Research Article Open Access
First Use of an Ingestible Sensor to Manage Uncontrolled Blood Pressure in Primary Practice: The UK Hypertension Registry
Naik R1, Macey N2, West RJ3, Godbehere P4, Thurston SC5, Fox R6, Xiang W7, Kim Y8, Singh I9, Leadley S10 and DiCarlo L11*1Department of Medicine, Far Lane Medical Centre, United Kingdom
2Department of Medicine, StoHealth, Violet Hill House, United Kingdom
3Department of Medicine, Woolpit Health Centre, United Kingdom
4Department of Medicine, North Brink Practice, United Kingdom
5Department of Medicine, Wymondham Medical Practice, United Kingdom
6Department of Medicine, The Health Centre, United Kingdom
7Data Management, Proteus Digital Health, London, United Kingdom
8Data Analytics, Proteus Digital Health, London, United Kingdom
9Clinical Operations, Proteus Digital Health, London, United Kingdom
10Clinical Support, Proteus Digital Health, London, United Kingdom
11Medical Affairs, Proteus Digital Health Ltd., London, United Kingdom
- *Corresponding Author:
- Lorenzo DiCarlo, MD
Head, Global Clinical Affairs, Proteus Digital Health, Inc.
2600 Bridge Parkway, Redwood City, California, 94109, USA
Tel: +1 415 806 9000
E-mail: ldicarlo@proteus.com
Received date: February 21, 2017; Accepted date: February 27, 2017; Published date: February 28, 2017
Citation: Naik R, Macey N, West RJ, Godbehere P, Thurston SC, et al. (2017) First Use of an Ingestible Sensor to Manage Uncontrolled Blood Pressure in Primary Practice: The UK Hypertension Registry. J Community Med Health Educ 7:506. doi:10.4172/2161-0711.1000506
Copyright: © 2017 Naik R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Background: Primary care physicians used a CE-marked ingestible sensor in a real world setting to assess patients with persistent hypertension. The objectives were to (1) characterize the pattern of their medication use, (2) differentiate medication use from pharmacologic unresponsiveness as a potential underlying cause for uncontrolled hypertension; (3) categorize and summarize subsequent management decisions; and (4) assess the usability and acceptability of passive electronic monitoring for managing hypertension.
Methods: There were 167 patients with uncontrolled hypertension whilst chronically prescribed 2 to 5 antihypertensives; 40% were chronically prescribed ≥ 3 antihypertensives. Patients were instructed to ingest a pharmacologically inert tablet containing the ingestible sensor whenever they took their prescribed medications. A wearable sensor on the patient's torso passively recorded the dates/times of tablet ingestion. Taking adherence (i.e., the total number passively detected divided by the total number prescribed) and timing adherence were determined for a period of two weeks with no change in therapy.
Findings: Taking adherence ranged from 53-100%; timing adherence ranged from 21-100%. Systolic blood pressure decreased from 154 ± 13 to 145 ± 18 mmHg (mean -9.7 mmHg; 95% CI: -12.5, -7.0 mmHg, p<0.001). Diastolic blood pressure decreased from 85 ± 11 to 80 ± 12 mmHg (mean -5.0 mmHg; 95% CI: -6.5, -3.5 mmHg, p<0.001). Thirty-two percent (32%) of participants achieved their treatment goals using their existing therapy. Subsequent management included: medication reviews and/or adherence counseling with no anti-hypertensive changes in 81%, anti-hypertensive changes in 13%, referrals to specialists in 1%, and no additional actions were taken in 6%. Practitioners found the offering easy to utilise, and the majority of patients expressed a positive experience in using it.
Conclusion: In patients with uncontrolled blood pressure, the inclusion of an ingestible sensor in everyday practice helped practitioners to identify potential factors contributing to persistent hypertension and to determine subsequent patient-specific interventions.
Keywords
Anti-hypertensive; Blood pressure; Digital health; Digital medicine; Hypertension; Medication adherence; Non-adherence; Telehealth
Introduction
One third of adults in the United States and United Kingdom have hypertension [1,2]. Current control rates are below the Healthy People goal of 50%, which was originally set as the year 2000 goal and has since been extended [3]. There is a range of interventions that can be used to support patients in medicines adherence [4,5]. However at the core of clinical decisions for individual hypertension management is the distinction between non-adherence and pharmacologic unresponsiveness as the potential root cause for individuals with uncontrolled blood pressure, or who appear to require a considerable pharmaceutical burden to achieve blood pressure control [6].
