Findings on the Relationship between Cardiovascular Disease and Coronavirus Disease 2019: A Systematic Review
Received: 21-Feb-2022 / Manuscript No. JIDT-22-55035 / Editor assigned: 23-Feb-2022 / PreQC No. JIDT-22-55035(PQ) / Reviewed: 09-Mar-2022 / QC No. JIDT-22-55035 / Revised: 16-Mar-2022 / Manuscript No. JIDT-22-55035(R) / Published Date: 23-Apr-2022
Abstract
Coronavirus disease 2019 (COVID-19) presents as the main cause of death, respiratory and heart failures, especially in the elderly, immunosuppressed, and those with cardiovascular comorbidities. Therefore, a better understanding of these findings is needed. A systematic review was carried out looking for articles published on the MEDLINE/PubMed using the following descriptors: ("cardiovascular disease") OR ("acute myocardial infarction") OR ("coronary artery disease") OR ("acute coronary syndrome") OR ("atherosclerosis") OR ("cardiac insufficiency") OR ("pericarditis") OR ("myocarditis") AND ("COVID-19") OR ("SARS-CoV-2") and considering inclusion and exclusion criteria. Of the total number of patients included in the 10 studies selected for this review, 40% of patients infected with SARS-CoV-2 had hypertension or other cardiovascular comorbidities, while 27% presented cardiovascular complications, mainly acute cardiac injury, arrhythmia, and heart failure. The hypotheses of involvement of an intense inflammatory response, decreased immunity and greater expression of ACE2 in the heart, associated with more severe heart conditions, were discussed in this study. The increase in cardiac and inflammatory markers was associated with worse clinical outcomes and risk of death, confirming the need to evaluate them since admission to the hospital. The articles analyzed presented as a limitation the small number of patients inserted, to the detriment of the pandemic state. We warned about the need for better clinical management of patients with cardiovascular comorbidities aiming at reducing the number of fatal cases due to infection.
Keywords: Cardiovascular disease; Novel coronavirus; SARS-CoV-2
Introduction
In 2019 the first cases of a new betacoronavirus were reported, caused by the agent called SARS-CoV-2 [1]. The outbreak of Coronavirus Disease 2019 (COVID-19) started in China, more precisely in Wuhan, Hubei province, and took proportions on a global scale leading the World Health Organization (WHO), on March 11, 2020, to declare a pandemic state [2,3]. The spread of the disease has as its main characteristic the person-to-person transmission which allows its epidemiological picture to evolve daily, having as updated data on January 05, 2022, the global number of cases exceeding 290,000,000, and the number of deaths exceeding 5,500,000 [4,5]. After gene sequencing, it was verified that SARS-CoV2 shares about 79.6% of the SARSCoV genome and, the pathogenesis mechanism of both are similar. Angiotensin-converting enzyme 2 (ACE2) is used as a receptor [6], a protein that has expression in several human organs such as intestine, kidneys, lung and, heart, making them targets susceptible to infection. In the myocardium, besides the ACE2 being strongly expressed in pericytes, the enzyme showed a higher expression in patients with heart failure, contributing to an increase in the probability of adverse prognosis in this group in the case of SARS-CoV-2 contagion [7].
The SARS-CoV-2 infection has taken different forms: asymptomatic; mild to moderate; severe; critical and lead to death [8]. It is observed that, initially, the appearance of non-specific symptoms such as fever, cough, myalgia, or fatigue is more common. Studies show that the most present comorbidities in hospitalized patients are diabetes, hypertension, and cardiovascular diseases, the latter two being more frequent in ICU (intensive care units). Moreover, heart failure and acute heart injury are the most reported complications in deceased patients [9-11]. In this review, we discuss the association between cardiovascular diseases and COVID-19 considering risk factors and main complications found in infected patients.
Methodology
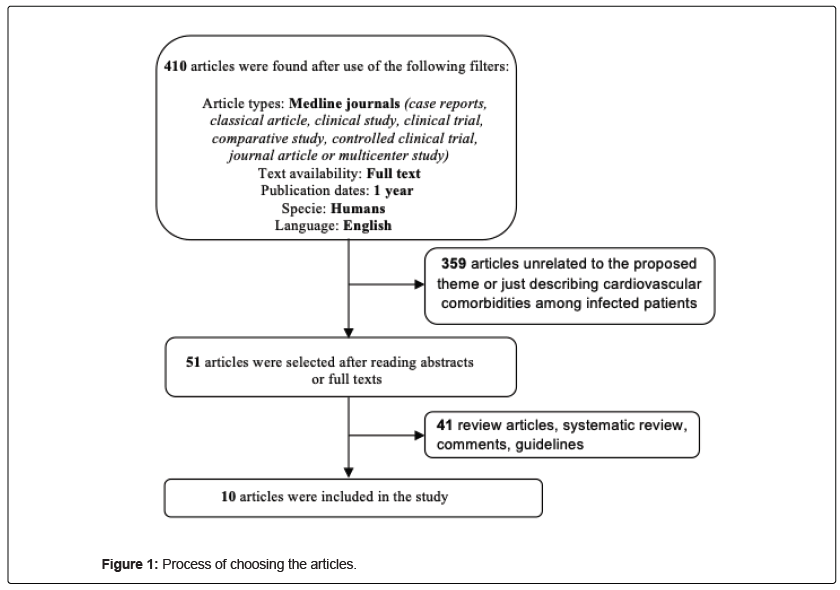
The study is a systematic review of the literature in search of an updated answer to a central and guiding question: what relationships have been found so far between cardiovascular diseases and COVID-19? The descriptors were organized using the Boolean operators “AND” and “OR” according to the sequence: (("cardiovascular disease") OR ("acute myocardial infarction")) OR ("coronary artery disease")) OR ("acute coronary syndrome")) OR ("atherosclerosis")) OR ("cardiac insufficiency")) OR ("pericarditis")) OR ("myocarditis")) AND ("COVID-19")) OR ("SARS-CoV-2"). The following filters were applied as inclusion criteria: full text, case reports, classical article, clinical study, clinical trial, comparative study, controlled clinical trial, journal article or multicenter study, articles published between January 2019 and May 8, 2020, research with humans, in English, MEDLINE. The exclusion criteria were: duplicate articles, reviews, Meta-analyzes, and those that presented a main theme or methodologies that did not contribute to the study proposal. So, the descriptors together with the Boolean operators were entered on the MEDLINE platform via PubMed, inclusion criteria were selected in the search for eligible articles and, exclusion criteria were observed by reading abstracts and full text.
