or other routes such as through nervous tissue (neurotropic route) or connective tissues [29]. Using SRXTM, we visualized and characterized the internal structures of the foregut, specifically focusing on the cibarium and precibarium, which play critical roles in sap ingestion and phloem feeding. The identification of these structures and their function provides a basis for further research on how RRSV interacts with the vector within these anatomical compartments. The present findings shed light on the internal complexities of the BPH’s feeding apparatus and provided valuable information for future investigations into virus acquisition, retention and transmission mechanisms within insects. Based on these findings, we examined the mouthparts and foregut structures using SRXTM to virtually dissect the foregut to determine the practicality of using this technology for future experimental applications.
Conclusion
In conclusion, the SRXTM valuable insights into the internal structures of the BPH. These techniques offer advantages over traditional methods and present new avenues for understanding vector-pathogen interactions and developing innovative pest management strategies. As we continue to explore the intricate relationships between insects and plant pathogens, the application of advanced imaging and behavioral analysis techniques will undoubtedly drive significant progress in entomology and agricultural science.
Funding Information
This work was financially supported by the Agricultural Research Development Agency (Public Organization) (Grant No. PRP6405030060) and Kittibandit scholarships (2021), Suranaree University of Technology.
Data Availability Statement
The datasets used to support the findings of this study are available online from GEO database. All data can be acquired from the corresponding authors on request.
Acknowledgments
We would like to thank Ubon Ratchathani Rice Research Center, Thailand for improving the colony of brown planthopper.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chetanachit D, Putta M, Disthaporn S (1978) Rice ragged stunt in Thailand. IRRN 3:14-15.
- Ling KC, Tiongco ER, Aguiero VM (1977) Transmission of rice ragged stunt disease. IRRN 2:11-12.
- Wu J, Du Z, Wang C (2010) Identification of Pns6, a putative movement protein of RRSV, as a silencing suppressor. Virol J 7:335
[Crossref] [Google Scholar] [PubMed]
- Agrios GN (2005) Chapter fourteen-plant disease caused by viruses.
- Cabauatan PQ, Cabunagan RC, Choi IR (2009) Rice viruses transmitted by the brown planthopper Nilaparvata lugens stal in planthoppers: New threats to the sustainability of intensive rice production systems in Asia. International Rice Research Institute S3:357-368.
- Jia D, Guo N, Chen H, Akita F, Xie L, et al. (2012) Assembly of the viroplasm by viral non-structural protein Pns10 is essential for persistent infection of rice ragged stunt virus in its insect vector. J Gen Virol 93:2299-2309.
[Crossref] [Google Scholar] [PubMed]
- Hogenhout SA, Ammar ED, Whitfield AE (2008) Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46:327-359.
- Almeida RPP, Purcell AH (2006) Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Ann Entomol Soc Am 99:884-890.
- Brodersen CR, Roddy AB (2016) New frontiers in the three-dimensional visualization of plant structure and function. Am J Bot 103:184-188.
[Crossref] [Google Scholar] [PubMed]
- Hörnschemeyer T, Beutel RG, Pasop F (2002) Head structures of Priacma serrata Leconte (Coleptera, Archostemata) inferred from X-ray tomography. J Morphol 252:298-314.
[Crossref] [Google Scholar] [PubMed]
- Greco M, Jones A, Spooner-Hart R, Holford P (2008) X-ray computerised Microtomography (MicroCT): A new technique for assessing external and internal morphology of bees. J Apic Res 47:286-291.
- Greco MK, Tong J, Soleimani M (2012) Imaging live bee brains using minimally-invasive diagnostic radioentomology. J Insect Sci 2:89.
[Crossref] [Google Scholar] [PubMed]
- Fischer G, Sarnat EM, Economo EP (2016) Revision and microtomography of the pheidole knowlesi group, an endemic ant radiation in Fiji (Hymenoptera, Formicidae, Myrmicinae). PLoS ONE 11:e0158544.
[Crossref] [Google Scholar] [PubMed]
- Walker SM, Schwyn DA, Mokso R (2014) In vivo time-resolved microtomography reveals the mechanics of the blowfly flight motor. PLoS Biol 12:e1001823.