In this registry, primary care physicians used a Commonwealth of Europe (CE)-marked ingestible sensor and a wearable sensor in the real-world setting of their everyday practice to assess patients with persistent hypertension. The objectives were to (1) characterize the pattern of their medication use, (2) differentiate medication use from pharmacologic unresponsiveness as a potential underlying cause for uncontrolled hypertension; (3) categorize and summarize subsequent management decisions; and (4) assess the usability and acceptability of passive electronic monitoring for managing hypertension. The registry also served to determine potential effect sizes for the design of a subsequent controlled study using cluster randomization.
Patients and Methods
The registry consisted of a single cohort of consecutive patients in a real-world setting of six primary care practices in the Midlands of England. The protocol for this registry was reviewed and approved by the National Research Ethics Service (NRES Committee East Midlands – Derby).
Patients were enrolled consecutively in the registry and were not randomized. They were required to have uncontrolled blood pressure whilst chronically prescribed 2 or more anti-hypertensives. Written informed consent was obtained from participants before enrollment in the registry.
The registry was open for up to 175 patients with an age of at least 18 years and grade 1, 2 or 3 essential hypertension, or grade 1 or 2 isolated hypertension, as described by the National Institute for Care and Excellence (NICE), were allowed [7]. Patients were excluded who had a history of hypersensitivity to adhesive medical tape, a history of acute or chronic dermatitis, or any acute or chronic condition that, in the practitioner’s opinion, would make a patient unable to participate fully in the registry.
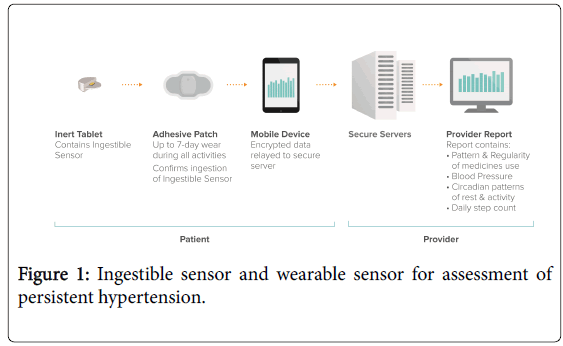
Patients were instructed to include ingestion of a pharmacologically inert tablet containing the ingestible sensor whenever they took their prescribed medications, and to wear a wearable sensor (also called “Patch”) on their torso continuously for two weeks, with replacement after one week, for passive recording of the dates/times of tablet ingestion and other activity patterns. The ingestible sensor and the wearable sensor that were used in this study consisted of CE-marked type 2 medical devices (Proteus Digital Health, Inc., USA; Figure 1).
The poppy seed-sized ingestible sensor is made of foodstuffs, and the wearable sensor may be utilized for seven-day wear during all activities including exercise and bathing. After entering the stomach, the ingestible sensor uses the gastric fluid to send a tiny and brief biogalvanic signal. The biogalvanic signal of each ingestible sensor is unique. The signal contains no radiation, is not pH-dependent, and lasts approximately 7 minutes. The remainder of the ingestible sensor is then inactive and is eliminated like a poppy seed husk in the faeces. Previously, more than 500 subjects have ingested more than 20,000 ingestible sensors in clinical studies that have included subjects with bipolar disorder, diabetes, heart failure, hypertension, renal transplantation, schizophrenia, tuberculosis, seniors with fragile skin and healthy volunteers. The most common side effect reported during ingestible sensor use has been nausea/vomiting (~1%). No devicerelated serious adverse events and no unanticipated adverse device effects have been reported. The ingestible sensor has been co-ingested with more than 400 different drugs without adverse interaction [8].
The biogalvanic signal of each ingestible sensor is unique. In addition to passively recording the date and time of ingestible sensor ingestions the wearable sensor also passively records activity patterns that include daily step count, sleep duration and quality, and circadian pattern. All of the information that is collected on an individual’s wearable sensor is anonymous and secure. Each patient owns all of the health data in his or her bespoke Proteus account. Control over access to the data is enforced through this ownership, and technically enforced through encryption and secure servers. No one is allowed to decrypt these health data without the permission of the patient.
A two week time period of ingestible sensor use was supported by a meta-analysis demonstrating that the estimation of maximal effect can be made between 1 and 2 weeks after initiation of antihypertensive therapy, and was also consistent with existing practice patterns in the United Kingdom [9]. Data from the wearable sensor were downloaded for correlation with blood pressures that were obtained at the time of clinic visits at the beginning and after 2 weeks of ingestible sensor use.
