Financial Impact of Coagulase-Negative Staphylococcal (CoNS) Bacteremia at a Cancer Hospital
Received: 11-Jan-2014 / Accepted Date: 27-Jan-2014 / Published Date: 29-Jan-2014 DOI: 10.4172/2161-1165.1000147
Abstract
Objective: To determine the financial impact of Coagulase-Negative Staphylococcus (CoNS)- positive blood cultures.
Design: Retrospective chart review.
Setting: Tertiary care cancer hospital.
Patients: All episodes of CoNS bacteremia during the first 6 months of 2001 were identified. Episodes from the intensive care unit, those never treated, and those treated with antibiotics before the culture was drawn or became positive were excluded. Chart review was performed in a random sample of patients to estimate the cost of each component of medical care attributable to the management of CoNS. Each patient-episode was retrospectively designated as infection or contaminant by an expert reviewer.
Results: In the 6 month study period, 137 episodes of CoNS were treated. Medical records for 43 (39%) of 111 eligible patients were extracted. The average cost of managing the 43 CoNS episodes was $7,594. For the subset of 16 patients with increased length of stay (LOS) attributable to CoNS, the mean cost was $15,919, 77% due to hospital day charges. The 43 episodes were retrospectively designated either infection (n=31) or contaminant (n=12); attributable cost differed by assigned category. The mean cost for 31 patients with infection was $8,858. Six episodes, designated as contaminants by both primary team and expert review, had a mean cost of $1,881. Six additional episodes, treated as infection by the primary team but reassigned as contaminant on retrospective review, had a mean cost of $7,127. Applying the proportion of patients (6/43 or 14%) reclassified as contaminant to the annual number of treated blood cultures (2×137) would project to an annual savings of $201,237 ($7,127 minus $1,881 or $5,246/episode) at our hospital.
Conclusion: In the management of CoNS bacteremia, unneeded treatment of contaminants as true infection costs up to $200,000 a year at our hospital. Infectious disease or antibiotic management program management may result in a reduction of this cost.
Keywords: Bloodstream infection; Coagulase-negative staphylococcus; Cost and cost analysis
161230Introduction
Coagulase-Negative Staphylococcus (CoNS) is the third most common cause of Bloodstream Infection (BSI) [1] and the most common cause of nosocomial BSI [2]. In addition, the incidence and detection of serious nosocomial bacteremia due to CoNS is increasing, due to more frequent use of vascular access devices and more sensitive blood culture systems [3]. CoNS is also the most common cause of blood culture contamination, especially when cultures are obtained from Central Venous Catheters (CVC) [4-6]. Given that 72% of CoNS isolates in the United States in 1997 were methicillin-resistant, [1] many patients receive prolonged courses of vancomycin that may or may not be appropriate. Substantial hospital resources are dedicated to the management of CoNS isolated from blood cultures.
At our institution, CoNS is overwhelmingly the most common organism isolated in blood cultures, with 500-700 episodes per year or about 40% of all positive blood cultures. We sought to measure the financial impact of managing episodes of CoNS-positive blood cultures by defining all components of medical care that contribute to cost. Such information may help justify the need for programs to improve antibiotic management.
Methods
Patient population
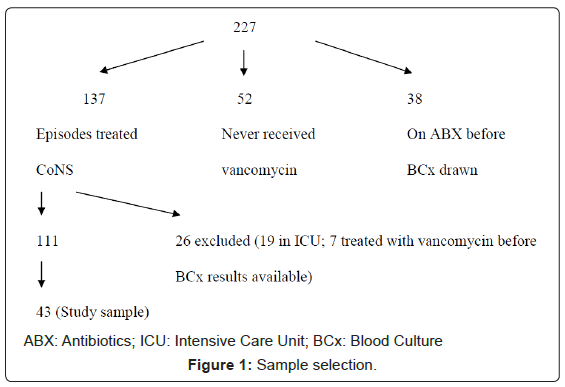
We retrospectively identified all blood cultures that were positive for CoNS between January and June 2001 at Memorial Sloan-Kettering Cancer Center, a 431-bed tertiary care cancer hospital in New York City (n=227). After exclusion of patients who were receiving antibiotic treatment prior to having blood cultures drawn (n=38) and patients who were never treated for the CoNS (n=52), there were 137 treated patient-episodes of CoNS in the 6-month study period. In addition, we excluded any patient who was in the Intensive Care Unit (ICU) when the incident culture was obtained (n=19) and those who received antibiotics after the blood culture was drawn but before it became positive for CoNS (n=7) (Figure 1). Of the remaining 111 episodes, 43 (39%) were selected at random and closely examined. Extracted variables are listed in Table 1. Each episode was classified as infection or contaminant in two ways: primary (defined as the opinion of the original treating team as documented in the medical record) and retrospective (based on chart review by a single observer [MJM] with confirmation by a second observer [GZ]).