[Crossref] [Google Scholar] [PubMed]
- Blanke A, Rühr PT, Mokso R, Stampanoni M, Uesugi K, et al. (2015) Structural mouthpart interaction evolved already in the earliest lineages of insects. Proc Biol Sci 282:20151033.
[Crossref] [Google Scholar] [PubMed]
- Metscher BD (2009) MicroCT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol 9:11.
[Crossref] [Google Scholar] [PubMed]
- Betz O, Wegst U, Weide D (2007) Imaging applications of synchrotron X-ray phase-contrast microtomography in biological morphology and biomaterials science. I. General aspects of the technique and its advantages in the analysis of millimetre-sized arthropod structure. J Microsc 227:51-71.
[Crossref] [Google Scholar] [PubMed]
- Weintraub PG, Hoch H, Mühlethaler R (2014) Synchrotron X-ray micro-computed tomography as a tool for in situ elucidation of insect bacteriomes. Arthropod Struct Dev 43:183-186.
[Crossref] [Google Scholar] [PubMed]
- Westneat MW, Socha JJ, Lee WK (2008) Advances in biological structure, function and physiology using synchrotron X-ray imaging. Annu Rev Physiol 70:119-142.
[Crossref] [Google Scholar] [PubMed]
- Dierick M, Masschaele B, Hoorebeke V (2004) Octopus, a fast and user-friendly tomographic reconstruction package developed in labview®. Meas Sci Technol 15:1366-1370.
- Wang XQ, Guo J, Li DT (2021) Three-dimensional reconstruction of a whole insect reveals its phloem sap-sucking mechanism at nano-resolution. eLife 10:e62875.
[Crossref] [Google Scholar] [PubMed]
- Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, et al. (2014) A systematic nomenclature for the insect brain. Neuron 81:755-765.
[Crossref] [Google Scholar] [PubMed]
- Wipfler B, Weibing K, Klass KD (2016) The cephalic morphology of the American cockroach Periplaneta americana (Blattodea). Arthropod Syst Phylogeny 74:267-297.
- Spangenberg R, Friedemann K, Weirauch C (2013) The head morphology of the potentially basal heteropteran lineages enicocephalomorpha and dipsocoromorpha (Insecta: Hemiptera: Heteroptera) Arthropod Syst Phylogeny 71:103-136.
- Alves E, Leite B, Marucci RC, Pascholati SF, Andersen PC, et al. (2008) Retention sites for Xylella fastidiosa in four sharpshooter vectors (Hemiptera:Cicadellidae) analyzed by scanning electron microscopy. Curr Microbiol 56:531-538.
[Crossref] [Google Scholar] [PubMed]
- Ruschioni S, Ranieri E, Riolo P (2019) Functional anatomy of the preci-barial valve in Philaenus spumarius (L.). PLoS One 14:e0213318.
[Crossref] [Google Scholar] [PubMed]
- Killiny N, Brodersen CR (2022) Using X-ray micro-computed tomography to three-dimensionally visualize the foregut of the glassy-winged sharpshooter (Homalodisca vitripennis). Insects. 13:710.
[Crossref] [Google Scholar] [PubMed]
- Dietzgen RG, Mann KS, Johnson KN (2016) Plant virus-insect vector interactions: Current and potential future research directions. Viruses 8:303.
[Crossref] [Google Scholar] [PubMed]
- Whitfield AE, Falk BW, Rotenberg D (2015) Insect vector-mediated transmission of plant viruses. Virology 480:278-289.
[Crossref] [Google Scholar] [PubMed]
Citation: Chansawang N, Roddee J, Pakawanit P, Borikul N, Khangjoho S (2024) Foregut Structure and Physiology of Brown Planthopper, Nilaparvata lugens (Stål), Unveiled via Synchrotron Radiation X-ray Tomography. Diagn Pathol Open 9:238.
Copyright: © 2024 Chansawang N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 1434
- [From(publication date): 0-0 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 1104
- PDF downloads: 330