Results and Discussion
10 articles were selected using the filters on the PubMed platform and by consensus between two researchers after reading the abstracts or full texts (Figure 1). Table 1 shows a brief description of the selected articles. A limitation that we can observe in these studies is the small number of patients inserted in the face of a pandemic. Probably, the rapid spread of the virus found the medical scientific community with insufficient tools to deal with the problem more widely. The urgent interest in understanding COVID-19 in several aspects such as comorbidities, complications, transmission, treatment, among others, has accelerated publications, including many preliminary studies. This can be monitored daily by increasing the number of publications available per day on digital platforms since the beginning of the pandemic. Considering the 848 patients of the 10 articles included in this review, it was observed that approximately 40% of the cases presented cardiovascular comorbidities, while cardiac complications were reported in about 27% of the patients, being distributed in 17% of Acute Heart Injury and 10% of Heart Failure. In study 7, a retrospective and single-center case series of the 138 COVID-19 patients reported that 16.7% patients had arrhythmia. We also observed that the male gender was more prevalent, representing 62% of the patients.
| Our numbering authors, year of publication, journal | Country | Article title | Patients included/Sex | Average ages | Main findings and conclusion related to cardiovascular diseases |
|---|---|---|---|---|---|
| Chen et al., 2020 [9], The Journal of Clinical Investigation | China | Clinical and immunological features of severe and moderate coronavirus disease 2019. | 21/17 male | 61 years (severe cases) 52 years (moderate cases) |
Hypertension was comorbidity found in 36.4% of severe cases and 10% of moderate cases. 100% of severe cases (mortality of 36.4%) had acute respiratory distress syndrome and 83.3% respiratory failure. Secondary infection (27.3%), acute cardiac injury (9.1%), hypoxic encephalopathy (18.2%), acute kidney injury (18.2%), shock (9.1%), and acute liver injury (9.1%) were the least frequent findings in these cases. |
| Chen et al., 2020 [10] BMJ |
China | Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. | 274/171 male | 68 years (deceased patients) 51 years (recovered patients) |
Hypertension and cardiovascular disease were found in 48% and 14% of severe cases and 24% and 4% of moderate cases, respectively. The most frequent complications in patients who died were: acute respiratory distress syndrome (113; 100%), type I respiratory failure (18/35; 51%), sepsis (113; 100%), acute cardiac injury (72/94 ; 77%), heart failure (41/83; 49%), alkalosis (14/35; 40%), hyperkalaemia (42; 37%), acute kidney injury (28; 25%) and hypoxic encephalopathy (23; 20%). Cardiac complications were more found in patients with cardiovascular comorbidities. |
| Huang et al., 2020 [12] Lancet |
China | Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. | 41/30 male | 49 years | Hypertension and cardiovascular disease were comorbidities found in 15% and 23% of patients in the ICU and 14% and 11% of the cases without ICU care, respectively. The main complications presented by the patients were: acute respiratory distress syndrome (29%), acute cardiac injury (12%; among this 31% of patients in the ICU and 4% of the cases without ICU care), and secondary infection (10%). |
| Su et al., 2020 [13] Emerging Microbes & Infections |
China | The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. | 23:9 children/ 3 male and 14 adults/ 8 male |
Children: 4.5 years Adults: 42.9 years |
6 children and 4 adults had no symptoms e no child needed intensive care. An increase in CK-MB occurred in 66.7% of children and only in 14.3% of adults. |
| Barton et al., 2020 [14] American Journal of Clinical Pathology |
USA | Covid-19 autopsies, oklahoma, usa. | 2 male | 59.5 years | One of the findings in the cardiovascular system of a 77-year-old man (with obesity and history of hypertension) was an acute ischemic injury (coronary artery atherosclerosis) but without myocarditis. 42-year-old Man also was found coronary artery disease, mild and aorta intimal fatty streaking but without acute ischemic injury. Died with COVID-19, not from COVID- 19. |