All of the primary care practices who participate in the registry utilized NICE guidelines for blood pressure assessment and management [10]. Practitioners measured blood pressure using their existing office equipment. Before registry entry, individuals had been chronically prescribed treatment as appropriate to existing NICE treatment guidelines for hypertension management in adults. No changes were made in existing anti-hypertensive therapy during the two weeks of ingestible sensor use. Patients were fully aware that their medication and activity patterns were being monitored. A written report was generated automatically and provided to the practitioners who reviewed it with their patients and advised subsequent consequent steps for blood pressure management. Management decisions were left to each practitioner’s discretion according to usual and customary practice, and were collected for categorization and summarization. Patients and practitioners then completed a written satisfaction survey.
Taking adherence was defined as the total number of Ingestible Sensors that were detected by the Wearable Sensor, divided by the number of total number of doses that were prescribed). Timing (also known as “scheduling”) adherence was defined as the number of Ingestible Sensors that were detected within a ± 2 hour time window around the prescribed dosing time, divided by the total number of ingestible sensors that were detected during that dosing interval. Eighty percent (80%) was used as a threshold for adequate taking adherence, as this same threshold has been used in a majority of the studies that have been published in the literature on medication adherence [11]. Using existing NICE guidelines, blood pressure control was defined as a BP<140/90 without diabetes, and <130/85 with diabetes [12].
Changes in blood pressure after two weeks of ingestible sensor use were evaluated using a pre-post analysis. A sample size of approximately 150 subjects was identified as being sufficient to identify 25% of the registry population being capable of achieving normal daytime ambulatory blood pressure targets when compared to baseline (power ≥ 80% and type-1 error less than 0.05) [13]. Categorical data analyses were performed secondarily (1) to identify potential interactions between comorbidity, or antihypertensive drug class, and achievement of blood pressure control; and (2) to summarize patient and practitioner responses to the satisfaction surveys.
Results
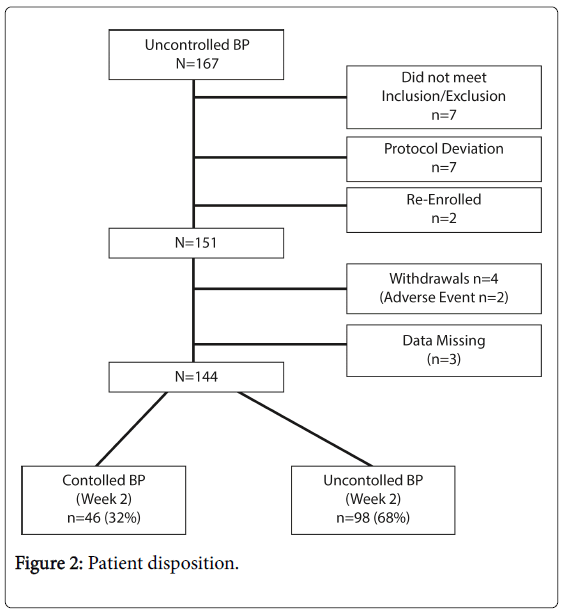
There were 167 participants in the registry. Figure 2 summarizes the numbers of individuals at each stage of the registry. All were Caucasian, more than half of patients were male, and the average age was 68 years. Thirty per cent had diabetes as co-morbidity. The range of chronically prescribed anti-hypertensives was two to five, and approximately 40% of individuals were prescribed ≥ 3 antihypertensives at the time of ingestible sensor use (Table 1).
| Gender [n, (%)] Male | 88 (58) |
| Age [years] Mean ± S. D. Range |
68 ± 9 31,90 |
| Ethnicity [n, (%)] Caucasian, Non-Latino | 151 (100) |
| Baseline blood pressure [mm Hg] Systolic [mean (range)] Diastolic [mean (range)] |
154 (125,207) 85 (47,110) |
| Diabetic [n (%)] | 45(30) |
| Anti-hypertensives [number of subjects (%)] Generic Generic and Branded Branded |
136 (90) 15 (10) 0 |
| 100 Anti-hypertensives/subject (range 2-5) [n (%)] 1 2 >2 |
0 93 (62) 58 (38) |
| Drug Class [number of subjects (%)] ACE inhibitor Angiotensin receptor blocker Beta-blocker Calcium channel antagonist Diuretic Fixed-dose combination Other |
82 (54) 57 (38) 43 (28) 93 (62) 72 (48) 3 (2) 29 (19) |
Table 1: Baseline demographics, blood pressures, and classes of prescribed medications and doses before Digital Health Service use.
