Research Article Open Access
Food Seed Health of Chick Pea (Cicer arietinum L.) at Panchgaon, Gurgaon, India
Narendra Kumar*
Amity Institute of Biotechnology, Amity University Haryana, Manesar, Gurgaon, Haryana, India
- Corresponding Author:
- Narendra Kumar
Amity Institute of Biotechnology
Amity University Haryana
Manesar-122 413, Gurgaon, Haryana, India
Tel: +919871288591
E-mail: narendra.microbiology@rediffmail.com
Received date: June 09, 2016; Accepted date: June 23, 2016; Published date: June 29, 2016
Citation: Kumar N (2016) Food Seed Health of Chick Pea (Cicer arietinum L.) at Panchgaon, Gurgaon, India. Adv Crop Sci Tech 4:229. doi:10.4172/2329-8863.1000229
Copyright: © 2016 Kumar N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Advances in Crop Science and Technology
Abstract
The chickpea (Cicer arietinum) is a legume of the family Fabaceae, subfamily Faboideae. It is also known as gram, or Bengal gram, garbanzo or garbanzo bean and sometimes known as Egyptian pea, ceci, cece or chana, or Kabuli chana (particularly in northern India). The 20 food seed samples of chick pea (Cicer arietinum L) were collected from farmer markets of Panchgaon (Gurgaon) Haryana. The mycobiota analysis through Std. blotter paper, Agar plate, Seed washates of food seed revealed the presence of 16 fungal species viz., Aspergillus flavus, A. fumigatus, A. niger, A. sydowi, A. ochraceous, A. terreus, A. nidulans, Cladosporium macrocarpum, F. oxysporum, F. semitectum, Macrophomina phaseolina, Penicillium notatum, Sclerotium rolfsii, Rhizoctonia solani, R. batatiocola, Rhizopus arrhizus. These species showed diversity in terms of Percent (%) frequency of fungi. In these species Aspergillus flavus, A. niger, A. ochraceous, A. terreus showed dominance in terms of Percent (%) frequency of fungi. The insect analysis revealed presence of one species of insect Callosobruchus chinensis. These species showed reduction in terms of weight loss, germination, protein content and carbohydrate content.
Keywords
Mycobiota; Callosobruchus chinensis; Chick pea (Cicer arietinum)
Introduction
Pulse seeds are reported to carry many moulds both in fields and during storage and association of this adversely affects quality and health of the seeds and under storage bring about several undesirable changes making them unfit for consumption and sowing. The chick pea (Cicer arietinum) is a legume of the family Fabaceae, subfamily Faboideae. It is also known as gram, or Bengal gram, garbanzo or garbanzo bean, and sometimes known as Egyptian pea, ceci, cece or chana, or Kabuli chana (particularly in northern India). Its seeds are high in protein chickpea seeds are eaten fresh as green vegetables, parched, fried, roasted, and boiled; as snack food, sweet and condiments; seeds are ground and the flour can be used as soup, dhal, and to make bread; prepared with pepper, salt and lemon it is served as a side dish and grown over 6.66 m ha of land in India.
Chickpea seed has 58.9% carbohydrate, 3% fiber, 5.2% oil, 3% ash, 0.2% calcium, and 0.3% phosphorus. It furnishes an important food for lower classes and the flour is quite nutritious. Many fungal species viz., Alternaria porri, A. alternata, Aspergillus amstelodami, A. flavus, A. fumigatus, A. nidulans, A. niger, A. sydowi, A. wentii, Botrytis cinerea, Cladosporium macrocarpum, Curvularia lunata, Fusarium equiseti, F. moniliforme, F. oxysporum, F. semitectum, Macrophomina phaseolina, Myrothecium roridum, Penicillium notatum, Rhizoctonia sp., and Rhizopus arrhizus been reported from chickpea Ahmad et al.[1].
Therefore present study was undertaken in order to study seed mycobiota in terms of frequency of the fungus and insect responsible for chickpea damage during storage.
Materials and Methods
The 20 seed samples of chickpea were collected from farmer stores of Panchgaon (Gurgaon).