| Borba et al., 2020 [15] JAMA Network Open |
Brazil | Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. | 81/61 male | 51.1 years | 37% of patients who received low doses of chloroquine and 53.6% of those who received high doses were hypertensive. Heart disease was found in 17.9% of patients who received high doses of chloroquine and none in the other group. The QTc interval corrected by the Fridericia method (QTcF) greater than 500 milliseconds was mostly found in the group that received high doses of chloroquine and 1 patient per group had myocarditis. |
| Gao et al., 2020 [16] Respiratory Research |
China | Prognostic value of NT-proBNP in patients with severe COVID-19. | 54/24 male | 60.4 years | Plasma NT-proBNP was positively correlated with age, urea, cardiac injury markers, and inflammation markers. NT-proBNP may be an independent risk factor for in-hospital death in critically ill patients with COVID-19. But also a history of hypertension (HP), myoglobin (MYO), creatine kinase-MB (CK-MB), and high-sensitivity troponin-I (HS-Tnl) were correlated with the risk of in hospital death. |
| Zhou et al., 2020 [11] Lancet |
China | Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. | 191/119 male | 56 years | Hypertension and coronary heart disease were found in 48% and 24% of non-survivors and 23% and 1% of survivors, respectively. Cardiac failure and acute cardiac injury occurred in 52% and 59% of non-survivors and 12% and 1% of survivors, respectively. 70% of those who died had septic shock and this did not occur in any survivors. |
| Wang et al., 2020 [17] Critical care |
China | Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. | 107 / 57 male | 51 years | Hypertension and cardiovascular disease were found in 52,6% and 36.8% of non-survivors and 18.2% and 6.8% of survivors, respectively. The acute cardiac injury occurred in 42.1% of patients who did not survive and in 4.5% of those who survived. Age and male gender were independent risk factors for death |
| Korean Society of Infectious Diseases and Korea Centers or Disease Control id Prevention. 2020 [18] Journal of Korean Medical Science |
Korea | Analysis on 54 Mortality Cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. | 54/33 male | 75.5 years | Cardiovascular disease was the most common comorbidity found among patients, 58% of those over the of 70, and 50% of the others. There was no significant difference in symptom duration between patients had comorbidities and those who did not. The highest number of fatal cases was in patients over 70 year: age. |
Table 1: Description of selected articles.
Cardiovascular comorbidities and worse prognosis in COVID-19
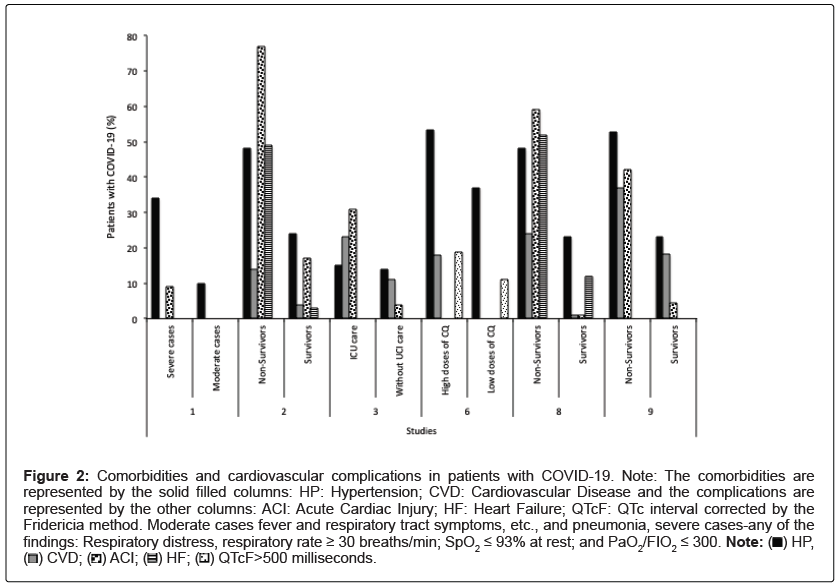
The findings from studies 1, 2, 3, 6, 7, 8, and 9 in Table 1 shows clinical outcomes (transfer to the ICU, respiratory and cardiac complications, for example, and death) in patients with COVID-19 and history of hypertension or another cardiovascular disease. Considering the studies that presented data, which enabled a joint assessment (comorbidities and cardiovascular complications compared between groups of patients with the better and worse clinical outcome), it can be noted that heart failure, acute cardiac injury, and elevation of the QTcF interval on the electrocardiogram of patients tend to be directly proportional to the presence of cardiovascular comorbidities (Figure 2) [9-12,15,17,19]. These findings reinforce the inclusion of patients with such comorbidities in the risk group for SARS-CoV2 infection.
Figure 2: Comorbidities and cardiovascular complications in patients with COVID-19. Note: The comorbidities are
represented by the solid filled columns: HP: Hypertension; CVD: Cardiovascular Disease and the complications are
represented by the other columns: ACI: Acute Cardiac Injury; HF: Heart Failure; QTcF: QTc interval corrected by the
Fridericia method. Moderate cases fever and respiratory tract symptoms, etc., and pneumonia, severe cases-any of the findings: Respiratory distress, respiratory rate ≥ 30 breaths/min; SpO2 ≤ 93% at rest; and PaO2/FIO2 ≤ 300. Note:  HP,
HP,  QTcF>500 milliseconds.
