Finding New and Ideal Formulation for Snow/ Water Foam Producing Tablets
Received: 03-Jan-2022 / Manuscript No. ico-22-43808 / Editor assigned: 05-Jan-2022 / PreQC No. ico-22-43808(PQ) / Reviewed: 19-Jan-2022 / QC No. ico-22-43808 / Revised: 24-Jan-2022 / Manuscript No. ico-22-43808(R) / Published Date: 31-Jan-2022 DOI: 10.4172/2469-9764.10002180
Abstract
The aim of this work is to find an ideal formulation for foam-producing tablets that would produce the maximum amount of bubbling per tablet, exclude some of the ingredients that might be harmful to the bubble forming ability of the tablet, and include some additives that would increase and cause persistence of the bubbling per tablet and also find an appropriate biding agent that would be inert towards the ingredients and also hold the tablet ingredients together. Also, find a method to make the tablets look smoother.
Introduction
Some of the companies in the US are looking to produce kid-safe foaming tablets from household chemicals that would produce bubbles when placed in snow. The ingredient included the following chemicals corn starch (pure starch), red beet powder (Betanin), salt (Sodium Chloride), cream tartar (Potassium Bitartrate), baking soda (Sodium Bicarbonate), Vitamin C (citric acid), drops of water (H2O). The amount of bubbling was minimal; also, the tablet didn’t hold together, was grainy and fragile. Since bubbling forms when the fragile tablet is placed in snow, that is evidence of a chemical reaction. In a chemical reaction, reactant substance change to product substance, the product substance will have different physical and chemical properties than the reactant substance. All chemical reactions involve a detectable change [1]. A chemical equation is used to describe a chemical reaction. A Chemical reaction a be represented by a molecular equation. Reactant species are always listed on the left side of a chemical equation and the product species are listed on the right side of the chemical equation and they are separated by an arrow. A chemical reaction can also be presented by ionic equation or net ionic equation [2]. 2.1Reactants Products. Reactions are classified into three main reactions, precipitation reactions, Acid base reactions and oxidation reduction reactions. Precipitation reactions would involve the formation of insoluble ionic compound forms from mixing a solution of two ionic substances. Acid base reactions involve the reaction of an acid with a base, a transfer of a proton between reactants are involved. Oxidation reduction reactions (redox reactions) involve transfer of electron between reactant species [3]. The reaction that takes place when placing the created tablet in water/ice is an acid-base reaction. Acid- Base reactions are widely known of its ability to produce bubbling if either reactant is a we5ek acid or week base that would produce a gas product. In an acid-base reaction, acid and base properties neutralizes, where the anion of the acid and the cation of the base combine to form the salt, and hydroxide anion, OH- combines with the hydrogen cation, H+ to form water [4]. The counting unit for number of atoms, ions or molecules in a laboratory seize sample is mole, abbreviated mol. [5]. A mole of samples of different substances has different masses. For example, 1 mole of 24mg and 1 mole of 12C. A single 24mg atom is twice as massive as, has a mass of 24 amu, as a single 12C atom, has a mass of 12 amu. The formula weight of any substance is numerically equals to the molar mass of that substance. For example, for table salt with chemical formula NaCl, the formula weight is 58.5 amu and its molar mass is 58.5 g/mol. The molar mass of substance in g/ mol can be used as a conversion factor to convert mass of substance into moles or moles of substance into grams [6].
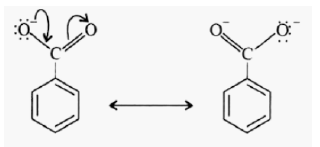
The relative number of molecules in a reaction is represented by coefficients in a balanced chemical equation. The relative number of moles and the relative number of molecules in a reaction are indicated by the coefficients in a balanced chemical equation [7]. A limiting reactant is the reactant that is completely consumed in a chemical reaction; it limits and determines the amount of product formed. The other reactants are called excess reactants. The quantities of product formed, and reactant consumed are restricted by the amount of limiting reactant. If one reactant, the limiting reactant is consumed, the reaction stops, and the amount of limiting reactant becomes zero at the end of the reaction [8]. Carboxylic acids are the largest category of weak organic acids that contain carboxylic groups (-COOH). The generic formula for carboxylic acids is R-COOH, where R= alkyl group. The acidic behavior of carboxylic acids is due to the oxygen atom bonded to carboxyl group carbon draws electron density from hydroxyl bond. It helps stabilize the conjugate base and increases the polarity of -OH bond. The carboxylate anion, conjugate base of acid has resonance which spreads the negative charge over several atoms and contributes to stability of the anion [9].