| Microbiology |
| Positive blood culture (Date of first positive, number of additional positive) |
| Negative blood culture (Date of first negative, number of negative*) |
| Urine culture* |
| Antibiotics (vancomycin and others†) |
| Start and stop dates, number of doses, total dosage given (g) |
| Additional tests* |
| Echocardiogram |
| Electrocardiogram |
| CVC placement and removal |
| Consultations |
| Dermatology, Infectious Disease, Nephrology |
| Length of stay |
| Home care |
| Vancomycin (Start and stop dates, number of doses, total dosage given (g)) |
| Visiting nurse services (# days) |
†Given if complications arose with vancomycin
Table 1: Summary of laboratory and clinical variables.
Component costs
Each microbiologic, laboratory and clinical variable was assigned an incremental dollar amount to allow calculation of the financial impact of an episode of a positive blood culture for CoNS (Table 2). Hospital charges rather than costs were used and actual dollar amounts were obtained from relevant hospital departments. Home care charges were obtained from the principal home care provider used by the institution (Coram Healthcare). Average Wholesale Prices (AWP) was used to estimate the incremental financial contribution of antibacterial therapy. For each patient, the number of doses and grams of vancomycin administered was calculated and used to determine the total cost of vancomycin. One patient received 5 doses of intravenous linezolid. The same in-hospital administration fees were used and the cost of intravenous linezolid was $71.88 per 600mg bag (AWP).
| Item | |
| Laboratory | Incremental cost (per item) |
| Negative blood culture | $170 |
| Positive blood culture (including susceptibility testing) | $197 |
| Urine Culture | $64 |
| Vancomycin levels (peak, random, or trough) | $68 |
| Antimicrobial | |
| Vancomycin in hospital or at home | $7.80/500mg vial |
| $15.60/1gm vial | |
| Inpatient antibacterial administration (15 minutes of nursing and pharmacy time, $39/hour) | $19.50/dose |
| Home antibacterial administration | $110/day for QD |
| $120/day for BID | |
| Nursing visits for home/catheter care (3 visits in week 1, then 1 visit per week) | $90/visit |
| Tests & procedures | |
| Tunneled catheter placement | $1445 |
| Tunneled catheter removal | $1100 |
| Triple lumen catheter or PICC line placement | $340 |
| Echocardiogram | $1579 |
| EKG | $141 |
| Consultation services | |
| Assuming initial and two follow-up visits | $695 |
| Hospital stay | |
| Initial hospital day | $3000 |
| Subsequent hospital days | $2369 |
Table 2: Items contributing to financial impact.
Only attributable costs, or those incurred in response to the positive blood culture, were included in the calculation of financial impact. A single observer (MJM) attributed cost as related or not to CoNS management. The first action that indicated a response to the positive blood culture, usually initiation of vancomycin treatment, was the point at which the assessment of financial impact began. The assessment of financial impact ended with the completion of antibacterial therapy. This study was conducted in accord with policies of the institutional review board.