QTcF>500 milliseconds.
In study 7 (Table 1), the group of patients who presented NTproBNP> 88.64 pg/mL had a higher percentage of cardiovascular comorbidities and elevations in other markers of cardiac injury (myoglobin, CKMB, and Hs-TnI). Mortality in this group was 60%, while in the group of patients with NT-proBNP<88.64 pg/mL there was no death. Although NT-proBNP was related to cardiac injury, it was also an independent factor for the risk of in-hospital death, as well as other markers of cardiac injury and hypertension. A more recent study also revealed higher levels of NT-proBNP in patients with cardiovascular comorbidity infected with COVID-19 [20]. It is known that high concentrations of cTnI, NT-proBNP and other cardiac biomarkers such as CK-MB and MYO are associated with a higher mortality rate in patients with COVID-19. Among the complications that lead to death, acute myocarditis, acute myocardial infarction and rapid-onset heart failure have higher mortality among those infected with COVID-19 [21].
In study 10 (Table 1), it is observed that among the fatal cases analyzed, the percentage of cardiovascular comorbidities (hypertension and other heart diseases such as myocardial infarction) was 8% more in the group of people over 70 years of age and the increase in mortality was mainly related to males in this age group. However, the main symptoms, biochemical tests, and complications that led patients to death were not detailed.
The ACE2 is an aminopeptidase bound to the cell membrane that is involved in cardiac function and the development of hypertension. This enzyme also functions as a receptor for entry into the cell for both SARS-CoV and SARS-CoV-2 [2]. The expression of ACE2 in human cardiac tissues was evaluated and it was noted that those obtained from patients with muscle heart disease showed greater expression of the enzyme, indicating in this group of patients a greater vulnerability to SARS-CoV-2 infection, which may develop lesions, more severe cardiac conditions, and contribute to a worse prognosis [7]. However, it is still unclear in the literature whether hypertensive patients with COVID-19 and treatment with an ACE inhibitor or angiotensin receptor blocker can contribute to an increase in ACE2 and consequently lead to an increased risk of infection in the myocardium by SARS-CoV-2 [2]. Despite that, it has been recommended that these patients should continue treatment unless a change in therapy is made on medical advice [22].
New studies show that ACE inhibitors (ACE) and angiotensin receptor blockers (ARB) increase the number of ACE2 receptors on the myocardial surface. However, lower rates of severe COVID-19 and lower levels of interleukin-6 (IL-6) in addition to a decreased viral load were observed in patients using ACE inhibitors and ARBs [23]. In addition, the study by Rossi et al., states that patients with hypertension who use ACE inhibitors and ARBs were not associated with mortality from COVID-19. This underpins the recommendations of not discontinuing or replacing treatment because of COVID-19 [24].
Another point to be considered is that hypertensive patients have high levels of plasminogen, which increases the ability of many viruses to bind to ACE2. The envelope proteins in SARS-CoV-2 are cleaved by furin-like intracellular proteases and this increases the ability of viruses to enter host cells. A type of non-furin cleavage can be exerted by plasmin, generated through plasminogen cleavage. However, despite the participation of plasmin in the entry of various viruses into cells, further in vivo studies are needed to prove this participation concerning SARS-CoV-2 [25].
Cardiac markers and prognosis
The studies from 1 to 4, 6 to 9 of Table 1 presented laboratory findings, among which some markers stand out, such as hypersensitive cardiac troponin I (hs-cTnI); Creatine Kinase MB (CK-MB); Creatine Kinase (CK); B-type Natriuretic Peptide (NT-proBNP); Lactate Dehydrogenase (LDH); High Sensitivity Reactive C Protein (hsCRP) and Myoglobin (MYO). It is observed that these markers were higher in the group of patients who presented more severe cases or who died as a result of infection, than in groups of moderate or recovered cases, thus showing the correlation of the high level of these markers with poor prognosis. It is also observed that cardiac markers had higher levels in elderly patients [16].
It is worth noting that advanced age is associated with the risk of death of patients infected by SARS-CoV-2, and in this age group, among the main comorbidities are hypertension and coronary heart disease [11]. Age-related dysfunctions also reverberate in the T-cell function, making it difficult to recognize new antigens and making the elderly more susceptible to infections. Additionally, an increase in the expression of pro-inflammatory cytokines is also observed in this group [26]. Both contribute negatively to the control of the replication of the coronavirus, besides prolonging the inflammatory response that demonstrates to be one of the main causes of the severity of COVID-19, that is, the cytokine storm is part of the pathological process of infection [9,11].
More recent studies agree with the findings described above, as increasing age continues to be observed as a major risk factor for severity in COVID-19. Although severe manifestations of COVID-19 have been reported in all age groups, about 90% of severely ill COVID-19 patients are over 30 years of age. In addition, high rates of comorbidity and mortality are consistently observed in older populations throughout the pandemic [27]. Elderly people have a disproportionate number of severe cases and deaths, with patients aged 75-84 having a 220-fold increased risk of mortality compared to young people aged 18-29. This is linked to the fact that older patients tend to have a dysregulated immune response, in addition to a higher frequency of comorbidities, facilitating viral spread and disease severity [28].