Alcohols are compounds that contain one or more hydroxyl or alcohol group (-OH). The -OH bonds are polar; bonds and alcohols are more soluble in polar solvents than hydrocarbons and OH groups can form hydrogen bonds. Examples of alcohols are
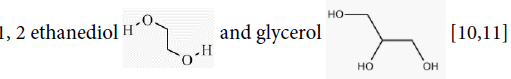
Experimental: Corn starch is pure starch with a formula of C27H48O20 and is known to be used as liquid thickener [12]. Red beet powder is used as dye that colors the tablet [13]. Table salt is sodium chloride with formula NaCl and molar mass of and this salt has a neutral PH, PH = 7 [14]. Cream tartar is potassium bitartrate with formula KC₄H₅O₆ and molar mass: 188.177 g/mol [15]. Baking soda is a weak base with a formula of NaHCO₃ and molar mass: 84.007 g/ mol [16]. Citric acid is a triprotic acid, a weak organic acid that has the formula of C₆H₈O₇ and molar mass: 192.124 g/mol [17] (Table 1).
| Household Chemical and Chemical Name | Chemical structure | Molecular Formula | Molecular Weight (g/mol) |
|---|---|---|---|
| Corn Starch (Pure Starch) |
|
C27H48O20 | 692.66 |
| Table Salt (Sodium Chloride) |
 |
NaCl | 58.44 |
| Cream Tartar (Potassium Bitartrate) |
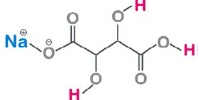 |
KC₄H₅O₆ | 188.177 |
| Baking Soda (Sodium Bicarbonate) |
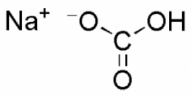 |
NaHCO₃ | 84.007 |
| Citric Acid (Vitamin C) |
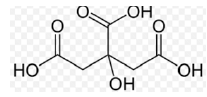 |
C₆H₈O₇ | 192.124 |
| Water (Dihydrogen Monoxide) |
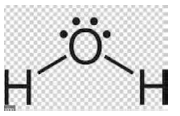 |
H2O | 18.01 |
| Red Beet (Betanin) |
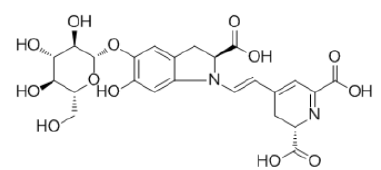 |
C24H26N2O13 | 550.47 |
| Glycerol | 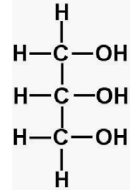 |
C3H8O3 | 92.094 |
Table 1: Lists the chemical name, molecular formula, and molecular weight of household chemicals in the ingredients.
The bubbling during creating the tablet using water a binding agent is due to the reaction between weak acid (citric acid) and potassium bitartrate with the weak base (baking soda) to form salt, water, and carbon dioxide, causes loss of bubbles while making the tablet. To get the maximum amount of yield, maximum amount of CO2 per tablet, intern the greatest amount of bubbling, number of moles of Cirtic acid to sodium hydrogen carbonate must be 1:3 and number of moles of sodium bitartrate to sodium hydrogen carbonate must be 1:1 according to the balanced chemical equations. Vitamin C [17] citric acid is a triprotic acid containing three acidic group and one hydroxyl group [17]. The hydroxyl groups (-OH) are alcoholic groups, and they don’t react with Sodium hydrogen carbonate, baking soda, while the acidic groups, carboxylic group (-COOH) can react with Sodium hydrogen carbonate to form salt, carbon dioxide and water. The bubbling is due to the formation of carbon dioxide as a product. The molar-amount of citric acid and sodium bicarbonate reactants must be 1: 3 to produce the maximum amount of bubbling in the formula per tablet:
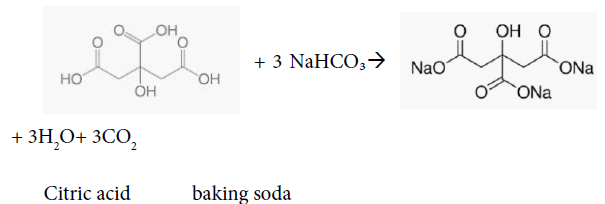
Using 1 g of vitamin C with formula CHO in the ingredients, the amount needed for reaction to go to completion and produce the maximum amount of carbon dioxide, and hence the maximum amount of bubbling would be 1.3 grams of baking soda as follows:
1 g /192.124 g/mol * 3 NaHCO /1 CHO * 84.007 g/mol= 1.312 g of NaHCO3
Cream tartar [15]. potassium bitartrate is a monoprotic acid containing on acidic group and two hydroxyl groups. The hydroxyl groups (-OH) are alcoholic groups, and they don’t react with Sodium hydrogen carbonate, baking soda, while the acidic group, carboxylic group (-COOH) can react with Sodium hydrogen carbonate to form salt, carbon dioxide and water. The bubbling is due to the formation of carbon dioxide as a product. Using 1g of cream tartar, potassium bitartrate with formula KCHO in the ingredients, the amount needed for reaction to go to completion and produce the maximum amount of carbon dioxide, and hence the maximum amount of bubbling would be grams of baking soda as follows:
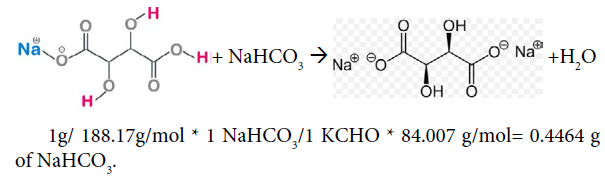
The number of grams of sodium hydrogen carbonate needed to completely neutralize 1 g of vitamin C and 1 g of sodium bitartrate must be 1.3117g+ 0.4464g = 1.76g.