Moisture content estimation
The weight (100-seeds) of Cicer arietinum were recorded on a randomly using an electronic balance. The seed moisture content was estimated following oven dry method using two replications each of 20 g ISTA [2]. After estimating the initial moisture content of seeds, about 200 g seed sample in each accession was kept in muslin cloth bags, to permit free flow of air, and placed in a seed drying room maintaining a constant temperature of 15°C and 15% RH. Seed samples were drawn at an interval of seven days to estimate the moisture content. Observations on mean 100-seed weight, moisture content are presented in Table 1.
| Seed size | 100-seed Weight(g) | Seed moisture content (%) | |
|---|---|---|---|
| 0 days | 7 days | ||
| Small Mean | 9.0 | 9.01 | 6.71 |
| Medium Mean | 17.5 | 9.27 | 7.32 |
| Large Mean | 39.0 | 9.71 | 6.94 |
| Overall Mean | 18.5 | 9.33 | 6.94 |
Table 1: Mean 100-seed weight, changes in moisture content and viability of small-, medium- and large-seeded chickpea under constant seed drying environment.
It is evident from Table 1 that the 100-seed weight of the seed of Cicer arietinum ranged from 9.0 to 39.0 g with a mean of 18.5 g which indicates the seed size diversity. Differences in seed moisture contents of Cicer arietinum under constant drying environment were significant among the three seed sizes. After seven days of incubation, small seeds showed moisture content of 9.01 to 6.71%, medium seeds from 9.27 to 7.32% and large seeds from 9.71 to 6.81%.
Detection of seed mycobiota
The mycobiota of small, medium and large sized seed was analysed following Standard blotter paper method, Agar plate method and Seed washates method.
Blotter method: The collected small, medium and large seed samples of chick pea (Cicer arietinum) were analyzed for the presence of major seed borne fungal pathogens by blotter method following the International rules for Seed Testing ISTA [3]. Two hundred mixed seeds were placed on three layers of moist blotting paper (Whatman No. 1) in each glass petridish containing 5 seeds. The petridishes were incubated at 25 ± 1°C under 12/12 hrs light and darkness cycle for 7 days. Each seed was observed under stereo microscope in order to record the presence of fungal colony. In doubtful cases temporary slides were prepared from the fungal colony and observed under compound microscope. For isolation of internal fungi seeds were treated with 1% sodium hypochlorite solution followed by 2-3 washings with sterile water. The developing fungal colonies were examined. The colour of the colony on the reverse side is also examined as it is characteristic for some fungi which are identified on this basis.
Agar plate method: In the agar plate method, two hundred mixed (small, medium and large) seeds of chick pea (Cicer arietinum) were tested for each maintaining four replications. For internal seed fungi Surface disinfected seeds (1% sodium hypochlorite solution) were plated on the Czapek Dox Agar medium and the plated seeds were usually incubated for 5-7 days at 28 ± 2°C under 12 h altering cycles of light and darkness. Sucrose 30.0; Sodium nitrate 2.0; Dipotassium phosphate 1.0; Magnesium sulphate 0.5; Potassium chloride 0.500; Ferrous sulphate 0.010; Agar 15.0 Gms / Litre; Final pH (at 25°C) 7.3 ± 0.2. These constituents were dissolved in 1000 ml distilled water. Heated to boiling to dissolve the medium completely, sterilized by autoclaving at 15 lbs pressure (121°C) for 15 minutes and mixed well and poured into sterile Petri plates. At the end of the incubation period, fungi growing out from the seeds on the agar medium were examined and identified. Identification was done based on colony characters and morphology of sporulation structures under a compound microscope. In the agar plate method more than one type of fungal colonies were produced. In this case, identification was done on the most frequently occurring colony present in all the petridishes and then the second most frequent, the third most frequent and so on. Thereafter, the identification of the different colonies were done visually and then under a stereomicroscope and followed by an examination of the fruiting structures under a compound microscope.