The studies 2 and 7 (Table 1) demonstrate that the levels of inflammatory markers such as hsCRP and Procalcitonin (PCT) are higher in critically ill or deceased patients compared to the group of recovered patients, as well as markers of cardiac injury-hs-cTnI, NT-proBNP, MYO, and CK-MB are also there. These findings indicate a possible association between inflammation and cardiac injury in COVID-19. Linked to this, as patients with cardiac comorbidities present a greater expression of ACE2 [7], this may favor the mechanism of direct injury to the cardiac tissue, caused by the toxicity induced by the virus [29].
Direct myocardial injury via SARS-CoV-2 and ACE2 interaction remains a possibility [30]. Direct endothelial infection was documented in autopsies of hearts. Furthermore, viral particles have been identified in endothelial cells of these hearts by electron microscopy, although the appearance and location of these particles within the cells do not appear to be typical of coronavirus-infected cells [31]. The tropism of the virus by the ACE2 enzyme can potentially mediate direct cardiac injury, however, further investigations are needed [32].
In studies 7 and 9 (Table 1), the cardiac markers hs-cTnI, CK-MB, MYO, LDH, and NT-proBNP were associated with mortality. Already in study 8 (Table 1) an association was also found between changes in hscTnI levels and the risk of death, with a rapid increase in its concentration after the 16th day of the disease being observed in deceased patients, as well as being present in more than half of them. The trials with hscTnI are effective in the diagnosis of Acute Coronary Syndrome (ACS), and its use allows a more precise report in the indication of myocardial injury. However, despite the alteration of the levels of this enzyme to predict suspicion of ACS, it was observed in patients with COVID-19 without heart symptoms, even though its elevation is more common after the onset of symptoms [33]. This raises the possibility of secondary cardiac injury related to an increase in oxygen demand caused by sepsis in subclinical coronary artery disease. Sepsis would lead to ischemia resulting in type 2 acute myocardial infarction [34]. This sequence of events may contribute to the elucidation of the findings of study 3 (Table 1), which included 113 patients who died as a result of the COVID-19, among which 100% had sepsis and 49% had heart failure. The same study points to higher levels of hs-cTnI, NT-proBNP, and LDH in deceased patients compared to survivors. In study 9 (Table 1), the values of these markers, including the CKMB, were also higher at the admission of non-survivors.
Interestingly, study 4 (Table 1) found changes in the concentration of CK-MB in 66.7% of children and 14.3% of adults. None of the children required intensive treatment or presented serious complications, although the increase in CKMB indicates cardiac injury. This finding can be explained by the fact that the ACE2 enzyme is less expressed in children, which can lead to a milder course of the disease. Myocardium seems to be a target of infection also in children [35,36].
New updates point to an association of SARS-CoV-2 with cases of multisystem inflammatory syndrome in children (MIS-C). Early reports during the early phase of the COVID-19 pandemic indicated that children were relatively spared from severe manifestations, with the low incidence of 2 to 6% having severe COVID-19. However, numerous reports of severe systemic hyperinflammation linked to COVID-19 cases led the WHO, in conjunction with other societies, to identify the cases as a new condition called multisystem inflammatory syndrome in children (MIS-C). Currently, it is known that cardiac involvement is highly present in patients with MIS-C, and that this syndrome is directly linked to cases of COVID-19 infection [37]. Inflammatory markers such as IL-6 and cardiac markers such as NTproBNP and troponin are present at high levels in patients with MIS-C [38]. Cardiovascular manifestations include ventricular dysfunction, coronary artery dilation and arrhythmias [37]. Most of these patients recover successfully and show good rates of improvement in myocardial dysfunction, but the underlying mechanism of myocardial dysfunction in MIS-C is still not fully understood [38].
In study 7 (Table 1) the NT-proBNP was an independent factor of risk of death in severe cases of infection, although it correlates with the other markers of acute myocardial injury and inflammatory markers. The elevation of cardiac markers seems to represent an adverse prognosis for the SARS-CoV2 patient, evidencing the need for their laboratory request since the patient's admission, aiming at the most appropriate clinical management.
Cardiac complications in COVID-19
After an average incubation period of approximately 4 to 5 days, the symptoms begin to appear, and COVID-19 tends to last for two weeks with worsening in the second week due to the inflammatory response that follows the virus's replication period. In this short time, the most severe complications of the disease can happen [39]. Acute cardiac injury and heart failure were the main cardiovascular complications found in patients infected with SARS-CoV-2 among studies selected for this review (Figure 2). In addition to the involvement of ACE2, which possibly can contribute to increasing the risk of cardiac complications in COVID-19, especially in the presence of cardiovascular comorbidities, as previously described, other hypotheses have been discussed, such as those mentioned by Adão and Guzik [40]. The highlight was given to inflammatory cytokines and hypoxia generated by the impairment of the respiratory system in COVID-19.