Results
The 43 patients had a broad range of cancer diagnoses, including 22 with hematologic malignancies (Table 3). Twenty-six were men and the median age was 50 (mean age 44 years). Patients had an average of 2 positive (range 1-11) and 4 negative (range 0-13) blood cultures. The average length-of-stay was 21 days (range 3-75). Home intravenous therapy was given to 12 patients (28%) for an average of 8 days.
| Median age (years) | 50 (range 3-84) |
| Female: Male | 17:26 |
| Positive blood cultures (average per patient) | 2 (range 1-11) |
| Negative blood cultures (average per patient) | 4 (range 0-13) |
| CVCs placed (total N) | 7 |
| Tunneled catheters placed (total N) | 2 |
| Tunneled catheters removed (total N) | 7 |
| Urine cultures performed (total N) | 1 |
| EKGs performed (total N) | 2 |
| Echocardiograms performed (total N) | 5 |
| Consultations performed (total N) | 11 |
| Home intravenous therapy (total N) | 12 |
| Duration of home intravenous therapy (average days) | 8 (range 3-22) |
| Increase in LOS attributable to CONS (average days) | 5 (range 1-13) |
Table 3: Overview of 43 patients with positive blood cultures for CoNS treated with vancomycin.
Catheter removal was required in 7 patients, all of whom had a tunneled device. Echocardiograms were done in 5 patients (8.6%) and 11 patients had a subspecialty consultation by Infectious Disease, Dermatology or Nephrology. Management of the CoNS episode required central venous catheter placement in 18 patients, including peripherally inserted central catheter (PICC) (n=9), triple lumen catheter (n=7), and tunneled catheter (n=2).
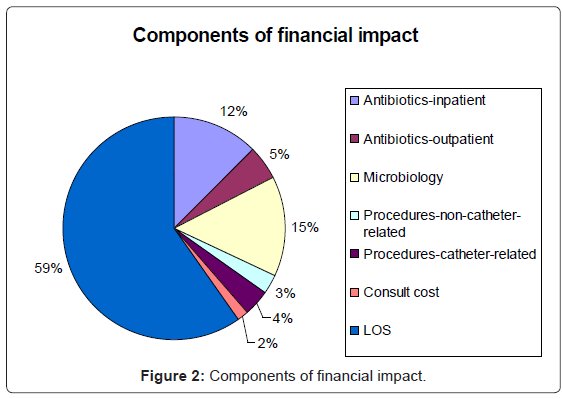
The average total cost of managing a CoNS-positive blood culture was $7,594 (range: $507-$38,437). Increase in length-of-stay was the largest component of the financial impact (59%), (Figure 2). Treatment with antimicrobials, including pharmacy services, administration, and vancomycin levels, both at home and in the hospital, accounted for an additional 17%. Microbiology costs, including attributable negative surveillance and positive blood cultures, accounted for 15% of the financial impact. Procedures (7%) and additional physician consultations (2%) accounted for relatively little of the financial burden.
Comparison of primary and retrospective review found that 37 episodes were assessed concordantly (31 with infection and 6 with contaminant) (Table 4). The financial impact of these concordantly assessed cultures averaged $8,858 and $1,881, respectively. Six episodes were evaluated discordantly (primary assessment of infection, retrospective assessment of contamination). These episodes had a mean cost of $7,127. Four of these six had an attributable increase in length of- stay (1,2,3 and 7 days) with an average financial impact of $10,211 (range $4,389- $20,468).
| Number of episodes (%) | Primary review | Retrospective review | Concordance | Mean Cost ($) |
|---|---|---|---|---|
| 31 (72%) | Infection | True Infection | Yes | $8,858 |
| 6 (14%) | Infection | Contaminant | No | $7,127 |
| 6 (14%) | Contaminant | Contaminant | Yes | $1,881 |
Table 4: Results of scoring infection versus contaminant (43 isolates).
Applying the proportion of patients (6/43 or 14%) reclassified as contaminants to the approximately 274 (2×137) CoNS episodes given vancomycin per year, we estimate that 38 episodes are misclassified annually. This misclassification is associated with an excess expenditure of $5,246 per episode ($7,127 minus $1,881) or $201,237 annually. Prospective identification of misclassified blood cultures could eliminate these expenditures.
Discussion
CoNS is frequently isolated in blood cultures and is the most common cause of nosocomial bacteremia in the United States. To determine the cost of managing this common isolate, we undertook the arduous task of carefully reviewing individual charts to assign cost as attributable to CoNS management or not.