Seed washates method: 100 mixed seeds (small, medium and large) of chick pea (Cicer arietinum) were taken in flask with sterile distilled water for their soaking. The flasks were subjected to mechanical shaker for 5-10 minutes. 1 mL of seed washing, thus obtained was placed on PDA medium for growth of individual spore of fungus. The seed washing contains spores of the fungi. The plates were incubated at room temperature for development of colonies and observations were made. Fungi developed within 3 days. These colonies were immediately transferred to Czapek Dox Agar slants for further study. The various moulds appeared on seeds in blotter tast, agar plates and seed washates were isolated and maintained on Czapek Dox Agar. For identification of fungi temporary slides were prepared from the fungal colony and observed under compound microscope at 100X and 400X and identified with the help of keys suggested by Ref. [4-11]. The fungi from the incubated seeds were also transferred to PDA when needed.
The percent frequency was calculated by using following formulae.
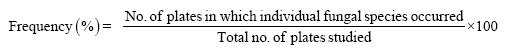
Histopathological techniques
To find the location of fungi in the seed, component plating was done. The seeds were soaked in water and thus obtained soft seeds were sectioned. The sections were stained and examined.
Culture of insects: The cultures of Callosobruchus chinensis (L.) were established from infested stored chick pea seeds collected from twenty farmer places and identified by literatures following Drees and Jackman [12] and Beck and Blumer [13]. The cultures of insects were maintained subsequently on insecticide free newly harvested chick pea seeds at laboratory (25 ± 2°C temperature) in darkness to obtain same aged insects.
Effect of storage pest on chick pea seeds
The deterioration caused by dominant fungal species viz., Aspergillus flavus, A. niger, A. ochraceous, A. terreus with respect to weight loss and seed germination was evaluated. For this purpose freshly harvested sterilized chick pea seeds were taken in presterilized polyetylene bags (200 g seeds/bag) and inoculated by two disc (5 mm diam) of different fungal species separately. Likewise 5 insect – Callosobruchus chinensis were inoculated separately in presterilized polyetylene bags. The inoculated matar seed samples were stored for 20 days under laboratory conditions at room temperature. Experiments were revised and contained five replicates. The protein content was studied following Lowry [14] using bovine serum albumin as standard. The optical density of each specimen was measured at 650 nm. Carbohydrate estimation was done following anthrone method of Thimmaih [15].
Results and Discussion
It is evident from Table 1 that the 100-seed weight of the seed of Cicer arietinum ranged from 9.0 to 39.0 g with a mean of 18.5 g which indicates the seed size diversity. Differences in seed moisture contents of Cicer arietinum under constant drying environment were significant among the three seed sizes. After seven days of incubation, small seeds showed moisture content of 9.01 to 6.71%, medium seeds from 9.27 to 7.32% and large seeds from 9.71 to 6.81%.
A total of 16 fungal species viz., Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Aspergillus sydowi, Aspergillus ochraceous, Aspergillus terrus, Aspergillus nidulans, Cladosporium macrocarpum, Fusarium oxysporum, Fusarium semitectum, Macrophomina phaseolina, Penicillium notatum, Rhizoctonia solani, Rhizoctonia batatiocola, Rhizopus arrhizus were isolated (Table 2). In these species Aspergillus flavus, Aspergillus niger, Aspergillus ochraceous and Aspergillus terreus showed higher per cent frequency. All fungi developed on agar and blotter paper, except for P. notatum which developed on agar plate only. Blotter method showed greater incidence of fungi on different parts of seeds followed by agar plate method and seed washates method.