The rapid replication of the virus can cause massive death of epithelial and endothelial cells, and vascular leakage, triggering the exuberant production of pro-inflammatory cytokines and chemokines [41]. In studies 1, 2, 7 and 8 (Table 1), higher levels of inflammatory markers were found (IL-6, IL-10, and TNF-α; IL-6, IL-18, IL-10, IL- 2, and TNF-α receptors; PCR; ferritin, and IL-6, respectively) in the group of patients with more severe cases of the disease. TNF-α, IL- 1, IL-6, IL-8, IL-12, IL-18, and interferon-gamma (IFNγ) are proinflammatory cytokines, which act as mediators of the immune system. Anti-inflammatory cytokines, such as interleukins 4, 5, 10, 11, and 13, are also produced and the regulation of this balance is complex. The pro-inflammatory cytokines effectively initiate the inflammatory process against infectious agents. TNF-α, IL-1, IL-6 are responsible for raising protein levels in the acute inflammatory phase, which includes C-reactive protein (CRP) during infection [42,43].
When a set of cytokines is considered, the predictive value for cardiac complications increases, since it is known that cytokines work together leading to a complex inflammatory response [44]. IL-6 participates in the activation of T lymphocytes and the cytotoxicity of these cells leads to an intense inflammatory response that can damage the heart muscle [45]. The T-lymphocytes present a cardiotropism by an interaction between the heart-produced hepatocyte growth factor (HGF) and c-Met, the HGF receptor on naïve T-cells [46]. Also, clinical trials showed that both TNF-α and IL-1 are associated with impaired systolic and diastolic function and, also leading to adverse cardiac remodeling and decreased contractility of the heart muscle due to a reduction in oxygen consumption of the respiratory chain [47-50]. The 77-year-old man (with obesity and a history of hypertension) in study 5 (Table 1) had an acute ischemic injury (coronary artery atherosclerosis), but no evidence of myocarditis was found. So, in patients in whom heart failure is caused by inflammatory mechanisms, there is an expectation that well-targeted anti-inflammatory therapies can be used to obtain a better clinical outcome for patients [51].
One of the therapeutic options being analyzed for COVID-19 are chloroquine (CQ) and hydroxychloroquine (HCQ), antimalarial drugs and also used in treating some chronic rheumatic condictions due to immunomodulatory properties, for example, the inhibition of the production of proinflammatory cytokines such as IL-1, IL-6 and TNF-α [52-54]. Although CQ has antiviral properties tested in vitro, it has not always been confirmed during the treatment of patients with viral infections and the mechanisms of action of this drug may vary according to the pathogen. Among these mechanisms stand out the alkalinization of endosomes affecting the proper maturation of the viral protein and the recognition of the viral antigen by dendritic cells. This recognition occurs through a pathway that involves the Toll-like receptor that requires endosomal acidification. And finally, QC showed an anti-SARS-CoV-1 effect in vitro, decreasing the glycosylation of ACE2 [54,55].
In study 6 (Figure 2), which included patients in severe COVID-19, was observed that the group of patients who received higher doses of QC had a higher percentage of acute cardiac injury, but it should be considered that this group also presented a higher percentage of cardiovascular comorbidities and a higher mean age. Added to this, the group that received high doses of QC (i.e., 600 mg twice daily for 10 days) presented more instance of QTc interval greater than 500 milliseconds compared with the low-dosage group (i.e., 450 mg twice daily on day 1 and once daily for 4 days). Therefore, suggesting that the QC should be administered with caution, especially in severe cases of the infection. At doses beyond the safety margin, chloroquine can show pro-arrhythmic activity mainly because of its ability to inhibit the cardiac potassium current from the internal rectifier and, consequently, induce lethal ventricular arrhythmias [56].
Recent randomized evidence regarding the use of hydroxychloroquine for post-exposure prophylaxis and among hospitalized patients reveals no significant benefits. In 2020, in the United States, more than 890,000 chloroquine and hydroxychloroquine prescriptions were made for the prevention, post-exposure prophylaxis and treatment of COVID-19. In 2021 there were more than 560 thousand prescriptions. This was due to the fact that biological mechanisms support the inhibition of the virus that causes COVID-19. Some case series without comparison groups and observational groups reported possible benefits of use. However, randomized trials published in high quality peer-reviewed journals have shown disappointing results regarding the positive effects of administering these drugs in patients with COVID-19 [57].
A randomized, double-blind, placebo-controlled trial that included 821 subjects tested hydroxychloroquine for post-exposure prophylaxis. The study concluded that after moderate or highrisk exposure to COVID-19, HQ did not prevent COVID-19-compatible disease or confirmed infection when the drug was used as prophylaxis within 4 days of exposure. This study also reported that collaterals effects were more common in those taking the drug than those taking placebo [58]. Another randomized clinical trial also tested the prophylactic effects of HQ. It included 2,314 individuals, of which 1,116 were randomly assigned to receive HQ and 1,198 to receive usual care. The study presented a similar conclusion: Post-exposure therapy of healthy people to a PCR positive patient with HQ did not predict symptomatic SARSCoV- 2 or COVID-19 infection [59].
Regarding the care of hospitalized patients, a randomized study of 4,716 patients concluded that those who received HQ had no lower incidence of death than those who received usual care. This study also showed that those in the HQ group had a higher frequency of invasive mechanical ventilation or death. It also showed that there was no difference in the incidence of new cardiac arrhythmias among patients who received hydroxychloroquine [59]. On March 28, 2020, the US Food and Drug Administration (FDA) issued an emergency use authorization for HQ on COVID-19. Since then, no significant benefit has been seen in the randomized evidence, either for post-exposure prophylaxis or for patients hospitalized with COVID-19. Current evidence points to the lack of efficacy and possible harm of HQ in the treatment and prevention of COVID-19 [57].