A number of studies have investigated the cost of managing blood culture contaminants, [6-8] but few have focused solely on CoNS. Bates et al. [6] found that the total incremental increase in charges (laboratory, microbiology, and pharmacy) associated with contaminant blood cultures was $4,385. Our estimate ($5,246) is higher than in the Bates study performed in 1989. This difference may be accounted for by inflation and differences in institutional practice patterns. Although the study by Bates et al did not independently analyze cases of CoNS, the majority of patients (66/104, 63%) in this study had false-positive episodes of Staphylococcus epidermidis [6].
Waltzman and Harper [7] also evaluated charges associated with false-positive blood cultures in a population of febrile, but otherwise healthy young children. Of the contaminants isolated from 87 children who were evaluated for fever without a focus, 54 (63%) had blood cultures with staphylococcal species other than Staphylococcus aureus. In this series, 0.9% of all cultures was false-positives and yielded $32,230 in additional charges, which translated to an average additional charge of $3.40 per patient. The authors concluded that this excess charge was inconsequential in relation to the charges associated with the initial evaluation of young children with fever. They attributed this low rate of false positive blood culture results to the use of three isopropyl alcohol pads for skin preparation in their emergency room. Likely, this low rate is also due to the lack of vascular access devices in this healthy, young population [7]. Dunagan et al. [8], in a prospective study of 70 patients who had not undergone bone marrow transplantation with at least one positive blood culture, found that 22.3% of antimicrobial use was inappropriate treatment of false-positive blood cultures. After adjusting for severity of illness and diagnosis, this correlated with $5,368 in additional hospital charges per episode of bacteremia.
The majority of episodes (72%) of blood cultures positive for CoNS in our study were considered infection by both the primary treatment team and the retrospective review. This is higher than in published series, where the incidence of clinically significant CoNS bacteremia has been reported to be 24.7% [9] and 30% [10]. Although, as in the present study, these series used NNIS criteria as the basis for the definition of BSI, the unique cancer population in our study might have led to differences in blood culture interpretation. For example, treatment of a single blood culture positive for CoNS may be appropriate in a febrile neutropenic patient with an indwelling central venous catheter.
Overall, most positive cultures were handled properly, with 86% demonstrating concordance between the treating physician and expert reviewer. Other series have also found high concordance between retrospective review and the attending physicians’ clinical impression in the classification of a bacteremic episode as either BSI or contamination [9]. In most cases in our study, clinical decisions were made without consultation with an infectious disease subspecialist. Fourteen percent of the episodes were misclassified, demonstrating the need for improvement. Timely intervention in antibiotic management could limit the financial impact of these episodes.
We found that treatment cost of a true infection ($8,858) was higher than treatment of a contaminant ($1,881) or misclassified episode ($7,127). Misclassified episodes resulted in an excess expenditure of $5,246 per episode ($7,127-$1,881) or potentially $201,237 annually. This is higher than in a published series that exclusively considered CoNS and estimated the costs for treating patients with CoNS contaminants to be $1,000, entirely due to additional antibiotic administration [9]. However, it is not substantially different from the Bates et al ($4385 excess expenditure per episode) or Dunagan et al. ($5368 excess expenditure per episode) studies that included all falsepositive blood cultures [6,8].
Increases in length of stay accounted for the greatest proportion of the financial impact (59%) in our study while outpatient antibiotic therapy and home administration accounted for only 5%. Regardless of whether a case is considered to be a true BSI or contaminant, home intravenous therapy appears to be a cost-saving maneuver.
Species identification and selected strain genotyping of CoNS may reduce the misinterpretation of probable contaminants among patients with ≥ 2 positive blood cultures for CoNS [11,12]. However, real-time strain typing techniques, such as pulsed field gel electrophoresis, to aid in the analysis of blood cultures positive for CoNS may not be available or affordable at many institutions. Another approach to distinguishing between infection and contamination is the drawing of multiple cultures. In a study performed in a neonatal intensive care unit, percutaneous blood cultures drawn from two separate sites was shown to reduce misinterpretation of CoNS BSI and lead to reductions in the duration of vancomycin therapy in 5% of babies and a decrease of 8.2% in vancomycin usage [13].