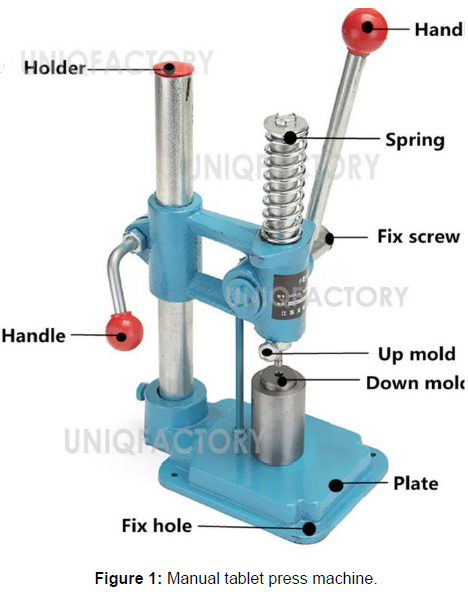
Water as a binding agent causes bubble loss during creating the tablet and glycerol would act as a better binding agent since sugars would cause bubbling to persist and the compounds in the ingredients are inert in glycerol. Sodium chloride reduces bubble formation, the amount of base used was insufficient to neutralize both citric acid and Potassium bitartrate in the formula. (Figure 1)
Conclusion
The tablets have been created using the correct molar amount for each chemical in the ingredients and the amount of bubbling as increased significantly, the number of bubbles formed has doubled using the correct formula. Using 1 g of Vitamin C and 1g of Sodium bitartrate, 1.76g of Sodium Hydrogen carbonate is needed in the ingredients to get the maximum amount of yield, maximum amount of CO2 in the product, in turn the maximum amount of bubbling per tablet in the formula. For the tablet to look smoother and for equal distribution of each of the compounds in the ingredient per table, the ingredients must be grinded to powder before adding the binding agent, glycerol. Sugars including glycerol, sugar alcohol and sucrose are good additives to cause to persistence of bubbling forming ability of the tablet created. It is also found that using sodium chloride would prevent forming bubbles, using greater number of moles of starch would cause tablet to be thicker on snow and glycerol acted as an ideal chemical binding agent for the ingredient.
References
- Ebbing and Gammon, 2015, General Chemistry, Cengage, P.103
- Ebbing and Gammon, 2015, General Chemistry, Cengage, P.108
- Ebbing and Gammon, 2015, General Chemistry, Cengage, P.111
- https://en.wikipedia.org/wiki/Acid%E2%80%93base_reaction
- Brown, LeMay, Burstein, Murphy, Woodward, Stottzfus, 2017, Chemistry the Central Science, Pearson, P.93
- Brown, LeMay, Burstein, Murphy, Woodward, Stottzfus, 2017, Chemistry the Central Science, Pearson, P.94-95
- Brown, LeMay, Burstein, Murphy, Woodward, Stottzfus, 2017, Chemistry the Central Science, Pearson, P. 102
- Brown, LeMay, Burstein, Murphy, Woodward, Stottzfus, 2017, Chemistry the Central Science, Pearson, P.106
- Brown, LeMay, Burstein, Murphy, Woodward, Stottzfus, 2017, Chemistry the Central Science, Pearson, P.703
- https://pubchem.ncbi.nlm.nih.gov/compound/Glycerol
- Brown, LeMay, Burstein, Murphy, Woodward, Stottzfus, 2017, Chemistry the Central Science, Pearson, P.1048
- https://www.molinstincts.com/formula/Cornstarch-cfml-CT1087471098.html
- https://en.wikipedia.org/wiki/Betanin
- https://en.wikipedia.org/wiki/Sodium_chloride
- https://en.wikipedia.org/wiki/Potassium_bitartrate
- https://en.wikipedia.org/wiki/Sodium_bicarbonate
- https://pubchem.ncbi.nlm.nih.gov/compound/Citric-acid
Citation: Wahab HA (2022) Finding New and Ideal Formulation for Snow/ Water Foam Producing Tablets. Ind Chem, 8: 180. DOI: 10.4172/2469-9764.10002180
Copyright: © 2022 Wahab HA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2346
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1851
- PDF downloads: 495