Conclusion
The presence of cardiovascular comorbidity points to an increased risk of complications in COVID-19, such as heart failure and acute cardiac injury. Besides, higher levels of inflammatory and cardiac markers were associated with a worse prognosis of infected patients. These findings seem to support the hypothesis that the probable higher expression of ACE2 in the heart of patients with such comorbidities allows greater entry of the virus into the cell, an intense inflammatory response that leads to decreased oxygen consumption and less contractibility. However, it is important to note that the studies discussed in this systematic review, included a small number of infected patients, considering a pandemic. These studies reinforce the need for attention to the group of cardiac patients, both for better clinical management and for new studies due to the poor prognosis that the infection promotes to these patients.
Acknowledgment
Everyone who has been working on the front lines in defense of life during this pandemic.
References
- Lake MA (2020) What we know so far: COVID-19 current clinical knowledge and research. Clin Med 20:124.
[Crossref] [Google Scholar] [PubMed]
- Zheng YY, Ma YT, Zhang JY, Xie X (2020) COVID-19 and the cardiovascular system. Nat Rev Cardiol 17:259-260.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization (2020) WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020.
- Chan JF, Yuan S, Kok KH, To KK, Chu H, et al. (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 15;395:514-523.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization (2022) Situation Report-121. Accessed on Jan 05, 2022.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270-273.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Li X, Chen M, Feng Y, Xiong C (2020) The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116(6):1097-1100.
[Crossref] [Google Scholar] [PubMed]
- Zhi ZL (2020) Epidemiology working group for NCIP epidemic response, chinese center for disease control and prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41:145-151.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Wu DI, Guo W, Cao Y, Huang D, et al. (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130:2620-2629.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Wu DI, Chen H, Yan W, Yang D, et al. (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj 26;368.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054-1062.
[Crossref] [Google Scholar] [PubMed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497-506.
- Su L, Ma X, Yu H, Zhang Z, Bian P, et al. (2020) The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. Emerg Microbes Infect 9:707-713.
[Crossref] [Google Scholar] [PubMed]
- Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S (2020) Covid-19 autopsies, oklahoma, usa. Am J Clin Pathol 153:725-733.
[Crossref] [Google Scholar] [PubMed]
- Borba MG, Val FF, Sampaio VS, Alexandre MA, Melo GC, et al. (2020) Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 3:208857.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Jiang D, Wen XS, Cheng XC, Sun M, et al. (2020) Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 21:1-7.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Yin Y, Hu C, Liu X, Zhang X, et al. (2020) Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care 24:188.
[Crossref] [Google Scholar] [PubMed]
- Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention (2020) Analysis on 54 Mortality Cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci 35:132.
- National Health Commission of the People's Republic of China (2020) Guidelines for Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia, Trial Version 7.
- Ma L, Song K, Huang Y (2021) Coronavirus disease-2019 (COVID-19) and cardiovascular complications. J Cardiothorac Vasc Anesth 35:1860-1865.
[Crossref] [Google Scholar] [PubMed]
- Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, et al. (2021) COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther 19:345-357.
[Crossref] [Google Scholar] [PubMed]
- Statement from the American Heart Association, the Heart Failure Society of America, and the American College of Cardiology (2020) Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician.
- Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P (2020) SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ 29:973-987.
[Crossref] [Google Scholar] [PubMed]
- Rossi L, Malagoli A, Biagi A, Zanni A, Sticozzi C, et al. (2021) Renin–angiotensin system inhibitors and mortality in patients with COVID-19. Infection 49:287-294.
[Crossref] [Google Scholar] [PubMed]
- Ji HL, Zhao R, Matalon S, Matthay MA (2020) Elevated plasmin (ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100: 1065‐1075.
[Crossref] [Google Scholar] [PubMed]
- Alves AS, Bueno V (2019) Immunosenescence: participation of T lymphocytes and myeloid-derived suppressor cells in aging-related immune response changes. Einstein (Sao Paulo) 17: 4733.
[Crossref] [Google Scholar] [PubMed]
- Kim YI, Yu KM, Koh JY, Kim EH, Kim SM, et al. (2022) Age-dependent pathogenic characteristics of SARS-CoV-2 infection in ferrets. Nat Commun 13:21.
[Crossref] [Google Scholar] [PubMed]
- Pirabe A, Heber S, Schrottmaier WC, Schmuckenschlager A, Treiber S, et al. (2021) Age Related Differences in Monocyte Subsets and Cytokine Pattern during Acute COVID-19—A Prospective Observational Longitudinal Study. Cells 10:3373.
[Crossref] [Google Scholar] [PubMed]
- Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, et al. (2020) Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep 22:32.
[Crossref] [Google Scholar] [PubMed]
- Patil M, Singh S, Henderson J, Krishnamurthy P (2021) Mechanisms of COVID‐19‐induced cardiovascular disease: Is sepsis or exosome the missing link?. J Cell Physiol 236:3366-3382.