|
S No |
Name of Fungi |
Percent (%) frequency of fungi |
||
|---|---|---|---|---|
|
Std. blotter Paper Mean± SE |
Agar PlateMean± SE |
Seed washates Mean± SE |
||
|
1 |
Aspergillus flavus Link |
50.7± 0.23 |
45.1 ± 0.21 |
35.6 ± 0.23 |
|
2 |
Aspergillus fumigatus Fresen |
20.1 ± 0.00 |
14.1 ± 0.11 |
10.1 ± 0.13 |
|
3 |
Aspergillus niger van Tieghem |
49.2 ± 0.12 |
44.3 ± 0.13 |
40.1 ± 0.14 |
|
4 |
Aspergillussydowi(Bainier and Sartory) |
5.1 ± 0.25 |
2.3 ± 0.11 |
2.1 ± 0.10 |
|
5 |
Aspergillus ochraceous Wilhelm |
45.1 ± 0.01 |
40.3 ± 0.12 |
37.1 ± 0.13 |
|
6 |
Aspergillus terreus Thom |
41.0 ± 0.13 |
38.1 ± 0.25 |
38.3 ± 0.26 |
|
7 |
Aspergillus nidulans (Eidam) G. |
10.0 ± 0.25 |
7.3 ± 0.23 |
3.3 ± 0.21 |
|
8 |
Cladosporium macrocarpum Preuss |
5.0 ± 0.00 |
3.0 ± 0.00 |
1.0 ± 0.00 |
|
9 |
Fusarium oxysporum von Schlechtendal |
8.1 ± 0.21 |
2.0 ± 0.22 |
1.1 ± 0.21 |
|
10 |
Fusarium semitectum Berk. and Ravenel, |
2.2 ± 0.00 |
1.1 ± 0.10 |
1.0 ± 0.00 |
|
11 |
Macrophomina phaseolina (Tassi) Goid |
1.0 ± 0.00 |
1.2 ± 0.01 |
0.8 ± 0.00 |
|
12 |
Penicillium notatum Thom |
- |
1.1 ± 0.00 |
1.0 ± 0.00 |
|
13 |
CephalosporumAcromonium Corda |
2.0 ± 0.00 |
1.5 ± 0.01 |
0.1 ± 0.00 |
|
14 |
Rhizoctonia solani J.G. Kühn |
1.6 ± 0.10 |
0.5 ± 0.00 |
|
|
15 |
Rhizoctoniabatatiocola(Taubenh.) E.J.Butler |
1.1 ± 0.00 |
1.0 ± 0.00 |
|
|
16 |
Rhizopusarrhizus A. Fisch |
1.2 ± 0.01 |
1.1 ± 0.02 |
0.3 ± 0.01 |
Table 2: Percent frequency of mycobiota on unsterilized seeds of chickpea.
The fungal species were reduced in surface sterilized seeds, which indicate that most of fungi were located on seed coat. Blotter method showed greater incidence of fungi on different parts of seeds followed by agar plate and deep-freezing method (Table 3). Component plating of chickpea seeds showed that seed coat and cotyledons were infected by greater number of fungi (16) followed by axis (radicle+plumule) (12). Aspergillus flavus, Aspergillus niger, M. phaseolina and R. solani were also isolated from seed coat, cotyledons and axis of seed.
|
S No |
Name of Fungi |
Percent (%) frequency of fungi |
||
|---|---|---|---|---|
|
Std. blotter paper Mean± SE |
Agar plate Mean± SE |
Seed washatesMean± SE |
||
|
1 |
Aspergillus flavus |
30.1 ± 0.31 |
34.1 ± 0.21 |
25.1 ± 0.23 |
|
2 |
Aspergillus fumigatus |
10.1 ± 0.01 |
10.1 ± 0.02 |
5.1 ± 0.03 |
|
3 |
Aspergillus niger |
39.2 ± 0.21 |
24.3 ± 0.23 |
10.1 ± 0.11 |
|
4 |
Aspergillussydowi |
5.1 ± 0.12 |
2.3 ± 0.02 |
2.1 ± 0.05 |
|
5 |
Aspergillus ochraceous |
35.1 ± 0. 07 |
30.3 ± 0.19 |
17.1 ± 0.17 |
|
6 |
Aspergillus terreus |
31.0 ± 0.75 |
20.1 ± 0.37 |
16.3 ± 0.21 |
|
7 |
Aspergillus nidulans |
7.0 ± 0.31 |
5.3 ± 0.21 |
1.3 ± 0.23 |
|
8 |
Cladosporium macrocarpum |
3.0 ± 0.31 |
2.0 ± 0.21 |
0.1 ± 0.34 |
|
9 |
Fusarium oxysporum |
3.1 ± 0.31 |
1.0 ± 0.32 |
0.1 ± 0.33 |
|
10 |
Fusarium semitectum |
1.2 ± 0.31 |
0.1 ± 0.22 |
|
|
11 |
Macrophomina phaseolina |
1.0 ± 0.21 |
0.3 ± 0.01 |
|
|
12 |
Penicillium notatum |
- |
0.4 ± 0.02 |
|
|
13 |
Cephalosporum acromonium |
1.8 ± 0.00 |
0.1 ± 0.00 |
|
|
14 |
Rhizoctonia solani |
1.7 ± 0.00 |
0.7 ± 0.00 |
|
|
15 |
Rhizoctonia batatiocola |
1.7 ± 0.03 |
1.0 ± 0.00 |
|
|
16 |
Rhizopusarrhizus |
1.5 ± 0.04 |
0.5 ± 0.00 |
|
Table 3: Percent frequency of mycobiota on sterilized seeds of chickpea.