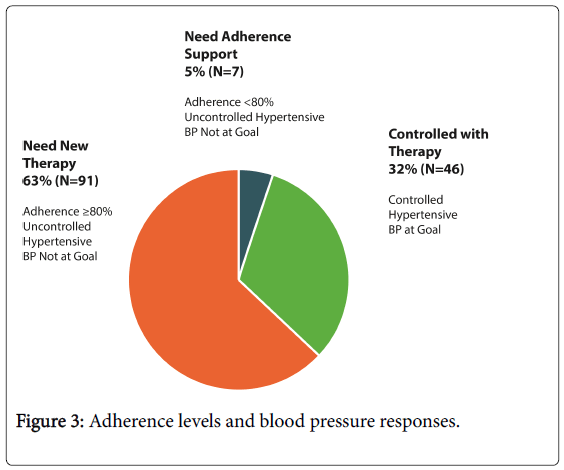
During two weeks of ingestible sensor use, taking adherence ranged from 53-100%, and timing adherence ranged from 21-100%. Systolic blood pressure decreased from 154 ± 13 to 145 ± 18 mmHg (mean -9.7 mmHg; 95% CI: -12.5, -7.0 mmHg, p<0.001) and diastolic blood pressure decreased from 85 ± 11 to 80 ± 12 mmHg (mean -5.0 mmHg; 95% CI: -6.5, -3.5 mmHg, p<0.001) (Figure 3).
Thirty-two percent (32%) of registry participants were found to be capable of achieving blood pressure control using their chronically prescribed medications. Secondary categorical analyses revealed that 40% of subjects without diabetes achieved blood pressure control, compared to 12% of subjects with diabetes. No correlations between comorbidities and inadequate anti-hypertensive use were found. There was also no correlation found between anti-hypertensive drug class and the root cause for persistent hypertension, with the exception of beta-blockers: 71% of patients who were prescribed beta-blockers had pharmacological resistance versus 51% who were not prescribed betablockers.
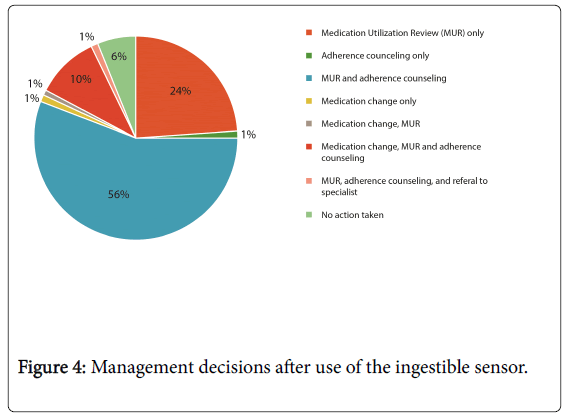
Subsequent management included: medicines reviews and/or adherence counseling with no anti-hypertensive changes in 81%, medication changes in 13%, and no additional actions were taken in 6%. One patient was referred to the hypertension specialist for additional assessment (Figure 4).
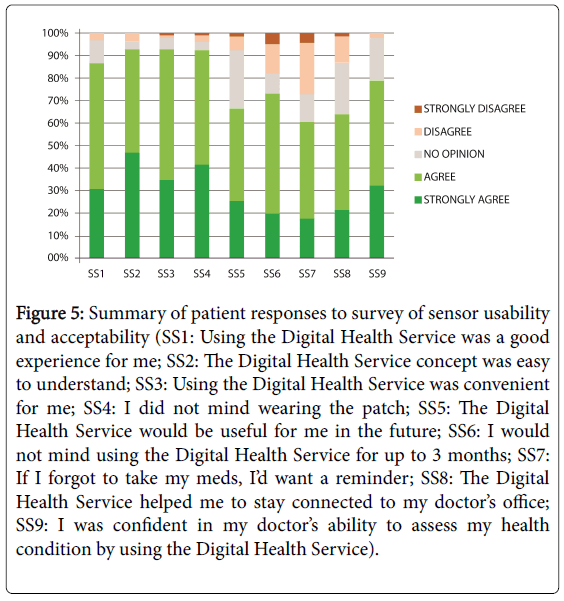
Ninety-two percent (92%) of patients reported that they did not mind wearing the wearable sensor. More than 87% of patients reported having a good experience from using the ingestible and thought that it was easy to understand and convenient to use (Figure 5). In a provider satisfaction survey (n=8), the majority of practices (75%) found that the use of the ingestible sensor added value to their practice, improved the conversations that they had with patients about treating their hypertension, and helped them stay connected their patients.