[Crossref] [Google Scholar] [PubMed]
- Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, et al. (2021) Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol 77:314-325.
[Crossref] [Google Scholar] [PubMed]
- Tschöpe C, Ammirati E, Bozkurt B, Caforio AL, Cooper LT, et al. (2021) Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 18:169-193.
[Crossref] [Google Scholar] [PubMed]
- Vasile VC, Jaffe AS (2017) High-sensitivity cardiac troponin for the diagnosis of patients with acute coronary syndromes. Curr Cardiol Rep 19:92.
[Crossref] [Google Scholar] [PubMed]
- Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, et al. (2020) Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 17:1463-1471.
[Crossref] [Google Scholar] [PubMed]
- Ludvigsson JF (2020) Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109:1088-1095.
[Crossref] [Google Scholar] [PubMed]
- Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, et al. (2020) Children’s heart and COVID-19: up-to-date evidence in the form of a systematic review. Eur J Pediatr 179:1079-1087.
[Crossref] [Google Scholar] [PubMed]
- Sperotto F, Friedman KG, Son MB, VanderPluym CJ, Newburger JW, et al. (2021) Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 180:307-322.
[Crossref] [Google Scholar] [PubMed]
- Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T (2021) COVID‐19 and multisystem inflammatory syndrome in children: A systematic review and meta‐analysis. Pediatr Pulmonol 56:837-848.
[Crossref] [Google Scholar] [PubMed]
- Sartor Z, Hess B (2020) Increasing the signal-to-noise ratio: COVID-19 clinical synopsis for outpatient providers. J Prim Care Community Health 11:2150132720922957.
[Crossref] [Google Scholar] [PubMed]
- Adao R, Guzik TJ (2020) Inside the heart of COVID-19. Cardiovasc Res.
[Crossref] [Google Scholar] [PubMed]
- Yang M (2020) Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019-nCoV Infection.
- Peng Y, Yuan Z, Li H (2005) Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns 31:623-628.
[Crossref] [Google Scholar] [PubMed]
- Schulte W, Bernhagen J, Bucala R (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators Inflamm 2013:165974.
[Crossref] [Google Scholar] [PubMed]
- Kristono GA, Holley AS, Lakshman P, Brunton-O'Sullivan MM, Harding SA, et al. (2020) Association between inflammatory cytokines and long-term adverse outcomes in acute coronary syndromes: A systematic review. Heliyon 6:03704.
[Crossref] [Google Scholar] [PubMed]
- Komarowska I, Coe D, Wang G, Haas R, Mauro C, et al. (2015) Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity 42:1087-1099.
[Crossref] [Google Scholar] [PubMed]
- Bozkurt B, Kribbs SB, Clubb Jr FJ, Michael LH, Didenko VV, et al. (1998) Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97:1382-1391.
[Crossref] [Google Scholar] [PubMed]
- Van Tassell BW, Toldo S, Mezzaroma E, Abbate A (2013) Targeting interleukin-1 in heart disease. Circulation 128:1910-1923.
[Crossref] [Google Scholar] [PubMed]
- Van Tassell BW, Seropian IM, Toldo S, Mezzaroma E, Abbate A (2013) Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm Res 62:637‐640.
- Zell R, Geck P, Werdan K, Boekstegers P (1997) TNF-α and IL-1α inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: evidence for primary impairment of mitochondrial function. Mol Cell Biochem 177:61-67.
[Crossref] [Google Scholar] [PubMed]
- Murphy SP, Kakkar R, McCarthy CP, Januzzi Jr JL (2020) Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol 75:1324-1340.
[Crossref] [Google Scholar] [PubMed]
- Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72-73.
[Crossref] [Google Scholar] [PubMed]
- Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56:105949.
[Crossref] [Google Scholar] [PubMed]
- Devaux CA, Rolain JM, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. Int J Antimicrob Agents 55:105938.
[Crossref] [Google Scholar] [PubMed]
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, et al. (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2:69.
[Crossref] [Google Scholar] [PubMed]
- Crumb Jr WJ, Vicente J, Johannesen L, Strauss DG (2016) An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods 81:251-262.
[Crossref] [Google Scholar] [PubMed]
- Hennekens CH, Rane M, Solano J, Alter S, Johnson H, et al. (2022) Updates on hydroxychloroquine in prevention and treatment of COVID-19. Am J Med 135:7-9.
[Crossref] [Google Scholar] [PubMed]
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, et al. (2020) A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 383:517-525.
[Crossref] [Google Scholar] [PubMed]
- Mitjà O, Corbacho-Monné M, Ubals M, Alemany A, Suñer C, et al. (2020) A cluster-randomized trial of hydroxychloroquine for prevention of Covid-19. N Engl J Med 384:417-427.
[Crossref] [Google Scholar] [PubMed]
- RECOVERY Collaborative Group (2020) Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 383:2030-2040.
[Crossref] [Google Scholar] [PubMed]
Citation: da Silva YH, Monteiro Júnior JGM, de Araújo RFF (2022) Findings on the Relationship between Cardiovascular Disease and Coronavirus Disease 2019: A Systematic Review. J Infect Dis Ther S2:005.
Copyright: © 2022 da Silva YH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2897
- [From(publication date): 0-2022 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 2308
- PDF downloads: 589