As evident from Table 4, Aspergillus flavus, A. niger and insect –Callosobruchus chinensis played important role in seed weight loss and seed germination. The Aspergillus flavus inoculated seeds showed 13%, A. niger 14% while insect inoculated showed 18% protein content respectively. Aspergillus flavus inoculated seeds showed 16.10, A. niger 16.37 g while insect inoculated 17.10 g/100 g respectively. On account of wide occurrence and their pathogenicity these were selected as test organisms.
| Fungal species/insect | Weight loss(in/g) | Germination% | Protein % | Carbohydate per 100g | ||||
|---|---|---|---|---|---|---|---|---|
| C | T | C Mean ± SE) | T Mean ± SE) | C Mean ± SE) | T Mean ± SE) | C Mean ± SE) | T Mean ± SE) | |
| Aspergillus flavus | nil | 0.186 | 84.43 ± 0.01 | 45.17 ± 0.21 | 25.3 ± 0.11 | 13 ± 0.12 | 27.40± 0.13 | 16.10 ± 0.12 |
| A.niger | - | 0.179 | 86.13 ± 0.13 | 49.30 ± 0.12 | 25.6 ± 0.12 | 14 ± 0.00 | 26.42 ± 0.03 | 16.37 ± 0.04 |
| A.ochraceous | - | 0.130 | 81.0 ± 0.00 | 74.31 ± 0.03 | 25.8 ± 0.50 | 20 ± 0.00 | 26.41 ± 0.04 | 23.40 ± 0.03 |
| A.terreus | - | 0.05 | 85.00 ± 0.00 | 75.23 ± 0.32 | 26.6 ± 0.23 | 20 ± 0.07 | 26.41 ± 0.07 | 22.10 ± 0.13 |
| Insect-Callosobruchus chinensis | - | 0.184 | 86.00 ± 0.00 | 45.23 ± 0.13 | 28.0 ± 0.11 | 18 ± 0.00 | 26.46 ± 0.17 | 17.10 ± 0.12 |
C:Control;T: Treatment
Table 4: Fungal species /insect species vis-à-vis weight loss,germination and protein content of chickpea seeds after 20 days storage.
Patil [16] observed that fungi associated with seeds of Chickpea were Alternaria alternata, Aspergillus flavus, Aspergillus. niger, Aspergillus carboniferus, Cladosporium herbarum, Chaetomium globosum, Curvularia lunata, Fusarium moniliforme, Fusarium. oxysporum, Fusarium. semitectum, Fusarium roseum, Mucor sp. Penicillium citrinium, Phytophthora sp., Pythiurn sp., Rhizoctonia solani, Rhizopus stolonifer, Trichoderma viride while Singh [17] found nine fungal species, namely, Alternaria alternata, Aspergillus flavus, Aspergillus niger, Curvularia lunata (Cochliobolus lunatus), Fusarium moniliforme (Gibberella moniliformis), Helminthosporium sativum (Cochliobolus sativum) Mucor sp, Penicillium notatum and Rhizopus nigricans (R. stolonifer), were observed on seven seed samples of chickpea. All fungi developed on agar and blotter paper, except for P. notatum which developed on agar plate only.