Figure 5: Summary of patient responses to survey of sensor usability and acceptability (SS1: Using the Digital Health Service was a good experience for me; SS2: The Digital Health Service concept was easy to understand; SS3: Using the Digital Health Service was convenient for me; SS4: I did not mind wearing the patch; SS5: The Digital Health Service would be useful for me in the future; SS6: I would not mind using the Digital Health Service for up to 3 months; SS7: If I forgot to take my meds, I’d want a reminder; SS8: The Digital Health Service helped me to stay connected to my doctor’s office; SS9: I was confident in my doctor’s ability to assess my health condition by using the Digital Health Service).
Side effects were minimal, consisting of mainly minor skin irritation (8%), or gastrointestinal symptoms (3%). Two patients (1%) withdrew due to what practitioners believed to be possibly-related adverse events: one patient had chest pain, diarrhea and abdominal pain, and the other patient had constipation. One patient discontinued the ingestible sensor due to abdominal discomfort possibly-related but continued the wearable sensor in the study.
Discussion
This report summarizes the first use of an ingestible sensor by primary practitioners in a real-world setting to evaluate persistent hypertension. Practitioners found the ingestible sensor useful in distinguishing medication use from pharmacological resistance as a potential underlying cause of persistent hypertension, and determining subsequent patient-specific interventions. Thirty-two percent (32%) of registry participants were found to be capable of achieving blood pressure control using their existing chronically prescribed medications.
This was an open-label registry and randomization to dummy devices was not utilized. Patients were fully aware that their daily medication adherence and activity patterns were being assessed by their providers. Whilst this reflects a different perspective than that of a randomized, controlled trial, it does nonetheless demonstrate how the product is used in actual practice. Patients may have increased their adherence whilst using the ingestible sensor due to a Hawthorne effect. Rather than being a limitation, it actually provided strength, as it facilitated determining whether blood pressure control was actually achievable on existing anti-hypertensive treatment. Ingestible sensor co-ingestion at the time of medication dosing was a surrogate for actual medication ingestion, and patients may have been selective in the medications that they actually ingested. Therefore, whilst use of the ingestible sensor successfully stratified one-third of patients who were capable of blood pressure control without therapy escalation, this effect size may be an underestimate of actual effect size when the ingestible and an anti-hypertensive are integrated together for oral administration. Such will be the case in the subsequently planned randomized study.
Of practical importance, for those patients who can be demonstrated by the ingestible sensor to be adherent and capable of achieving blood pressure control with existing therapy, controlled blood pressure can then be used as a legitimate surrogate for medication-taking for a period of subsequent months. Blood pressure elevation during this period of time can be addressed through medication reviews and adherence counseling by allied health professionals as an alternative to additional general practitioner visits and superfluous prescribing for blood pressure management.
The findings from the rural primary practices that participated in this registry complement findings from those in 15 rural community pharmacies [14]. Approximately 30% of patients with uncontrolled baseline blood pressure met their blood pressure goals after 2 weeks with no changes in existing anti-hypertensive prescribing. The majority of the pharmacists found that sharing the information collected from use of the ingestible sensor and wearable sensor with their patients helped to create a collaborative experience, and over 85% of the patients surveyed found the ingestible sensor and wearable sensor to be both usable and useful.
Non-adherence is a health problem of major relevance at a time when the National Health Service is facing immense financial challenges, and poor adherence to antihypertensive therapy is a major cause of lack of BP control [15,16]. In addition, medication wastage is a significant and largely unaddressed issue, as a significant amount of patients do not take their medications as intended [17]. NHS is looking to minimize medication wastage by improving prescribing and dispensing systems, and encouraging rational cost-effective prescribing [18]. Yet, whilst there is a need to identify and target those patients who do not take their medicines appropriately, physicians are unaware of how patients take their medications [19-21]. This registry has demonstrated that the use of an ingestible sensor is feasible for this purpose in a primary care setting.
Conclusions
In the day-to-day management of patients with uncontrolled blood pressure, the inclusion of ingestible sensor and accompanying wearable sensor were easy to incorporate in a primary care setting, were helpful in determining a potential underlying cause for persistent hypertension, and provided useful information for individually tailoring subsequent management decisions.