Our study had several potential limitations. We examined the charts for only 39% of the group of identified patients and so, despite random sampling, may not have obtained a representative sample. A second limitation was that determination of the components of the financial impact was made retrospectively from chart review, not prospectively. This might have led to an over-estimate of the component costs for responding to a positive blood culture because the active decision-making process by clinicians could not be considered. A third limitation is that hospital charges were used as a surrogate for actual costs, with the exception of antimicrobial costs. The summary values calculated are therefore composites of both cost and charges estimate. Lastly, our patient population may not be representative of patients treated at most other hospitals. Results therefore may be less generalizable. Nevertheless, it is evident that an intervention designed to reduce treatment of false-positive CoNS blood cultures could result in significant cost savings for the hospital.
In conclusion, management of blood cultures positive for CoNS is an expensive endeavor at our institution accounting for a mean expense of $7,594 per episode. Curtailment of inappropriate treatment by antibiotic management team review, infectious disease consultation, or automatic antibacterial discontinuation after 72 hours may save >$200,000 per year at our hospital. Given the increased use of CVCs in all medical settings, the identification of false-positive CoNS blood cultures and subsequent cessation of inappropriate therapy may result in lowering of health care costs.
Acknowledgement
NIA T32 AG019134 and the John A. Hartford Foundation (MJM).
References
- Pfaller MA, Jones RN, Doern GV, Sader HS, Kugler KC, et al. (1999) Survey of blood stream infections attributable to gram-positive cocci: frequency of occurrence and antimicrobial susceptibility of isolates collected in 1997 in the United States, Canada, and Latin America from the SENTRY Antimicrobial Surveillance Program. SENTRY Participants Group. Diagn Microbiol Infect Dis 33: 283-297.
- Jarvis WR, Martone WJ (1992) Predominant pathogens in hospital infections. J Antimicrob Chemother 29 Suppl A: 19-24.
- Bryan CS (1989) Clinical implications of positive blood cultures. Clin Microbiol Rev 2: 329-353.
- Everts RJ, Vinson EN, Adholla PO, Reller LB (2001) Contamination of catheter-drawn blood cultures. J Clin Microbiol 39: 3393-3394.
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, et al. (2001) Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis 32: 1249-1272.
- Bates DW, Goldman L, Lee TH (1991) Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA 265: 365-369.
- Waltzman ML, Harper M (2001) Financial and clinical impact of false-positive blood culture results. Clin Infect Dis 33: 296-299.
- Dunagan WC, Woodward RS, Medoff G, Gray JL 3rd, Casabar E, et al. (1989) Antimicrobial misuse in patients with positive blood cultures. Am J Med 87: 253-259.
- Souvenir D, Anderson DE Jr, Palpant S, Mroch H, Askin S, et al. (1998) Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol 36: 1923-1926.
- Finkelstein R, Fusman R, Oren I, Kassis I, Hashman N (2002) Clinical and epidemiologic significance of coagulase-negative staphylococci bacteremia in a tertiary care university Israeli hospital. Am J Infect Control 30: 21-25.
- Kim SD, McDonald LC, Jarvis WR, McAllister SK, Jerris R, et al. (2000) Determining the significance of coagulase-negative staphylococci isolated from blood cultures at a community hospital: a role for species and strain identification. Infect Control Hosp Epidemiol 21: 213-217.
- Seo SK, Venkataraman L, DeGirolami PC, Samore MH (2000) Molecular typing of coagulase-negative staphylococci from blood cultures does not correlate with clinical criteria for true bacteremia. Am J Med 109: 697-704.
- Struthers S, Underhill H, Albersheim S, Greenberg D, Dobson S (2002) A comparison of two versus one blood culture in the diagnosis and treatment of coagulase-negative staphylococcus in the neonatal intensive care unit. J Perinatol 22: 547-549.
Citation: Juthani-Mehta M, Seo SK, Bernstein P, Eagan J, Sohn S, et al. (2014) Financial Impact of Coagulase-Negative Staphylococcal (CoNS) Bacteremia at a Cancer Hospital. Epidemiol 4:147. DOI: 10.4172/2161-1165.1000147
Copyright: © 2014 Juthani-Mehta M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16158
- [From(publication date): 4-2014 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 11389
- PDF downloads: 4769