But Ghangoaker and Kshirsagar [18] reported many fungal species viz. Alternaria alternata, Aspergillus terrus, A. flavus, A. fumigatus, A. niger, Botrytis sp, Cladosporium, Curvularia lunata, Fusarium solani, F. moniliforme, F. oxysporum, Macrophomina phaseolina, Penicillium notatum, Rhizoctonia sp. and Rhizopus nigricans from Cicer arietinum Razia [19] reported twenty one fungal species viz., M. sphaerosporus, R. arrhizus, C. cucurbitarum, A. niger, A. flavus, A. terreus, Afumigatus, P. vermiculatum, A. alternata, A. sonchi, A. clamydospora, C. cladosporioides, C. herbarum, C. clavata, D. australiensis, D. hawaiiensis, D. halodes, H. fuscoatra, F. equiseti, F. oxysporum and Fusariella spp. from the external seed surface of damaged seeds of gram. Highest frequency value (12.50) and relative abundance (9.50) were recorded for A. niger and lowest frequency (1.00) and relative abundance (2.00) were recorded for M. sphaerosporus, A. fumigatus, C. clavata, and F. equiseti. During a seed borne mycoflora of five cultivars of Cicer under blotter paper method all varieties were found more susceptible to Fusarium, Aspergillus niger, Aspergillus flavus, Botrys cinerea, Sclerotium rolfisii Margeret et al. [20]. In a study Zaidi [19] isolated thirty fungal species were among these were Alternaria alternata, Chaetomium spp., Penicillium citrinum, Aspergillus niger, A. flavus, Rhizopus nigricans, Fusarium oxysporum. The no of fungal species were reduced in surface sterilized seeds which indicate of that many of the fungi were located on seed coat. Blotter method showed greater incidence of fungi on different parts of seeds followed by agar plate method. The seed mycoflora devalue the seed quality, reduce its nutritional value and cause a germination failure of the seedlings and of the crop raised from such infected seeds. Narayan [18] reported Alternaria alternata, Chaetomium spp., Penicillium citrinum, Aspergillus niger, A. fumigatus, A. flavus, Rhizopus nigricans, Fusarium oxysporum, F. moniliform, F. solani, Chaetomium sp, Curvularia lunata, Macrophomin sp, Monilia sp., Penicillium sp., Rhizoctonia sp, Trichoderma etc. from gram seeds. Sontakke and Hedawoo [21] found thirteen different fungi like Actinomucor repens, Alternaria alternata, Aspergillus flavus, A. fumigatus, A. niger, A. ochraceus, Cladosporium sp., Fusarium oxysporum, Fusarium sp., Mucor varians, Penicillium notatum, Phoma herbarum, Rhizopus stolonifer which were isolated in variable frequencies. Frequency of the individual species ranges between 1.11-8.19%. Of which, Fusarium oxysporum (8.19%), Rhizopus stolonifer (7.63%), Phoma herbarum (5.69%) and Aspergillus flavus (5.44%) were found to be predominant. Blotter paper method was found to be more effective than agar plate method. The percent germination of the Chickpea seeds was evaluated by the standard rolled paper towel method. Higher incidence of fungi on the seeds of chickpea adversely affected its germination. Many fungal species viz., Alternaria porri, A. alternata, Aspergillus amstelodami, A. flavus, A. fumigatus, A. nidulans, A. niger, A. sydowi, A. wentii, Botrytis cinerea, Cladosporium macrocarpum, Curvularia lunata, Fusarium equiseti, F. moniliforme, F. oxysporum, F. semitectum, Macrophomina phaseolina, Myrothecium roridum, Penicillium notatum, Rhizoctonia sp., and Rhizopus arrhizus have been reported from chickpea. These fungal diseases can kill chickpea crops and is difficult to remove once it sets in Ahmad [1] and Singh [22] also isolated seven fungal species such as Alternaria alternata, Aspergillus flavus, A. niger, A. fumigatus, Curvularia lunata, Fusarium monoliforme and Rhizocton ia solani.