Further evaluation is planned to determine the clinical and health economic impacts of using a direct means of confirming medication ingestion, through the utilization of “digital medication” dose forms having an ingestible sensor in actual combination with each antihypertensive medicinal. Ultimately, the ability to stratify individual patients with uncontrolled blood pressure to their appropriate treatment pathway is in their best interest. Identifying those who are capable of achieving treatment goals on their existing therapy is a benefit to them and a benefit to the health system as a whole if otherwise unnecessary primary practice visits, prescribed antihypertensive medicinal, specialist referrals, and specialty diagnostics can be eliminated. This would fit well with the NHS Right Care agenda to deliver value from healthcare over the remainder of this decade through evidence-based planning, delivery and monitoring of healthcare delivery [22].
Funding
This registry was sponsored, and any practitioner activities that were incremental to those of usual care were funded, by Proteus Digital Health, Incorporated (Redwood City, CA, USA).
Contributor Statement
All co-authors contributed to the design of the registry. R Nai, N Macey, RJ West, P Godbehere, S Thurston and R Fox served as principal investigators. S Leadley and I Singh assisted with study execution. Y Kim led the data analysis and W Xiang assisted with data integrity and the analysis. Lorenzo DiCarlo prepared the subsequent manuscript drafts, which the authors reviewed and revised.
Acknowledgements
The authors wish to thank Gregor Timlin, Liam Turner, and Lottie Crumbleholme for creating the figures for this manuscript.
References
- CDC (2011) Vital signs: prevalence, treatment, and control of hypertension-United States, 1999-2002 and 2005-2008. MMWR 60: 103-108.
- Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, et al. (1996) A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 9: 285-292.
- Centers for Disease Control (2010) Healthy people 2010.
- Report and Action Plan of the Steering Group on Improving the Use of Medicines (2012) Improving the use of medicines for better outcomes and reduced waste.
- The Royal Pharmaceutical Society (2013) Medicines optimisation, helping patients to make the most of medicines: Good practice guidance for health care professionals in England.
- Godbehere P, Wareing P (2014) Hypertension assessment and management: Role for digital medicine. J Clinical Hypertension 16: 235.
- National Institute for Care and Excellence [NICE] (2011) CG 127 Hypertension.
- Kim YA, Virdhi N, Raja P, DiCarlo L (2014) Modelling the impact of a digital health feedback system in uncontrolled hypertensive patients. Value in Health 17: A479.
- Lasserson DS, Buclin T, Glasziou P (2011) How quickly should we titrate antihypertensive medication? Systematic review modelling blood pressure response from trial data. Heart 97: 1771-1775.
- National Institute for health and Care Excellence [NICE] (2011) Hypertension: Clinical management of primary hypertension in adults [CG127].
- Ho PM, Bryson CL, Rumsfeld JS (2009) Medication adherence: its importance in cardiovascular outcomes. Circulation 119: 3028–3035.
- National Institute for health and Care Excellence (NICE) (2011) op cit.
- Parati G, Omboni S, Compare A, Grossi E, Callus E, et al. (2013) Blood pressure control and treatment adherence in hypertensive patients with metabolic syndrome: protocol of a randomized controlled study based on home blood pressure telemonitoring vs. conventional management and assessment of psychological determinants of adherence (TELEBPMET Study). Trials 14: 22-32.
- Noble K, Brown K, Medina M, Alvarez F, Young J, et al. (2016) Medication adherence and activity patterns underlying uncontrolled hypertension: assessment and recommendations by practicing pharmacists using digital healthcare. J Am Pharm Assoc 56: 310-315.
- NCCSDO (2015) Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R & D.
- Yiannakopoulou ECh, Papadopulos JS, Cokkinos DV, Mountlkalakis TD (2005) Adherence to antihypertensive treatment: a critical factor for blood pressure control. Eur J Cardiovasc Prev Rehabil 12: 243-249.
- NHS England Workshop Report (2013) Making medicines-taking a better experience.
- Parliamentary Secretary of State for Quality (2012) Steering group on improving the use of medicines.
- National Health Service [NHS] (2014) SBRI healthcare 2014.
- NHS England NICE (2009) Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence.
- McCormack T, Arden C, Begg A, Caulfield M, Griffith K, et al. (2013) Optimizing hypertension treatment: NICE/BHS guideline implementation and audit for best practice. Br J Cardiol 20: S1-S16.
- National Health Service (NHS) England (2015) Right care collaboration 2015/2019: Forward view.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 9582
- [From(publication date):
February-2017 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 8669
- PDF downloads : 913