Component plating of chickpea seeds showed that seed coat and cotyledons were infected by greater number of fungi followed by axis (radicle+plumule). Aspergillus flavus, Aspergillus niger, M. phaseolina and R. solani were also isolated from seed coat, cotyledons and axis of seed. Similarly Shahnaz et al. [23] reported that seed coat and cotyledons were infected by greater number of fungi followed by axis (radicle+plumule). M. phaseolina and R. solani were also isolated from seed coat, cotyledons and axis of seed. The fungal species were reduced in surface sterilized seeds, which indicate that most of fungi were located on seed coat.
Seed germination reduced significantly in artifically inoculated seeds with Fusarium oxysporum f. sp. ciceri as compared to uninoculated seeds Lily and Trivedi [24]. Similar, trend of results could be due to different response of different varieties due to their susceptible and resistant reactions and thus, indicating more seed borne infection in susceptible variety Singh et al. [17]. Rathod [25] studied standard blotter paper, agar plate and seed washates methods for seed mycoflora study. Among the three methods, the agar paper method was found to be suitable as in less incubation; there was higher percent incidence of seed mycoflora. The variation in fungal species may be due to different climatic conditions, isolation periods and different storage containers.
Use of Sodium hypochloride helped in minimizing the incidence of superficial and fast growing as well as common seed borne fungi like Aspergillus spp., Chaetomium spp., Cladosporium spp., Rhizopus spp., Cephalosporium spp. Similar results were obtained by Dawar and Ghaffar [26] on sunflower seeds. Surface disinfection of seed with 1% Na(OCl)2 reduced the incidence of Aspergillus spp. A number of fungi isolated in the present study are known to produce mycotoxins which are harmful for human health. Mycotoxins can cause severe damage to liver, kidney and nervous system of man even in low dosages Rodricks [27].
Aspergillus flavus produces aflatoxin B1, B2, G1, G2 which are carcinogenic and produce liver cancer Purchase, Diener and Davis (Pestka and Bondy) [28-30]. Fusarium solani cause corneal ulcer while F. oxysporum produce Zeralenone α and β causing haemorrhage and necrosis in bone marrow. F. proliferatum and F. verticillioides cause epidemiologically human esophageal cancer Desjardins et al. [31]. Significant decrease in protein content due to attack of seed-borne fungi like Aspergillus flavus and Fusarium semitectum has been observed in seeds of Black gram and Green gram Bilgrami et al. [32]. Prasad and Pathak [33] reported loss in protein content of cereals like Wheat, Maize and Barley seeds affected by Fusarium oxysporum and Fusarium semitectum under different storage condition. Similarly in present investigation Aspergillus flavus, A. niger showed a decrease in protein and carbohydrate content.
Conclusion
There is need for proper storage of chickpea seed to minimize the fungal infestation and mycotoxin production during storage and provide disease free food seeds for human consumption.
Acknowledgements
Author is thankful to Director, Amity Institute of Biotechnology, Amity University Haryana for providing Library and Laboratory facilities.
References
- Ahmad I, Iftikhar S, Bhutta AR (1993) Seed borne microorganism in Pakistan. A checklist 1991. Pakistan Agricultural Research Council, Islamabad, Pakistan,p: 32.
- ISTA (1993) International rules for seed testing. Seed Science and Technology 21: 1-288.
- ISTA (1966) International Rules of Seed Testing. ProcIntSeed Test Assoc 32: 565-589.
- MaloneGP, MusketteAE (1964)Seed Borne Fungi: Description of 77 Fungal Species.Proc Int Seed Test Assoc 29: 180-183.
- Booth C (1971) The genus Fusarium. Common Wealth Mycological Institute, Kew Survey, England, p: 237.
- RaperKB, ThomC (1949) A Manual of the Penicillia. Boulliere, Tindall and Cox, London,UK, p:875.
- Gillman JC (1967)A manual of soil fungi. Oxford and JBH Publishing Co, India.
- RaperKB, FennellDI(1965) The genus Aspergillus. The Williams and Wilkins Company, Baltimore,USA, p:686.
- Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England,UK, p: 608.
- Ellis MB (1976) Moredematiaceous hyphomycetes. Common Wealth Mycological Institute, Kew Surrey, England.
- ChidambaramPS, Mathur SB (1975) Deterioration of Grains by Fungi. Ann Rev Phytopathol 3: 69-89.
- DreesBM, JackmanJ (1999) Field Guide to Texas Insects. Gulf Publishing Company, Houston, Texas, USA.
- BeckCW, BlumerLS (2007) A hand book of bean beetles, Callosobruchus maculatus. Bean Beetles.
- Lowry OH, RosebroughNJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- ThimmaiahSK (1999) Standard methods of Biochemical analysis-Anthrone method. Kalyani Publishers, Ludhiana,India, pp: 54-55.
- PatilDP, PawarPV, MuleySM (2012) MycofloraassociatedwithPigeonpeaandChickpea. International Multidisciplinary Research Journal 2:10-12.
- SinghK, SinghAK, SinghRP (2005) Detectionofseedmycoflora of chick pea (Cicer arietinum L.).Annals of Plant Protection Sciences 13:167-171.
- NarayanM, Ghangaoker A,KshirsagarD (2013) Study ofSeed Borne Fungi of Different Legumes.Trends in Life Sciences 2: 32-35.
- Razia K, Zaidi, PathakN (2013)Evaluation of seed infection of fungi in Chickpea. e-Journal of Science & Technology, p:8.
- Margaret, NeerajaPV,RajeswariB (2013) Screening of Seed Borne Mycoflora of Cicer arietinum. Int J Curr Microbiol App Sci2: 124-130.
- SontakkeN,Hedawoo (2014)Mycofloraassociated with seeds of chickpea. Journal of Life Sciences 2: 27-30
- SinghVK (2014) Detection of mycoflora associated with Cicer arietinum seeds by agar plate method with PDA. Weekly Science Research Journal 1: 1-4.
- ShahnazD, FarzanaS, Ghaffar A (2007) Seed borne fungi associated with chickpea.Pak J BotPakistan 39: 637-643.
- Lily T, Rathi YPS (2015) Detectionofseedmycoflorafromchickpea wilt complex seedborne Fusarium oxysporum f.sp. ciceri diseased seeds. World Journal of Pharmacy and Pharmaceutical sciences 4:1242-1249
- RathodLR, JadhavMD, ManeSK,MuleySM, DeshmukhPS (2012)Seed Borne Mycoflora of Legume Seeds. International Journal of Advanced Biotechnology and Research3: 5
- Dawar S, Ghaffar A (1991) Detection of Seed borne mycoflora of sunflower. Pak J Bot 23: 173-178
- Rodricks JV (1976)Mycotoxinsandotherfungusrelatedfoodproblems. Advance in Chemistry, Series 149. American Chemicals Society, Washington,USA, p:239.
- PurchaseIRH (1974)Mycotoxin. Elsevier Scientific Publ. Amsterdam, p:443.
- Diener UL, Davis ND (1969) Relation of environment to aflatoxinproduction from Aspergillusflavus. pp:15-34.
- PestkaJJ, BondyGS (1990) Alteration of immune function following dietary mycotoxin exposure. Can J Physiol Pharmacol68: 1009-1016.
- Desjardins AE, Busman M, Proctor R, Stessman R (2006) Wheat kernel black point and fumonisin contamination by Fusarium proliferatum. National Fusarium Head Blight Forum Proceedings,p: 115.
- Bilgrami KS, Jamaluddin, RizviMA(1979)Fungi of India. Today and Tomorrow’s printers and Publishers, New Delhi, India.
- Prasad T, PathakSS (1987)Impact of various storage systems on biodeterioration of cereals. Indian Phytopath 40: 39-46.
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 11976
- [From(publication date):
August-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10999
- PDF downloads : 977
